Tetracycline sensitizes TiO2 for visible light photocatalytic degradation via ligand-to-metal charge transfer
Caidie Qin,Juanjuan Tang ,Ruxia Qiao ,Sijie Lin,*
a College of Environmental Science and Engineering,Biomedical Multidisciplinary Innovation Research Institute,Shanghai East Hospital,Tongji University,Shanghai 200092,China
b Key Laboratory of Yangtze River Water Environment,Shanghai Institute of Pollution Control and Ecological Security,Tongji University,Shanghai 200092,China
Keywords:Ligand-to-metal charge transfer sensitization Tetracycline TiO2 Photocatalytic degradation Antibiotic resistance gene
ABSTRACT Treatment of antibiotics contaminated water remains a global environmental challenge.In this study,tetracycline(TC)was found to effectively sensitize pure TiO2 for visible light photocatalytic degradation via a ligand-to-metal charge transfer mechanism.The sensitization was attributed to the formation of TC-TiO2 complex and the overlap of the molecular orbitals of TC and the conduction band of TiO2.The intermediate degradation products of TC,however,did not sensitize TiO2,which was the reason for the low mineralization rate.Nevertheless,our results showed that the intermediate degradation products of TC had significantly reduced bactericidal effects and less induction of antibiotic-resistance genes(ARGs).This study showcases an effective treatment of antibiotics-containing wastewater using the most common photocatalyst TiO2 with reduced risk in the spread of ARGs.
Tetracycline(TC)and TC antibiotics are heavily used to treat bacterial infections in humans and animals.Since TC are usually not fully absorbed and more than half of them are released into the environment,it remains an environmental challenge to address the risk of antibiotics owing to their retained biological activity[1].Studies have suggested that the accumulation of TC in wastewater treatment plant lowers the effectiveness of microbial treatment process and leads to the promotion of resistant strains[2].The continuous release of TC into aquatic environment further promotes the evolution of antibiotic resistance and exerts detrimental effects to the untargeted organisms[3,4].Therefore,it is essential to explore effective treatment technology meanwhile to evaluate their side effects,e.g.,promotion of antibiotic resistance.
In recent years,photocatalytic technology based on visible light-responsive semiconductor has been widely recognized as one of the most promising strategies to solve the increasingly serious environmental problems[5].In order to improve the photocatalytic performance of semiconductors,different heterojunction structures have been widely studied in the fields of photocatalysis,photoelectrochemistry and so on[6–8].Titanium dioxide(TiO2),the mostly studied photocatalyst has limited practical application due to the inability to be activated by visible light[9].By contrast,sensitization has recently emerged as an effective strategy as it does not require additional equipment or material modification[10–12].Among various sensitization methods,ligand-metal charge transfer(LMCT)enhances the visible light reactivity of TiO2through the formation of ligand-TiO2complexes[12].Although there were some studies mentioned the sensitization effect of antibiotics[13–15],the underlining mechanism was not yet clear to allow us to take full advantages of such sensitization strategy.
Not only the formation of TC-TiO2complex,but the overlap between the molecular orbitals of TC(i.e.,the highest occupied molecular orbital(HOMO)and lowest occupied molecular orbital(LUMO))and the conduction band(CB)of TiO2could play a critical role in facilitating the electron transfer between TC and TiO2.To validate this hypothesis,we set out to explore the sensitization effect of TC to TiO2,the resulted visible-light activated photocatalytic degradation,and the extent of mineralization.The bactericidal effects and the potential to induce antibiotic resistance genes of the degradation products were also evaluated.Other typical antibiotics,i.e.,norfloxacin(NOR)and sulfisoxazole(SSX)were selected for comparison purpose.
