Environmental applications of graphene oxide composite membranes
Yihu Li ,Jio Jio ,Qidong Wu,Qi Song ,Wncen Xie ,Bicng Liu,*
a Key Laboratory of Pollution Contro l Chemistry and Environmental Functional Materials for Qinghai-Tibet Plateau of the National Ethnic Af fairs Commission,School of Chemistry and Environment,Southwest Minzu University,Chengdu 610041,China
b Key Laboratory of Deep Earth Science and Engineering(Ministry of Education),College of Architecture and Environment,Institute of New Energy and Low-Carbon Technology,Institute for Disaster Management and Reconstruction,Sichuan University,Chengdu 610207,China
Keywords:Graphene oxide Membrane separation Water purification Desalination Gas separation
ABSTRACT Graphene oxide(GO)has been widely used in the modification of membranes due to its excellent properties,i.e.,huge specific surface area,good electrical conductivity,good hydrophilicity and various functional groups.The addition of GO in membranes were proved to exhibit improved properties in water permeability,molecular selectivity,membrane fouling mitigation and contaminants decomposition.Recently,the development of laminated GO in membranes achieved both high selectivity and high water permeability,conquering the limitations of conventional polymeric or inorganic membranes.By analyzing the separation mechanisms and the performance of GO composite membranes,this review systematically summarized the applications of GO composite membranes in three highlighted areas of environmental fields:desalination,gas separation and wastewater treatment,with challenges discussed faced with GO composite membranes.
1.Introduction
Membrane technology has played a crucial role in the environmental market in recent decades due to its high retention and easy operation[1].The performance of membranes includes water permeability,chemical stability,mechanical strength,fouling resistance,casting ability,pore distribution and so on.In general,membranes are classified as inorganic membranes and polymeric membranes[2]The inorganic membranes have extraordinary selectivity but limited permeability[3].While the polymeric membranes have the advantage of high water flux but low mechanical strength[4–7].
Up to now,nanomaterials including metal-organic frameworks(MOFs)[8],gold nanoparticles[9],graphene oxide(GO)[10],carbon nanotubes(CNTs)[11],SiO2[12]and TiO2[13]have been used as substrate modifiers to improve membrane properties.Among the two-dimensional nanomaterials,the stability and ultrahigh permeability of carbon nanomaterials have drawn substantial attention to researchers[14],especially GO nanosheets,which have been widely used as a nanofiller material embedded in active layers[15].Firstly,the presence of a large number of oxygen-containing groups such as carboxyl,hydroxyl and epoxy groups makes GO compatible with polymers easily[16].Secondly,the ultra-thin and two-dimensional structure of GO nanosheets enable to form ultra-thin polymer-GO nanosheet composite layers.Moreover,GO nanosheets have the characteristics of a twodimensional monoatomic layer plane structure,huge specific surface area,high tensile strength,rapid electron transmission and abundant surface functional groups[17].The above characteristics make GO nanosheets exhibit low transfer impedance,high water flux and high electron transmission efficiency[18].GO can also perform as additions in the membrane preparation,forming a composite membrane,which can achieve excellent permeability and selectivity[19].Recent studies showed that GO had the potential to prepare highly selective and permeable separation membranes compared to inorganic membranes or polymeric membranes.Low-dimensional GO nanomaterials with tunable functionality and enhanced surface properties facilitate the modification between membrane permeability and selectivity[20,21].
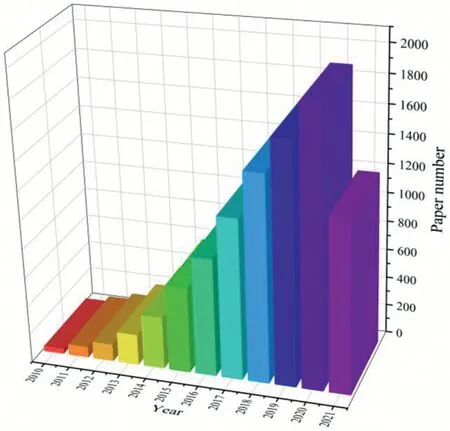
Fig.1.Numbers of publications about GO composite membranes from 2010 to 2021(topic keywords”graphene oxide membrane"searched from web of science),data updated by September 2021.
In recent decades,papers focusing on GO composite membranes are growing exponentially(Fig.1).The data indicates that GO has been widely used in membrane manufacture to improve the properties of membranes[22,23]and enhance the treatment efficiency[24],which are also in consistence with the concept of environmental sustainability[25].Laminates made of GO have attracted extensive attentions in gas separation and ion separation since they are easy and scalable preparation,with exhibiting high permeability.In the laminates of GO layers,some molecules or ions could pass through both the channels bounded by the layered GO and the nanopores inside the GO sheets.Based on the recovery,studies focused on the GO composite membranes for desalination in the treatment of source water and seawater spring up.The related results indicated GO exhibited considerable rejection towards ions like Na+,Cl−,Mg2+and SO42−with excellent permeability[26–28].The laminates of GO layers can be achievedviacross-linking with small molecules,layer-by-layer assembling,spin coating and vacuum filtration[14].
