Transcranial photobiomodulation with near-infrared light: a promising therapeutic modality for Alzheimer’s disease
Hanli Liu, Damir Nizamutdinov, Jason H. Huang
Transcranial photobiomodulation (tPBM) is a noninvasive neuromodulation technique that delivers near-infrared (NIR) light with low irradiance (i.e., power density in mW/cm2) in the wavelength range of 800–1070 nm. Several recently published books or collected literature (Hamblin, 2019; Gonzalez-Lima, 2021) and papers (Nizamutdinov et al., 2022) offer comprehensive reviews of the mechanism of action and potential clinical translations of tPBM for the treatment of a variety of diseases, including neurodegenerative diseases (Alzheimer’s disease (AD), and Parkinson’s disease), traumatic brain injury, stroke, and psychiatric disorders (depression and post-traumatic stress disorder). This communication focuses on the feasibility and benefits of tPBM in treating patients with AD. The socio-economic burden of AD is significant and will only increase with longer life expectancy unless effective interventions are developed. Currently, none of the therapeutic strategies have been successful in treating or alleviating the symptoms of AD.
In this paper, we review the well-accepted tPBM mechanisms of action, and summarize key experimental findings of tPBM-induced neurophysiological enhancement (Wang et al., 2017, 2021; Shahdadian et al., 2022; Truong et al., 2022) and behavioral improvements in healthy young (Zhao, 2022) and older adults (Qu et al., 2022), and provide tPBM mechanistic insights in living human brains. In addition, we demonstrate our clinical study with evidence-based safety and cognitive improvements in patients with dementia using daily tPBM therapeutic protocol at home (Nizamutdinov et al., 2021). Finally, we also discuss the mechanistic rationale of tPBM supported by a recent AD animal study (Yang et al., 2022) and documented literature.
Mechanism of action of PBM:The most welldocumented and accepted mechanism underlying the effects of PBM is based on the reports that complex IV of cytochrome C oxidase (CCO) in the mitochondrial respiratory chain can absorb NIR light at the local stimulation site (Wang et al., 2017; Hamblin, 2019; Gonzalez-Lima, 2021). The absorbed light stimulates cellular adenosine triphosphate, manages or counteracts reactive oxygen species, Ca2+, and the release of nitric oxide (NO) (Hamblin, 2019). Accordingly, this signaling reaction promotes energy supplementation to the disease-affected brain tissue with prominent mitochondrial dysfunction.
Another reported mechanism contributing to the local restoration of brain functionality is vasodilation stimulated by the endothelial secretion of NO with subsequent regional elevated blood perfusion resulting in increased cortical oxygenation. This mechanism contributes to oxidative stress management and supports other reported effects of tPBM, including neuroprotection, neurogenesis, synaptogenesis, and angiogenesis. Engaged signaling cascades and corresponding changes in the brain parenchyma promote positive support of energy metabolism and neuron-to-neuron communication networks, enhancing brain clearance through the improved local function of the vascular, glymphatic, and lymphatic systems (Nizamutdinov et al., 2022). Overall, tPBM intervention improves brain performance in healthy adults and patients affected by chronic neuropathology. Interestingly, local tPBM also triggers a cascade of systemic responses with positive immune and prominent anti-inflammatory outcomes (Nizamutdinov et al., 2022).
Significant neurophysiological effects of tPBM on healthy humans:Since 2017, Liu and her group have reported a series of neurophysiological measurements from healthy humansin vivoby quantifying cerebral hemodynamic, metabolic, and electrophysiological responses during and after tPBM on the forehead using a 1064-nm laser stimulation (Wang et al., 2017, 2021; Shahdadian et al., 2022; Truong et al., 2022). These findings offer novel experimental evidence and mechanistic insights into how tPBM affects the living human brain at the site of stimulation, distant, and deeper cerebral regions.