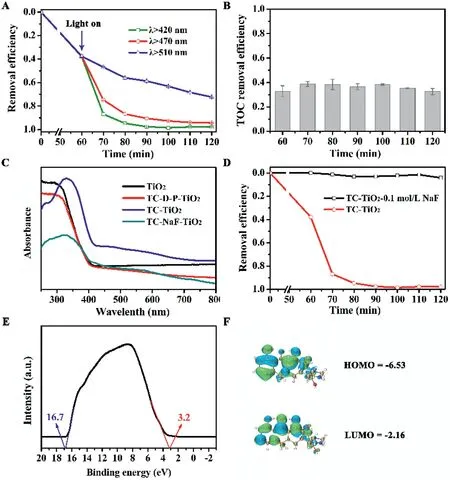
Fig.1.(A)Photocatalytic degradation of TC by pure TiO2 under different wavelengths:λ>420 nm,λ>470 nm,andλ>510 nm.(B)TOC removal efficiency of TC under visible light irradiation with TiO2.Conditions:[TiO2]=0.5 g/L,[TC]0=10 mg/L,and pH 5.5,λ>420 nm.(C)UV–vis absorbance spectra of pure TiO2,TC-TiO2,TC-NaF-TiO2,and the TC degradation products(named as TC-D-P)-TiO2 complex powders.(D)Effect of fluorides on removal efficiency of TC with pure TiO2 under visible light irradiations.Conditions:[TiO2]=0.5 g/L,[TC]0=10 mg/L,[NaF]=0.1 mol/L,and pH 5.5,λ>420 nm.(E)Full UPS spectrum of TiO2.(F)Frontier electron densities of LUMO and HOMO of TC.
Degussa P25 was used in this study to represent the most common TiO2,with typical transmission electron microscopic image and X-ray diffraction pattern shown in Fig.S1(Supporting information).Adsorption under dark after 60 min exhibited approximately 40%of TC(10 mg/L)being adsorbed by TiO2(0.5 g/L)(Fig.1A).The following photocatalytic degradation upon light irradiation at different wavelengths(i.e.,λ>420 nm,λ>470 nm,andλ>510 nm)showed an increasing trend of degradation efficacies with decreasing wavelengths.Interestingly,even with the irradiation wavelength well into the visible range,TiO2still displayed a significant degradation compared to the effect of visible light alone(Fig.S2A in Supporting information).These results suggested that the adsorption of TC on TiO2rendered the complex responsive to visible light,which could be attributed to the LMCT sensitization mechanism.However,with a clear degradation observed,the extent of total organic carbon(TOC)removal remained 35%−40%(Fig.1B),showing limited extent of mineralization.Similar phenomena were also observed in the case of SSX and NOR,where both antibiotics could sensitize TiO2but with little success in complete mineralization(Figs.S2B-D in Supporting information).
To verify the formation of TC-TiO2complex played a key role in the resulted sensitization,the ultraviolet-visible(UV–vis)absorbance spectra of TC-TiO2complex was obtained.As Fig.1C and Fig.S3A(Supporting information)showed,TC-TiO2complex had clear light absorbance in the visible range(λ>420 nm)while the pure TiO2and antibiotics only absorbed in the UV region.Pretreatment of NaF that prevented the adsorption of TC on the surface of TiO2resulted no visible light absorbance,confirming that the visible light responsiveness was a result of TC-TiO2complex formation.This was consistent with previous studies that showed fluoride preferentially adsorbed on the surface of TiO2and inhibited the adsorption of other molecules[16].In addition,NaF also led to a significantly reduced degradation of TC,a consequence of the reduced visible light responsiveness(Fig.1D).Consistently,pretreatment of NaF to TiO2also significantly reduced the sensitization effects of SSX and NOR antibiotics(Figs.S3B-D in Supporting information).
In order to demonstrate that the energy state overlap between the HOMO/LUMO of the antibiotics and the CB of TiO2determines the occurrence of sensitization,the CB and valence band(VB)levels of TiO2were measured using ultraviolet photoelectron spectroscopy(UPS).Based on the UPS spectrum of TiO2(Fig.1E),theEcutvalue was 16.7 eV and the estimated Fermi level(EF)value was−4.52 eV[17].The distance from EF to the VB level(EVB)was approximately 3.2 eV,and the band edge ofEVBwas−7.72 eV[18].As the calculated TiO2bandgap was 3.0 eV based on the UV–vis absorbance measurement(Fig.1C),the CB level(ECB)of the TiO2used in this study was−4.72 eV.Using discrete Fourier transform calculation,the HOMO and LUMO values of TC were−6.35 and−2.16 eV,respectively(Fig.1F).The details of the molecular orbital of SSX and NOR were summarized in Figs.S3E,F and Table S2(Supporting information).These results confirmed that the HOMO/LUMO of the antibiotics did overlap with the CB of TiO2,hence allowing the electron to flow from the antibiotics to the TiO2upon adsorption to facilitate the LMCT sensitization.