The reason why laminated GO became popular in gas separation is also attributed to its high efficiency.The challenge faced with membrane for gas separation is how to achieve ultrahigh selectivity towards gas mixtures.Studies discovered laminated GO layer with two-dimensional structure had strong in-plane chemical bonds and weak interlayer interactions,which is capable to exert nanometer control over membrane thickness,affording minimized transport resistance and maximized gas flux[29].In this case,many studies used GO assembled membranes to separate CO2from CO2/H2,CO2/CH4or CO2/N2gas mixtures.
The excellent electronic transmission functional groups of GO make it also an outstanding material for membrane fouling mitigation.The introduction of GO to membranes in wastewater treatment could not only reduce membrane fouling,but also accelerate the electrochemical reactions in many advanced oxidation processes(AOPs),for instance,electro-fenton process and photocatalysis process.
Therefore,GO showed excellent mechanical,electrical and optical properties in membranes.Since there are few reviews on the application of GO in membranes,this review provides a comprehensive summary of GO composite membranes in environmental applications in three aspects:desalination,gas separation and wastewater treatment,providing a quick access to researchers on similar areas.In the area of desalination and gas separation,the separation mechanisms of GO composite membranes towards different compounds were reviewed.The improved properties of GO as additive in membranes used in wastewater treatment were analyzed in detail containing hydrophilicity,pore size distribution,catalytic activity and antimicrobial property.Finally,future prospects and current challenges of GO composite membranes were summarized.
2.Desalination
Nowadays,the problems of limited water resources and water pollution drive researchers to explore advanced,effective and energy-efficient technologiesviaan environment-friendly way.Membrane technologies like nanofiltration(NF),reverse osmosis(RO)and forward osmosis(FO)processes were proved to show high removal rates,with thin film composite(TFC)polyamide(PA)membranes performing as the dominated membranes in recent decades.Towards the traditional TFC membranes,although they have salient advantages of high capacity and wide pH conditions,the disability of chlorine resistance and fouling resistance are the existing limitations restricts their practical application considering the operation cost.In addition,in the process of desalination,the commonly used TFC RO membranes have a permeation rate threshold that limits the breakthrough of high permeation rates[30,31].The above permeability rate threshold would make studies focused on condition modifications contribute little to energy saving[32].
Considering the excellent characteristics of nano-pores,interlayer spacing and various functionality of GO,GO composite membranes have been widely used as high-flux and cost-effective membranes for water purification.Compared with traditional polymer membranes,two-dimensional GO composite nanomaterial membranes provide a promising performance of both high retention rate towards salt ions and ultra-high permeability rate.Studies have already verified the water flux of GO layered membranes have no relationship with the number of GO layers,suggesting the thickness of GO coating affected slightly on the water resistance of membranes[1].Various GO composite membranes have been used in desalination in recent years.The preparation methods of GO composite membranes mainly include vacuum filtration method[33,34],layer-by-layer self-assembly method[35],dipcoating method[36,37],spin coating method[38],spray-coating method[39],etc.Among them,vacuum filtration method is simple operation,and the prepared membranes have uniform thickness.However,the area of the prepared membrane is small.The layerby-layer self-assembly method can accurately control the number of layers and achieve large-area membranes,which is cost effective and environment-friendly.However,it requires to construct electrostatic attraction between material layers.
The separation rate of GO composite membranes are mostly above 80%,stating that GO composite membranes are desired membrane materials for salt retention and water purification[40–54].Fig.2 exhibits the water flux and salt rejection rate of various GO composite membranes prepared by different works.As mentioned above,different preparation methods of GO composite membranes exhibit different properties[1,41,44,45,55–61]].The preparation methods and separation performance of GO composite membranes were summarized in Table 1,respectively.