Improvement of mitochondrial metabolism and hemodynamics:To investigate local changes caused by tPBM in cerebral neurophysiology, Wang et al. (2017) reported a sham-controlled human study that delivered a low-power 1064-nm laser beam to the forehead of healthy participants for 8 minutes and broadband NIR spectroscopy was utilized to acquire light reflectance from the light-treated region before, during, and after tPBM (Figure 1A). Quantitative analysis of time-dependent spectroscopic readings resulted in: (1) a significant increase in cerebral concentrations of oxidized CCO (P< 0.01), oxygenated hemoglobin ([HbO],P< 0.01; Figure 1B), and total hemoglobin ([HbT],P< 0.01) during and after tPBM, (2) linear interplay between increases of [CCO] and [HbO] and between increases of [CCO] and [HbT]. This is the first study to demonstrate that tPBM causes up-regulation of oxidized CCO in the local human brain, exhibits a linear correlation between enhanced mitochondrial metabolism and hemodynamics, and contributes important insights into physiological mechanisms.
To better understand the neurophysiological effects of tPBM across the entire human brain, a 111-channel functional near-infrared spectroscopy system (Figure 1C) was utilized to map cerebral hemodynamic responses to 8-min stimulation with 1064-nm tPBM on the forehead of 19 healthy participants (Truong et al., 2022). Truong et al. (2022) investigated the frequency-specific effects of tPBM on three infraslow oscillation components consisting of endogenic, neurogenic, and myogenic vasomotions. Significant changes induced by tPBM in spectral power of [HbO] and functional connectivity at each of the three infra-slow oscillation frequency bands were mapped topographically. The novel findings revealed that tPBM significantly increased endogenic [HbO] powers over the right frontopolar area near the stimulation site. In particular, the results showed that tPBM greatly enhanced endogenic and myogenic functional connectivity across the cortical regions (Figure 1D).
Combining the results from studies by Wang et al. (2017) and Truong et al. (2022), Figure 1E summarizes the two metabolic-hemodynamic events induced by tPBM. On the one hand, photooxidation of CCO enhances CCO redox metabolism and adenosine triphosphate synthesis, leading to a significant increase in [HbO]. On the other hand, tPBM activates the release of NO, which results in changes in endogenic and myogenic oscillations. Furthermore, vasodilation caused by an increase in NO levels leads to an improvement in cerebral blood flow.
tPBM improvements caused by non-thermal effects:It is a common question whether the reported outcomes of tPBM result from the thermal effects. A recent study (Wang et al., 2021) was designed using a 64-channel electroencephalogram (EEG) (Figure 1F). Two sets of sham-controlled measurements were performed in healthy adults by simultaneous recordings of EEG before, during, and following 8-min-long thermal stimulation (thermo_stim;n= 11) and tPBM stimulation (n= 46), respectively. After power analysis, the study demonstrated: (1) tPBM significantly increased EEG alpha and beta powers (Figure 1G), (2) thermal stimulation had opposite effects on EEG power topographic patterns compared with tPBM, and (3) tPBM and thermal stimulation induced significantly different topographies of changes in EEG alpha and beta waves (Wang et al., 2021).
Another human experiment proved the opposite and differed the impact of tPBM from the effect of heat on cerebral metabolic and hemodynamic changes measured by broadband NIR spectroscopy. In addition, magnetic resonance thermometry (Dmochowski et al., 2020) confirmed no significant increase in temperature between the tPBM and sham conditions. Overall, we conclude that the low intensity and irradiance of NIR brain stimulations transfer very low light energy to surrounding tissues and therefore do not contribute to beneficial outcomes associated with the treatment of tPBM through the thermal effect in the tissue. It is also worth noting that in our evidence-based observation from the clinical trial (Nizamutdinov et al., 2021), the heat of surrounding tissues or associated discomfort in the area of stimulation was never reported by our patients or family caregivers.