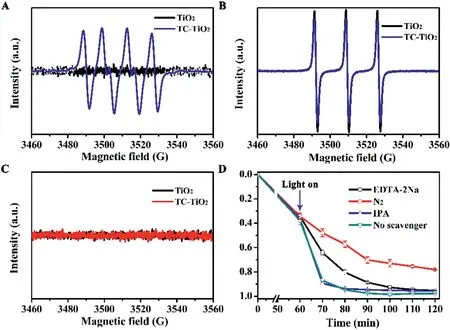
Fig.2.ESR spectra of pure TiO2 and the TC-TiO2 complex under visible light irradiations,(A)•O2−,(B)h+,and(C)•OH.Conditions:[TiO2]=0.5 g/L,[DMPO]=50 mmol/L,[TEMPO]=50 mmol/L,andλ>420 nm.(D)Effects of TBA(1 mmol/L),N2(introducing time 10 min)or EDTA-2Na(1 mmol/L)on degradation of TC under visible light irradiations.Condition:[TiO2]=0.5 g/L,[TC]0=10 mg/L,pH 5.5,andλ>420 nm.
To further investigate the degradation process of TC upon LMCT sensitization,the generation of reactive oxygen species,including superoxide(•O2−)radicals,hydroxyl(•OH)radicals,and photogenerated hole(h+)under visible light(λ>420 nm)irradiation was identified using electron spin resonance(ESR)spectroscopy.5,5-Dimethy-1-pyrrolineN-oxide(DMPO)and 2,2,6,6-tetramethylpiperidine 1-oxyl(TEMPO)were used as trapping agents.The results revealed that•O2−was generated in all three types of antibiotic-TiO2complexes(Fig.2A and Fig.S4A in Supporting information).As Fig.2B and Fig.S4B(Supporting information)showed,the signal from the antibiotic-TiO2complexes upon visible light irradiation decreased,indicating that h+was generated in the system,resulting in TEMPO being oxidized toN-oxoammonium salt[19,20].However,the sensitization process did not produce•OH(Fig.2C and Fig.S4C in Supporting information).To clarify the main radicals involved in the degradation of the antibiotics,EDTA disodium salt(EDTA-2Na),isopropanol(IPA),and N2were used to quench h+,•OH,and•O2−,respectively.It is worth mentioning that a LMCT complex could be formed between EDTA-2Na and TiO2[11].But due to the adsorption of antibiotics on TiO2surface,the impact of EDTA-2Na on the sensitization was limited.The results showed that N2had the most significant effect on the degradation rate,indicating that•O2−played a vital role in the degradation reaction of the three antibiotics(Fig.2D and Fig.S5 in Supporting information).In addition,the degradation efficiency of the antibiotics decreased after the addition of EDTA-2Na,indicating that h+also played a minor role in this process.However,the addition of IPA had no significant effect on the degradation of the antibiotics,confirming that•OH was not involved in the degradation mechanism,consistent with the ESR results.This was in contrast to the UV-activated scenario,where•OH was the main radicals produced by TiO2(Fig.S6 in Supporting information)[21].
Despite the clear evidence on the LMCT between antibiotics and TiO2,the question that remained to be answered was the low TOC removal rate.To assess whether the sensitization effect still existed after TC degradation occurred,solution containing the degradation product(TC-D-P)after visible light irradiation for 120 min was filtered and mixed with uncoated TiO2for 1 h in the dark.The resulted mixture was subjected to UV–vis and ESR characterizations.The light absorbance spectrum of the mixture showed no absorbance in the visible light range,similar to that of pure TiO2(Fig.1C,red line).Similar phenomena were also observed in the case of SSX and NOR antibiotics(Figs.S3B and C).The ESR spectra revealed that the key radical O2−that facilitated the photocatalytic degradation was no longer generated in the mixture of TC degraded products with TiO2(Fig.S7 in Supporting information).