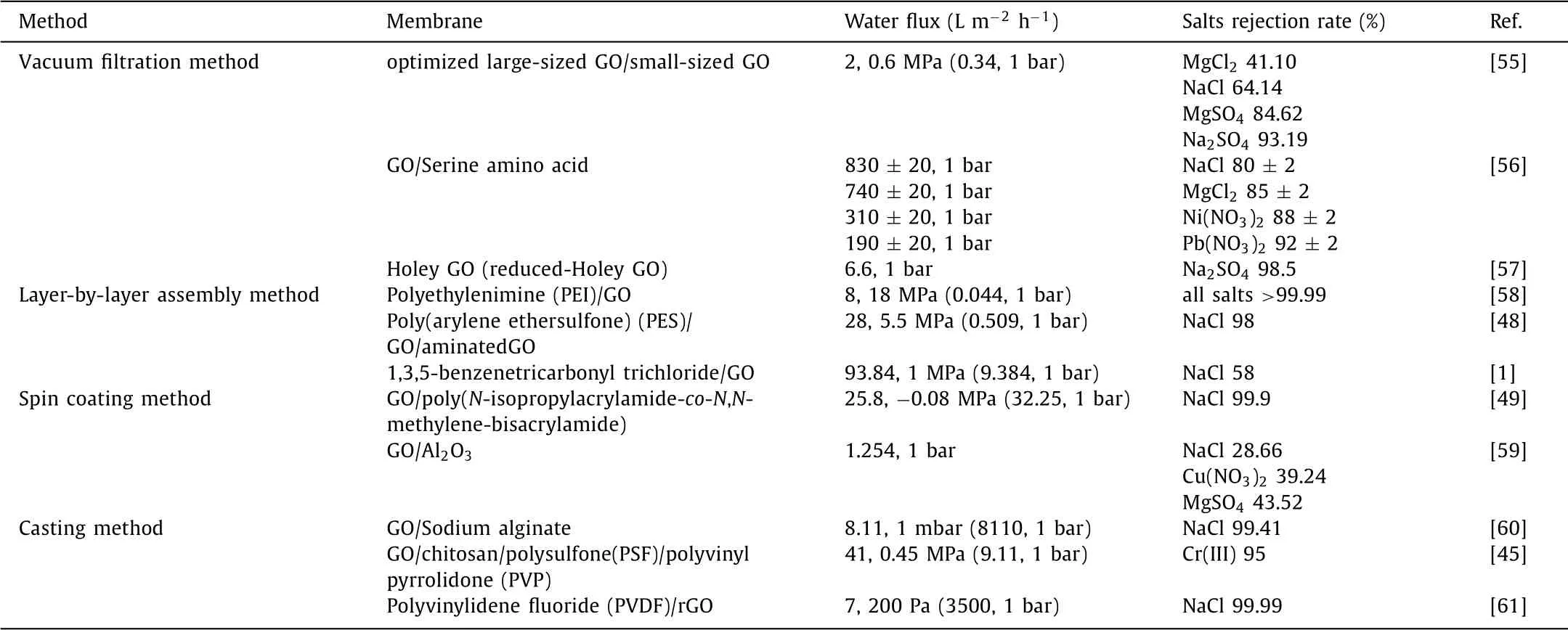
Table 1 The preparation methods and properties of GO composite membranes for desalination.
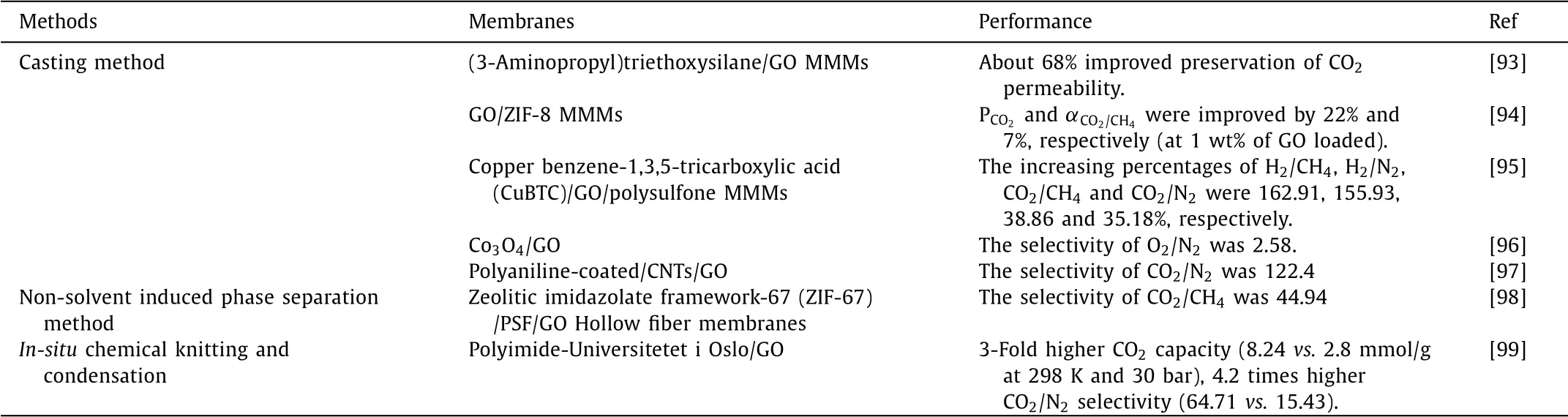
Table 2 The preparation methods and performance of GO composite membranes for gas separation.