Improvement of electrophysiological power and connectivity:Wang et al. (2021) could demonstrate that right prefrontal tPBM with a 1064-nm laser stimulation enables the significant increase of alpha and beta powers in EEG rhythms (Figure 1G), and observed changes are not heat-induced but lightinduced effects of tPBM.
In addition, the same EEG data were investigated by graph-theory analysis as a topological approach for quantifying brain connectivity metrics at global and nodal/cluster levels (Shahdadian et al., 2022). The beta band, the nodal graphical analysis demonstrated significant increases in local information integration and centrality over the frontal clusters, accompanied by a decrease in segregation over the bilateral frontal, left parietal, and left occipital regions induced by tPBM (Figure 1H).
Thus, frontal tPBM increases EEG alpha and beta powers in the frontal-central parietal regions, enhances the complexity of the global beta-wave brain network and augments the local information flow and integration of beta oscillations across prefrontal cortical regions. These observations can translate the electrophysiological effects of tPBM on human cognitive improvements.
Significant augmentation of human cognition:In the past decade, Gonzalez-Lima and his group published more than 12 studies that reported significant argumentation of neurocognitive functions (Gonzalez-Lima, 2021). Six of these publications investigated 333 healthy young adults (177 females, ages 17–40 years). They reported tPBM-enabled acute enhancement in attention and working memory, attention bias modification, executive skills, category learning, comparison of tPBM with exercise, and overall cognitive rate correct score (Gonzalez-Lima, 2021). Also, acute tPBM effects in older participants (n= 21, 11 females, ages 49–90 years) suggested beneficial neurocognitive effects of repeated tPBM (Gonzalez-Lima, 2021). More recently, two independent groups have presented significant improvement in working memory in healthy young (n= 79, age 23 ± 4 years) (Zhao, 2022) and older (n= 86, age 65 ± 6 years) adults (Qu et al., 2022) by use of repeated tPBM.
Clinical effects of tPBM in patients diagnosed with AD:Dementia is a complex syndrome with various presentations depending on the underlying pathology. In recent years, Huang and his group have completed several placebo-controlled, randomized, double-blinded clinical trials using tPBM, focusing on testing the safety, feasibility of therapeutic protocol, and efficacy to treat patients with dementia and AD-related dementia. One of these clinical trials had fifty-seven completed subjects and tested therapeutic protocol using two-month-long, twicea-day daily use of low-power transcranial 1072 nm light stimulations resulted in promising outcomes based on neuropsychological evaluations and caregivers’ feedback assessment in patients’ behavior (Nizamutdinov et al., 2021). It is worth noting that some patients with moderate dementia showed improvement in several cognitive readings as an outcome of this treatment. Thus, while some patients demonstrated 80% improvement in the clock drawing tests, others scored up to 80% on the clock copying test (Figure 2), up to 75% improvement in Mini-Mental State Exam readings, up to 52% improvement in the logical memory subtest, up to 73% on executive function using A and B trail making tests, and 35% improvement on verbal learning and memory tests, respectively (Nizamutdinov et al., 2021). The use of the tPBM therapeutic protocol reflected in the longevity of night sleep as early as day 6 of treatment and improvement in episodes of recurrent nightmares, anxiety, and mood swings. Researchers also reported beneficial effects of tPBM in patients with dementia in managing attention deficits, lack of concentration, and improvement of executive function, patient engagement, and energy to be involved in daily routines.