To further understand the photocatalytic degradation process,the degradation products of TC were identified using highperformance liquid chromatography with tandem mass spectrometry(HPLC-MS).The possible TC degradation pathways were summarized in Fig.S8(Supporting information)[11].Upon visible light irradiation,TC lost its hydroxyl and methyl groups at first[23,24].Subsequently,the loss of N dimethyl groups and the ring cleavage reactions occurred.Lastly,continued ring cleavage and hydroxylation resulted in products with a smallerm/z[23].The detailed spectral information of multiple degradation products with different mass to charge(m/z)was shown in Fig.S9(Supporting information).Previous studies proposed a more comprehensive degradation pathway analysis based on the radicals attacking mechanism of pollutantsviaDFT calculation with Fukui index and mass spectrometry data,which is helpful to determine the structure of the intermediates[25,26].
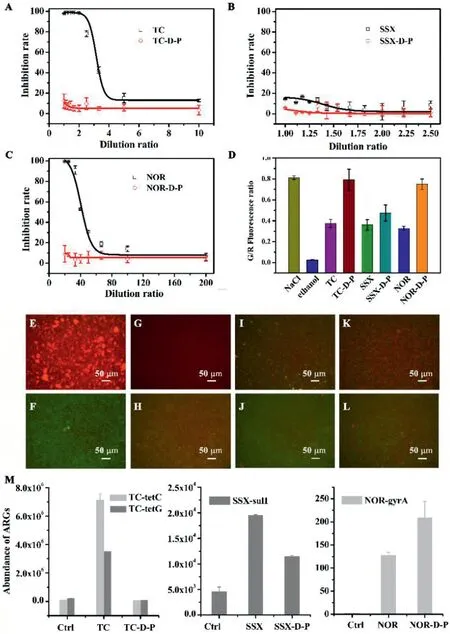
Fig.3.Concentration-response curves of Bacillus subtilis treated with TC(A),SSX(B),and NOR(C)and their degradation products(20 h exposure).Conditions:[NOR]0=10 mg/L,[TC]0=10 mg/L,[SSX]0=10 mg/L,pH 7.0 and degradation time=120 min.(D)SYBR Green I/propidium iodide(PI)staining of the resistant and sensitive strains of Bacillus subtilis treated with various antibiotics and their degradation products to show the live/dead cell ratios.*indicates P<0.05 compared with antibiotic and its degradation product.Conditions:[NOR]0=0.25 mg/L(EC50),[TC]0=3 mg/L(EC50),[SSX]0=10 mg/L,and pH 7.0.Representative fluorescence microscopic images of Bacillus subtilis stained with SYBR Green I/PI exposed to(E)ethanol(positive control),(F)sodium chloride(negative control),(G)TC,(H)TC-D-P,(I)SSX,(J)SSX-D-P,(K)NOR,and(L)NOR-D-P.Dead bacteria in red and live bacteria in green.(M)The abundance of ARGs after the treatment of three antibiotics and their degradation products,respectively.Conditions:[NOR]0=10 mg/L,[TC]0=10 mg/L,[SSX]0=10 mg/L,and pH 7.0.
Moreover,three degradation products proposed in Fig.S8 were selected to calculate their HOMO/LUMO orbitals(Table S3 and Fig.S10 in Supporting information).The results showed that the HOMO of the three degradation products was lower than that of TC,which made the photogenerated electrons easily recombined and could not be transferred to the CB of TiO2.This was also likely the reason why h+could be detected on the mixture of degradation products and TiO2(Fig.S7C).These results suggested that the LMCT sensitization no longer existed after the initial degradation of TC or other types of antibiotics,likely due to the incompatible energy state overlap or no adsorption/complex formation between the degradation products and TiO2.This result offered an explanation on why the photocatalytic degradation under visible light irradiation failed to proceed to further the mineralization.