2.1.Mechanism of desalination
Studies[62]verified that GO composite membranes in aqueous solution formed a capillary channel with the size at about 0.9 nm under hydration,which could prevent the transmission of ions or molecules with the hydration radius greater than 0.45 nm.The above characteristic can not only separate ions or molecules accurately,but also achieve higher diffusion rate than the classical
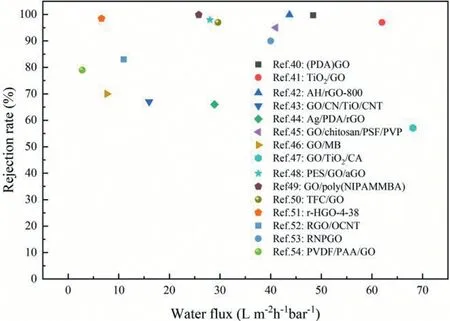
Fig.2.The water flux and salt rejection rate of various GO composite membranes prepared by different works[40–54].
materials.These discoveries have broadened the application of GO composite membranes in the fields of seawater purification,sensing technology and energy conversion.
Viveket al.[63]used different sized GO sheets to prepare GO membranes to permeate water.The results indicated although the sizes of GO sheets ranged in two orders of magnitude,the water permeability changed little.However,when changing the pore defects of GO membranes,the water permeability exhibited much difference.The above results indicate water flows not only along the gaps between the GO sheets,but also through the nanopores(Fig.3).When the surface of GO membranes was not the regularly layered structure,different water permeability will be obtained when using the same sized GO sheets.The results indicate both sheets structure and nano channel of GO membranes play crucial roles in water permeability.
Elimelechet al.[64]summarized the interception of GO membranes towards salt ions proceeded in two aspects:nanoporous GO membranes and stacked layered GO membranes(Fig.3).
2.1.1.Nanoporous go membranes
For the nanoporous GO membranes,nanopores are artificially maken on the surface of GO sheets to allow molecules,ions or atoms that smaller than the nanopores to pass through.The separation mechanism includes size sieving and electrostatic repulsion.The preparation methods include plasma etching[65],ion bombardment[66],template etching[67],electron beam etching[68],etc.For instance,Karniket al.[69]prepared nanoporous graphene membranes with high-density and sub-nanometer pores through ion irradiation-oxidation etching techniques.The results indicated with the extension of the oxidation etching time,the size of the nanopores gradually increased,and finally stabilized in the range of(0.40±0.24)nm.This nanoporous GO membranes was certified to allow salt ions transporting across the membrane.With the ultra-thin membrane thickness and unique pore structure,the nanoporous GO membranes exhibited excellent water permeability compared with other materials[70].
2.1.2.Stacked layered GO membranes
For the stacked layered GO membranes,researchers used physical and chemical modifications on the surface of GO sheet to improve the pore structure of the two-dimensional nanochannel and the chemical property of the membrane surface[71].Thereby,a GO membrane with high water flux,excellent rejection selectivity and good chemical stability is obtained.At present,there are three main methods to modify the layer spacing of the GObased membranes,which are weak reduction method[72],small molecule cross-linking method[73,74]and macromolecular intercalation method[75].Miet al.[76]discovered that by adjusting the gap size between the GO sheets,the pores of GO membranes can be precisely controlled.The regulation methods can be effectively realized through changing the charges between the layers or adding small-sized polymers(or nanoparticles)between the layers.The 1 nm spacing between GO layers sandwiched by 1,3,5-benzenetricarbonyl trichloride has already showed respectable rejection rates towards NaCl(60%),Na2SO4(80%)and organics(95%towards Methylene blue(MB)).The main separation mechanisms include size exclusion and electrostatic effect,and adsorption also occurred in the process.
Lately,Cohen-Tanugiet al.[77]discovered that the hydrogenated modified GO membrane and the hydroxylated modified GO membrane exhibited both the improvement of water flux and salt ions rejection.The above phenomenon also occurred with the increase of pore size or applied pressure.Due to the stronger hydrogen bond between hydrophilic hydroxyl groups and water molecules,the water flux of hydroxylated GO membranes is much higher than that of hydrogenated membranes(Fig.3c).