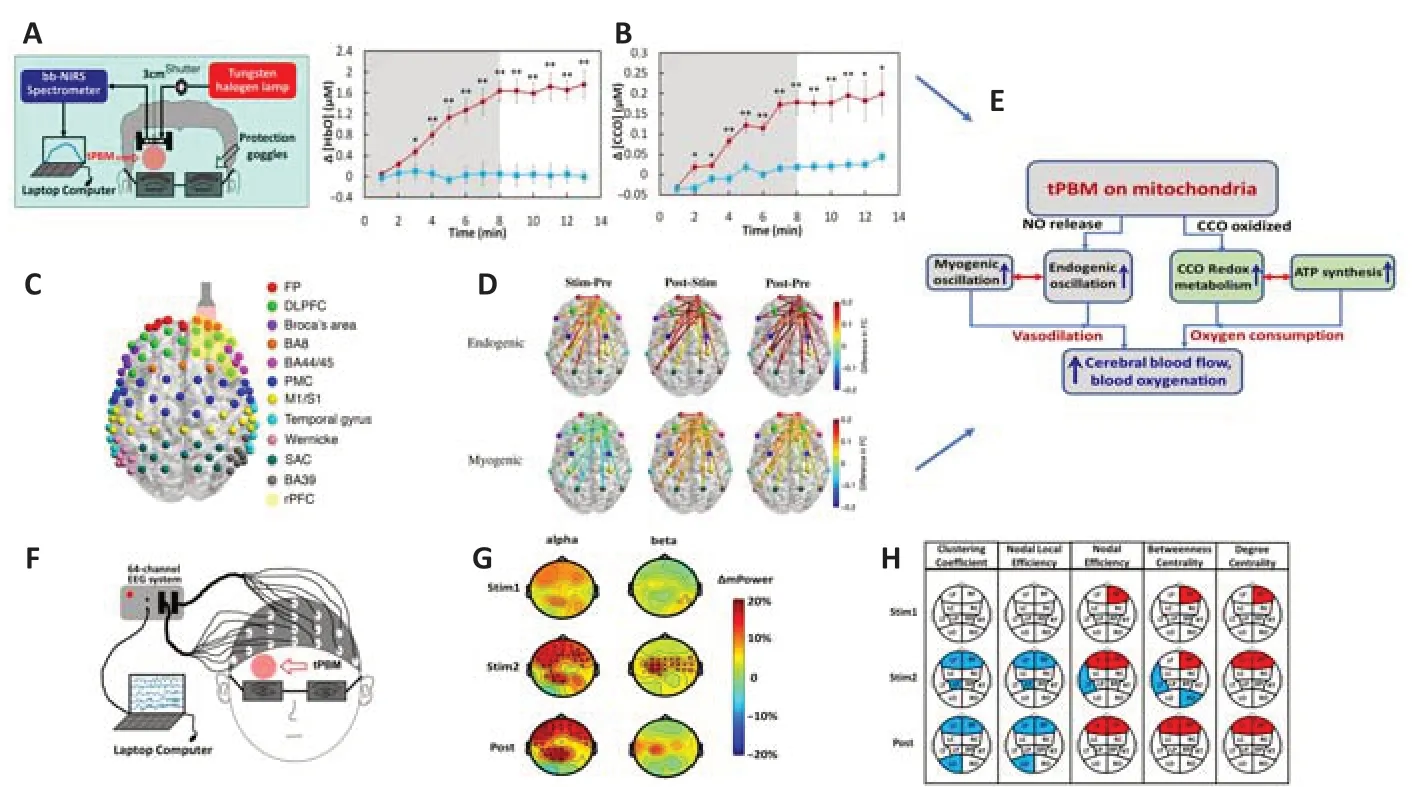
Figure 1|Summarized results of tPBM-induced neurophysiological alterations by 1064-nm right forehead laser treatment in healthy participants.