To depict a clearer picture,Scheme 1 summarized the mechanism of LMCT sensitization.The major steps involved in this LMCT sensitization was shown in Scheme 1A,including(1)the formation of a coordination complex between the antibiotics and TiO2,(2)the generation of electrons and h+viathe photoexcitation of the antibiotic molecules,(3)the transfer of the excited electrons to the CB of TiO2and the subsequent formation of•O2−radicals[22].The disappearance of LMCT sensitization effect was attributed to two reasons.One,there was a change in the antibiotic-TiO2interface as degradation proceeded,including the disappearance of the adsorption layer between the degraded products and TiO2,consequently,the mixture containing degraded products and TiO2exhibited no response to visible light(Scheme 1B).Second,the degradation products sensitized TiO2and produce photogenerated electron under the excitation of visible light;however,owing to the change in the molecular structure,the HOMO/LUMO of the degradation products was no longer matched with the CB of TiO2.As a result,the electrons failed to transfer but recombined with the h+(Scheme 1C).Due to the limited amount and the diversity of the intermediate products generated during photocatalytic degradation,the molecular structure of the main byproducts could not be accurately analyzed in this study.Further analysis on the intermediate products and calculation of their HOMO/LUMO orbitals would certainly help further understand the sensitization mechanism.
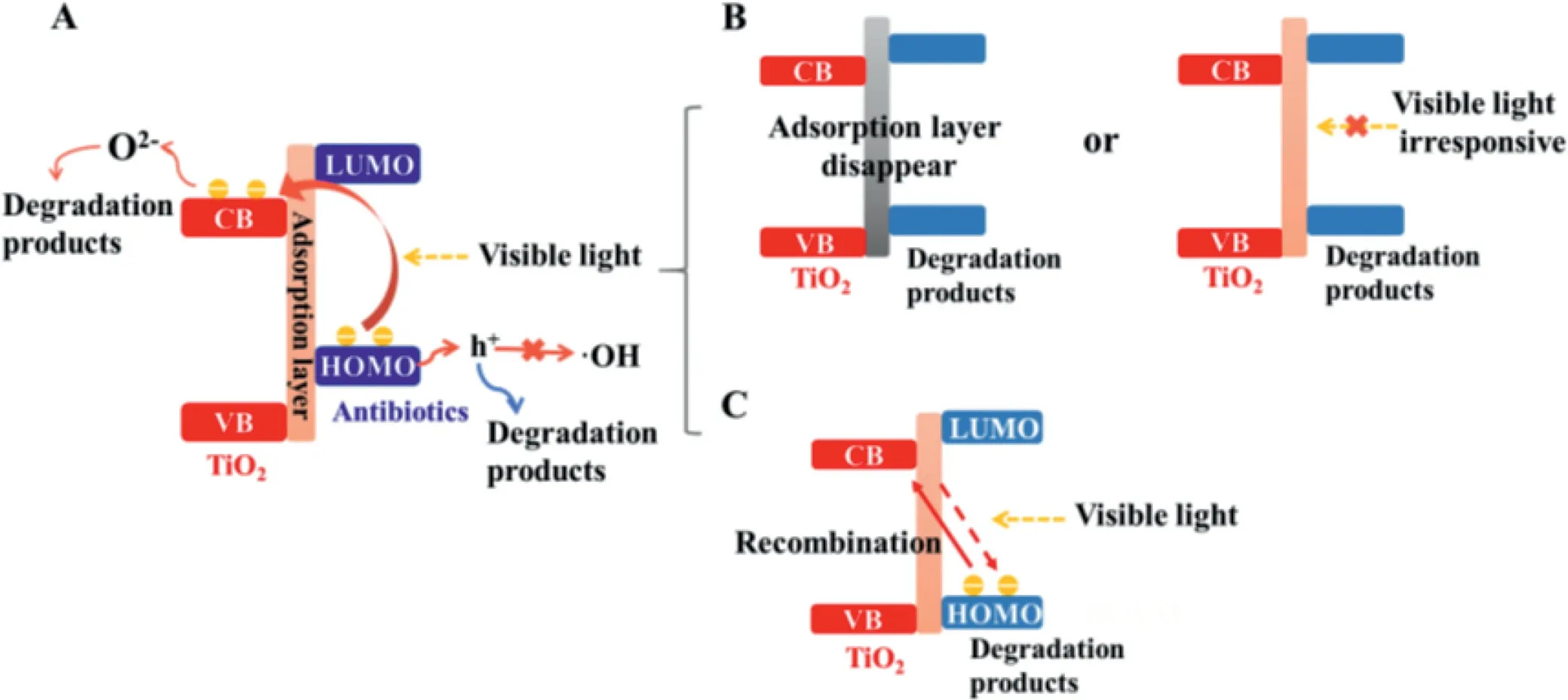
Scheme 1.Proposed mechanism of TC degradation by TiO2 through LMCT sensitization.