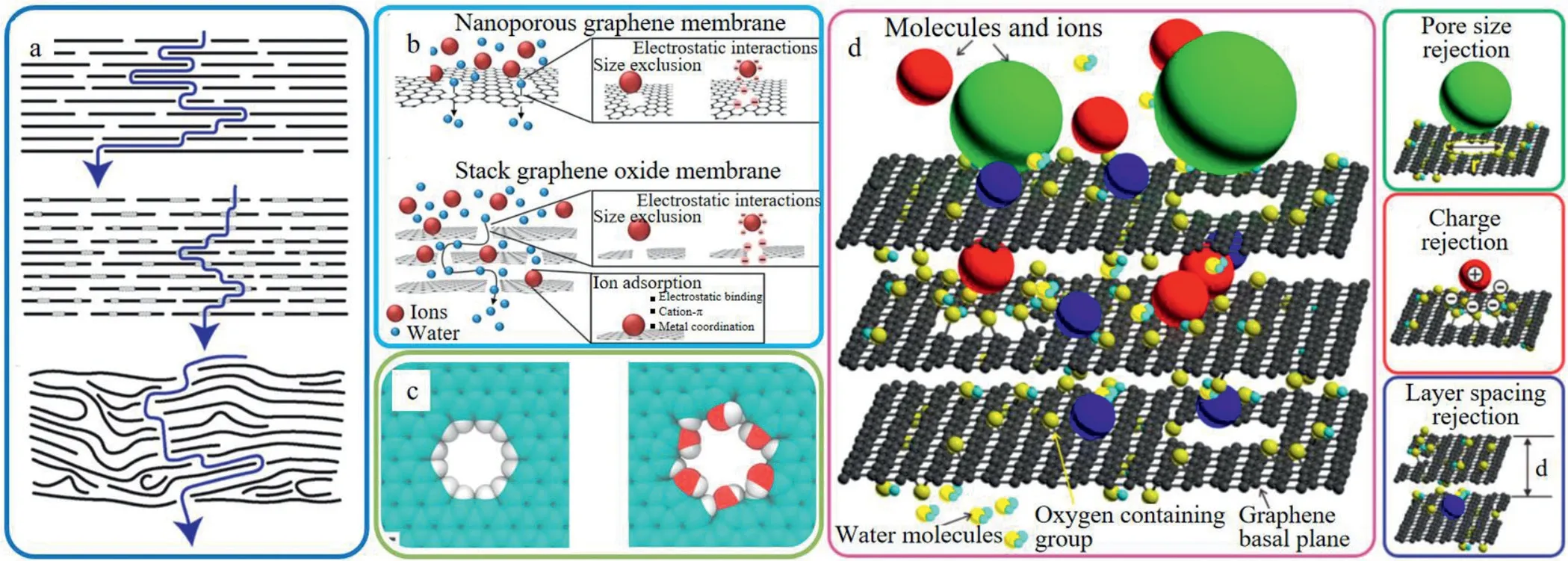
Fig.3.The schematic diagram of(a)water penetration pathways of GO sheet membranes.Reproduced with permission[63].Copyright 2018,American Chemical Society;(b)molecules penetration through nanoporous GO membrane and stacked GO membrane.Reproduced with permission[2].Copyright 2015,Royal Society of Chemistry;(c)hydrogenated GO membrane and hydroxylated GO membrane.Reproduced with permission[77].Copyright 2012,American Chemical Society;(d)molecular retention mechanisms by GO composite membranes.Reproduced with permission[78].Copyright 2019,American Chemical Society.
Hence,based on the above studies,the retention effect of salts by the GO composite membranes can be concluded in three factors in detail:(1)Pore size retention;(2)charge rejection;(3)layer spacing rejection(Fig.3d)[78].Using the unique properties of GO,by making nanopores on the surface of GO or modifying the GO composite membranes material,membranes with high water flux and high salt rejection rate can be prepared.
2.2.Condition optimization
Among all the influenced conditions,the first consideration for GO composite membranes used in desalination is its surface charge and interlayer spacing,and the separation of water from salt can be achieved by optimizing above properties during the preparation process.Yanget al.[78]found that the separation performance of GO membranes towards salts depended on the type and concentration of charges on the surface of GO membranes.The separation performance of GO membranes can be optimized by adjusting the pH,salt concentration and applied pressure of the filtering condition.At low pH condition,the interlayer spacing would be reduced by the protonation of carboxylic acid and the increase of ion concentration,resulting in a decrease in water flux and an increase of rejection rate.At high pH condition,the ion screening effect caused by the increase of ion concentration would cause the GO nanochannel to shrink.By analyzing the influence of applied pressure on the rejection rates of the target molecules,it is found that the generated pressure can change the nanochannels of GO membranes.Therefore,when GO composite membranes are used for desalination,the performance of GO can be optimized by adjusting pH,salt concentration and filtration pressure.
Computational chemistry method is an important way for understanding the mechanism of GO composite membranes in water desalination,which can provide structural information and process information at atomic level.Huanget al.[79]adopted the molecular dynamics simulations to study graphene membrane.They found that the pore size of chemically modified graphene membrane was smaller than the nanometer scale,which can effectively separate salt ions from water molecule.Giriet al.[80]employed the molecular dynamics simulations to study how water transport through GO nanochannels.They found that water molecules preferentially flowed through the non-oxidized area of the narrow channel,while no preferential path happened when flowing through the wide channel.The calculation results showed that the best channel for desalination of GO membrane is a channel with a layer spacing at 0.8 nm and an oxidation degree of 10%or less.