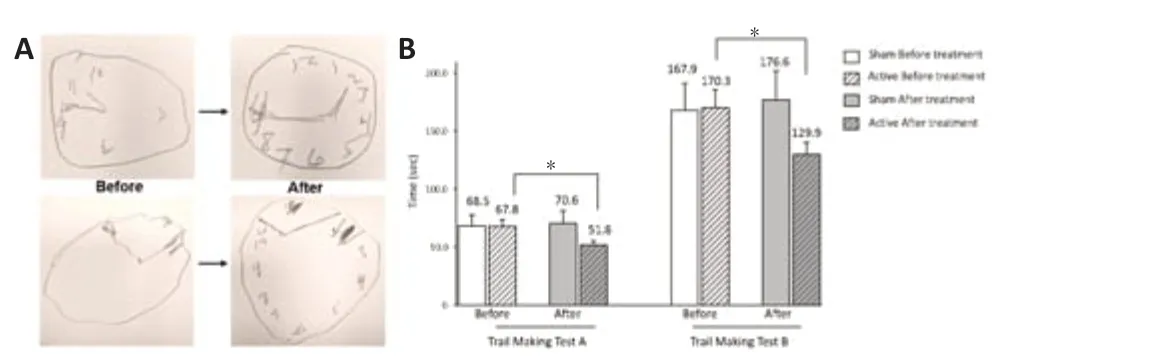
Figure 2|Cognitive improvements after treatment with 1072 nm tPBM twice daily for 8 consecutive weeks (Nizamutdinov et al., 2021).
A new view of the mechanism of action in treating AD with tPBM:A recent animal study tested a novel and detailed view of the mechanism of action of tPBM in treating AD (Yang et al., 2022). This study used tPBM for 2 minutes per session, three times per week, and 16 months long for 2-month-old transgenic AD rats. All animals with AD underwent a battery of behavioral tests to measure the effect of tPBM on cognitive dysfunction. The changes in AD-associated brain pathologies (amyloid plaques, intracellular neurofibrillary tangles, neuronal loss, apoptosis, neuronal injury, and neurodegeneration) were also quantified. The mechanisms of action were tested using molecular techniques and primary cell cultures (Yang et al., 2022). This study unveils the beneficial effects of tPBM on (1) preservation of mitochondrial dynamics, (2) inhibition of neuroinflammation by regulating glial cell polarization, and (3) management of neuronal oxidative damage of DNA, proteins, and lipids in transgenic AD rats.
Our human studies summarized in previous sections are indirectly consistent with findings in this animal study supporting the effects of tPBM on (1) mitochondrial activity and metabolism and (2) an increase in hemoglobin concentration, which mediates neuroprotection.
Summary:tPBM with NIR light stimulations presents an excellent and promising therapeutic modality to treat AD and AD-related dementia. The provided mechanistic understanding and tested protocol can be a powerful tool for a long-awaited therapeutic intervention of AD. Large mechanistic, placebocontrolled, randomized clinical trials are necessary to determine efficacy and achieve the full potential of tPBM.
This work was supported in part by the National Institute of Mental Health at the National Institutes of Health under the BRAIN Initiative (RF1MH114285) (to HL).
*Correspondence to:Jason H. Huang, MD, Jason.Huang@bswhealth.org.
https://orcid.org/0000-0002-4426-0168 (Jason H. Huang)
Date of submission:November 4, 2022
Date of decision:November 29, 2022
Date of acceptance:December 5, 2022
Date of web publication:January 5, 2023
https://doi.org/10.4103/1673-5374.366499
How to cite this article:Liu H, Nizamutdinov D, Huang JH (2023) Transcranial photobiomodulation with near-infrared light: a promising therapeutic modality for Alzheimer’s disease. Neural Regen Res 18(9):1944-1945.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Mesenchymal stem cells, extracellular vesicles, and transcranial magnetic stimulation for ferroptosis after spinal cord injury
- Inducing prion protein shedding as a neuroprotective and regenerative approach in pathological conditions of the brain: from theory to facts
- Use of mesenchymal stem cell therapy in COVID-19 related strokes
- Brain organoids are new tool for drug screening of neurological diseases
- Emerging roles of astrocytes in blood-brain barrier disruption upon amyloid-beta insults in Alzheimer’s disease
- External anal sphincter electromyography in multiple system atrophy: implications for diagnosis, clinical correlations, and novel insights into prognosis