As previous studies reported,the antibiotic activity of TC depended on the linear arrangement of the four rings and the two chromophoric keto-enol systems[27].Based on the analysis of the degradation products,it was reasonable to expect that despite the low mineralization of TC,the degradation would result in reduced bactericidal effects.To verify,the degradation products of TC and the other two antibiotics were exposed toBacillus subtilisafter which fluorescence signals were recorded to determine the live/death ratio of the bacteria.Significant decrease in the inhibition rate of the degradation products of all three antibiotics was observed(Figs.3A-C).The live/dead ratios ofBacillus subtilisexposed to the degradation products were all significantly higher than the ones exposed to the antibiotics(Fig.3D).The representative fluorescence images of the bacteria were shown in Figs.3E-L.
Another important aspect related to the environmental risk of antibiotics was its ability to induce antibiotic-resistance genes(ARGs).To this end,biomass collected from an anoxic tank in the Quyang wastewater treatment plant(Shanghai,China)was subjected to the exposure of antibiotic solution(10 mg/L)before and after photocatalytic degradation.Specific experimental method was summarized in Supporting information.The results revealed that the abundance of the ARGs of the biomass treated with the degradation products of TC and SSX decreased significantly(Fig.3M).However,the degradation products of NOR showed a higher induction of ARGs compared to the NOR.Similar trend was reported in other studies[28].It is speculated that although the molecular structure had changed,the NOR degradation products could still bind to the active site of DNA gyrase,and even with a greater binding affinity,resulting in an enhanced stimulation of resistance mutations.This was another important point for future investigations.
In summary,our study demonstrated the visible light degradation performance of TC by TiO2viaa LMCT sensitization mechanism.The degree of sensitization was determined by the formation of a visible light responsive TC-TiO2complexviasurface adsorption and the overlap of HOMO/LOMO of TC and the CB of TiO2.Interestingly,only TC but not the degradation products had the sensitization effect.Although the photocatalytic degradation did not achieve high mineralization,the degradation products showed significantly reduced bactericidal effects and less induction of ARGs,except for NOR.This study provided a case study on the formation of LMCT complexes that initiated photocatalytic conversion or self-degradation.It also paved the way for an effective treatment of antibiotics-containing wastewater using the most common photocatalyst TiO2.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work received the financial support from the National Natural Science Foundation of China(No.21777116)and the Fundamental Research Funds for the Central Universities.We would like to express our gratitude to Professor Xia Siqing’s research group and Dr.Si Pang of the College of Environmental Science and Engineering of Tongji University for their assistance in the detection of antibiotic-resistance genes.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.01.067.
 Chinese Chemical Letters2022年12期
Chinese Chemical Letters2022年12期
- Chinese Chemical Letters的其它文章
- Diverse strategic approaches en route to Taxol total synthesis
- Recent advances in gold-complex and chiral organocatalyst cooperative catalysis for asymmetric alkyne functionalization
- Unmodified methodologies in target discovery for small molecule drugs:A rising star
- Recent advances in single-crystalline two-dimensional polymers:Synthesis,characterization and challenges
- Environmental applications of graphene oxide composite membranes
- Recent advances in the application of metal organic frameworks using in advanced oxidation progresses for pollutants degradation