In conclusion,the demineralization ability of the GO composite membranes depends on the pore size of the membrane surface,the external pressure and the chemical properties of the membrane.With the desalting of GO composite membranes,the performance of membrane can be optimized by adjusting pH,salt concentration and filtration pressure.
2.3.Distillation desalination
Researchers also found that GO composite membranes prepared by vacuum filtration method absorbed more than 94%of sunlight with wavelengths in the range of 250–2500 nm(calculated as the frontal average illumination of solar radiation to the earth’s surface)[81].Based on this,an absorber based on GO membranes for desalination in solar steam generators was designed[82].In the process,GO acted as a light absorber and did not directly contact with the native water.The results showed that the GO composite membranes absorbed sunlight and converted it into thermal energy through optically excited electrons,thus heating and evaporating water.The device achieved a high conversion effi-ciency(78%)in a one-dimensional water path The deposition of large amounts of salt on the GO membranes affected little on the property.It was also found that after increasing the hydrophilicity of the reduced GO(rGO)membranes,the energy conversion effi-ciency of the membrane for sunlight-water evaporation increased by about 15%.Therefore,the property of GO on the conversion of light energy into heat makes it in the usage of water evaporation,providing a new idea for membrane distillation.
Xuet al.[83].synthesized a PVDF/polydopamine(PDA)/GO composite membrane for distillation.In this work,the relative content of different oxygen-containing groups of GO was adjusted by varying the oxidation temperature.Then the GO nanosheets deposited on the PVDF/PDA to fabricate a composite membraneviathe evaporation-assisted deposition method.The results showed that when the oxidation temperature was 70°C,the composite membrane exhibited the best performance,the salt rejection rate of the membrane reached 99.9%when the water flux at 17.8 L m−2h−1.Moreover,with the oxidation temperature increased,the oxygen-containing functional groups of GO increased,while the hydroxyl groups decreased.Therefore,transforming hydroxyl groups into epoxy groups of GO is an approach to the high water flux of GO composite membranes.
In summary,GO composite membranes can be applied for desalination through pore size retention,charge rejection andlayer spacing rejection mechanisms.At the same time,it provides new ideas for membrane distillation.Compared with other twodimensional nanomaterials,GO has the advantages of cleaning,high efficiency and energy saving.
3.Gas separation
In the field of environmental energy,separating pure gas from mixture is always used to improve the purity of products or reduce environmental pollution.Among the gas products,oxygen gas has the function of catalyzing and improving the efficiency of pollution abatement.H2is a frequently-used gas of energy storage[84,85].CH4is well known as a new alternative energy source to petroleum.In most chemical production processes,CO2is performed as the by-product.Besides,the excessive greenhouse gas CO2in the environment has changed the climate.In this case,how to separate CO2from the gas mixture is an important issue of environment protection.Thus,it is of great significance to develop gas separation membranes to obtain gas with high-purity.
In decades,membranes based on different two-dimensional materials such as MXenes,MoS2,C3N4and other nanosheet materials have been successfully used for gas separation[86,87]to achieve efficient purification of H2or CO2[88].Compared to common gas separation membranes,GO composite membranes become popular in the field of gas separation due to its unique atomic layer thickness and high mechanical strength.Besides,it has relative inertness and impermeability towards standard gas[89].GO and its modified derivatives have positive potential as membrane nanofillers,which can help to improve gas separation propertiesviamodifying the molecular sieving,the interlayer space channels and the diversity of functional groups[90,91].Meanwhile,the high specific surface ratio and high mechanical,thermal properties of GO also helped it become the best nanofiller candidates for CO2separation[92].Lots of studies confirmed GO composite membranes exhibited excellent desalination performance,the detailed results are listed in Table 2[93–99],which summarizes the preparation methods and the performance of GO composite membranes used for gas separation.Tsouet al.[100]reported that the microstructure of GO sheets in GO composite membranes could be obtained by three different deposition ways:pressurized filtration,vacuum filtration and solvent evaporation.The results indicated that different driving pressures resulted in different microstructures of the formated GO composite membranes,which would further resulted in different properties of GO composite membranes.
3.1.Influencing factors
The performance of GO composite membranes for H2/CH4separation was investigated theoretically by Zhenget al.[101]through molecular dynamics simulations.They found that the layer spacing,oxidation degrees and initial gas pressures of the GO composite membranes had significant effects on the gas permeability and selectivity.The experimental results showed that H2was difficult to pass through the GO sheet as the oxidation degree of GO sheet increased.And with the increase of temperature and initial gas pressure,H2passed through the GO sheet more easily.Similarly,Grossmanet al.[102]utilized molecular simulation techniques to investigate the formation of nanopores on the surface of reduced GO(abbreviated as rGO)and their performance in separation applications.They found that the formation of nanopores can be controlled under suitable conditions.When the oxidation degree and epoxide/hydroxyl ratio of GO were low,it required a relative high reduction temperature to form nanopores with certain sizes.Therefore,the conditions of synthesizing GO composite membranes are of great importance to achieve effective gas separation.
3.2.Mechanism of gas separation
The main mechanisms of GO composite membranes for gas separation can be concluded in four aspects(Fig.4):(1)molecular sieving;(2)Knudsen transport mechanism[103];(3)charge sieving effect[104]and(4)crosslinking degree between the electron density equipotential surfaces of GO and gas molecules[105].
3.2.1.Molecular sieving
Membranes with different pore sizes allow smaller molecules to pass through,while molecules larger than pore sizes will be intercepted,so that the molecules with different sizes can be sieved.The mechanism was called molecular sieving(Fig.4a).Qiet al.[106]successfully deposited ultrathin,defect-free and robust GO membrane on multivacancy stainless steel hollow fibers by a simple electrophoretic deposition method.In the experiments,the oxygen-containing functional groups of GO were selectively reduced,achieving a controlled reduction of the 2D channels of the stacked GO layers,thus leading to a selective sieving of gas molecules with different pore sizes.By excluding gas molecules larger than ethane(0.39 nm),an accurate molecular sieving performance was achieved.The as-prepared GO composite membranes can separate C2(ethane and ethylene)from C3(propane and propylene)precisely.
3.2.2.Knudsen transport mechanism
Studies[107]proved that graphite or GO sheets can be designed into membranes to exhibit ideal gas separation property.Selective gas separation could be achieved by designing stacking methods to control membrane pores and gas pathways.For the layered(3–10 nm)GO membrane,the adjustable efficiencies of gas separation
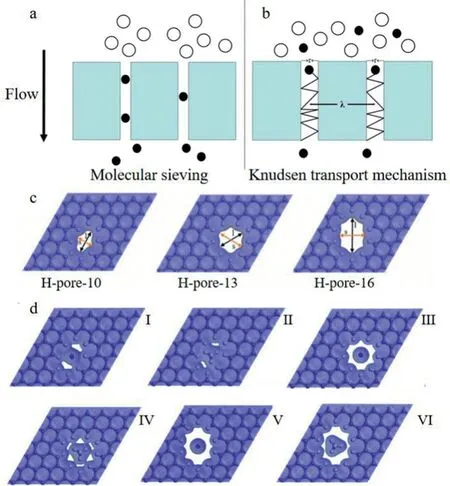
Fig.4.Diffusion mechanisms:(a)molecular sieving;(b)Knudsen transport mechanism.(c)The pore electron density isosurfaces of H-pore-10,H-pore-13 and Hpore-16.Reproduced with authors’permission[104].Copyright 2018,China Doctoral Dissertations Full-text Database,Chinese National Knowledge Infrastructure.(d)The electron density equipotential surface of gas passing through graphene nanopores:(I)N2/H-pore-10;(II)CH4/H-pore-10;(III)N2/H-pore-13;(IV)CH4/Hpore-13;(V)N2/H-pore-16;(VI)CH4/H-pore-16.Reproduced with authors’permission[105].Copyright 2016,China Doctoral Dissertations Full-text Database,Chinese National Knowledge Infrastructure.The copyright of the figures have been obtained from the authors.
were dependent on the interlocking degree within the GO stacking structure.The high selectivity of CO2/N2was achieved by a good interlocked GO membrane under relative high humidity.The experimental results also confirmed that the separation mechanism is the Knudsen transport mechanism(Fig.4b),which depends on the relationship between the pore size(r)and the molecular average free path(λ).When r/λ>5,the pore size is large.In this case,the viscous interaction between gas molecules and the viscous flow were the dominant force,and there is no separation effect.When r/λ<1,the pore size is small.In this case,the collision resistance between gas molecules and the pore wall is the dominant force.The resistance is proportional to the square root of molecular weight,and gasses with different molecular weights can be separated.
3.2.3.Charge sieving ef fect
Theoretical calculations showed the third gas separation mechanism of functionalized graphene is charge sieving effect.Wanget al.[104]used density functional theory and molecular dynamics simulation method to research the gas separation performance of porous graphene(graphene-like materials).It found that the Hpore-13 porous(Fig.4c)graphene membrane with a pore size of 4.06˚A can separate CO2/N2mixtures efficiently.The mechanism is the charge sieving effect caused by the entrance of H atoms.Further analysis showed that the position of adsorption potential hydrazine had an important influence on the separation performance of gas molecules passing through the graphene membrane.By adjusting the position of the adsorption potential hydrazine of gas molecules,the selective characteristic of the porous graphene membrane towards gas could be reversed.And the adjustment of the position of the adsorption potential hydrazine could be achieved by functionalizing the graphene pores with its charged state.The adsorption potential hydrazine location sieving effect was another form of the charge sieving effect in fact.
3.2.4.The degree of crosslinking between the electron density equipotential surfaces of graphene and gas molecules
Study[105]analyzed the performance of porous graphene membrane in the separation of N2/CH4mixture.After comparing three graphene structures of H-pore-10(with the pose size at 10 nm),H-pore-13(with the pose size at 13 nm)and H-pore-16(with the pose size at 16 nm),it was found that the H-pore-13 graphene membrane had the best separation performance towards CH4/N2mixture.The H-pore-13 sized graphene membrane can selectively transmit N2,with the permeability was 105 gas permeation unit(GPU)(1 GPU=3.35×10−10mol s−1m−2Pa−1),which could realize the purification of natural gas.After analyzing the density functional theory and dynamics simulations,it was shown that the transmission performance of graphene membrane towards gas molecules mainly depended on the degree of crosslinking between the electron density equipotential surfaces of graphene membrane and gas molecules.The pore size of H-pore-10 was small,and the electron density equipotential surfaces of graphene membrane and gas molecules(CH4and N2)were overlap greatly,thus the energy barrier of the graphene membrane was too large to let CH4or N2pass through.When it comes to H-pore-13,the electron density equipotential surface of H-pore-13 and CH4molecule overlapped a lot,while it overlapped less with N2molecule.Therefore,the N2molecule needed lower energy barrier to pass through,while CH4was intercepted.As to Hpore-16,the pore size of H-pore-16 was too large,and the degree of crosslinking with the electron density equipotential surface of CH4molecules and N2molecules were both small,so CH4and N2can both pass through the H-pore-16 sized graphene membrane easily,and there was no selectivity for gas separation(Fig.4d).It provides a theoretical basis for the preparation of porous graphene membranes.
3.3.The application in gas separation
Computational chemistry has been used to predict the separation of gasses and explain the related mechanisms by GO composite membranes.Leiet al.[107]prepared graphene-carbon nanotube hybrid structure(GNHS)and separated the CO2/H2S/CH4ternary mixture under Monte Carlo Simulation.The size of the threedimensional structure was 4.064 nm×4.401 nm×2.656 nm.The distances of the carbon nanotubes in the x and y directions were 2.032 nm and 2.198 nm,respectively,and the width of graphene slit was 0.664 nm.In the calculation process,CH4,CO2and H2S were all the all-atom model.The force of short-range van der Waals was simulated using the Lennard-Jones model with a cutoff radius of 1.2 nm.The simulated gas was composed of natural gas developed from an actual gas field,and the contents of CO2and H2S were 9%and 15%,respectively.The results showed that GNHS can separate CO2and H2S efficiently.With the pressure increasing,the force between the adsorbate and the adsorbent decreased,while the force between the adsorbates increased.When the pressure increased contiguously,the interaction between the adsorbate and adsorbent would keep in a relative stable state and not change any more.With the temperature increasing,more of the adsorbate molecules might detach from the adsorbent possibly due to the improved thermal motion of adsorbate molecules,especially for CO2and H2S with a large amount of adsorption.The desorption of CO2or H2S promoted the adsorption of CH4and also reduced the selectivity of adsorption.Further analysis of the mutual influence of adsorbates in the adsorption process showed that H2S could improve the separation of GNHS towards CO2significantly,while CO2had little effect on the separation of H2S.
There are lots of experiments verified that GO composite membranes can be used for gas separation.Kumaret al.[108]prepared a composite membrane of GO and zeolitic imidazolate framework-8(ZIF-8)and its derivative composite material GO/ZnS.With the increasing content of GO in the composite material,the structure of the ZIF-8 in the composite material changed from a hexagonal shape to a spherical shape.It showed that the amount of GO content can change the morphology and porosity of nanopores.Moreover,with the increase of GO concentration,the CO2storage capacity of the GO/ZnS increased gradually.The results also indicated that ZnS on the surface of GO had better CO2storage performance than that of ZIF-8.Liet al.[109]synthesized a high-performanced mixed-matrix membrane(MMM)with the composition of ZIF-8 and GO using solution-casting method,which achieved a good separation of CO2/CH4.What is more,the addition of GO could improve the mechanical strength of MMMs.The results showed that GO composite membranes had great potential for biogas upgrading and natural gas purification.
4.Wastewater treatment
Since the early 1960s,organic polymer membranes have dominated the membrane industry.Most of membrane processes for wastewater treatment relied on the development of TFC PA membranes and polymer membranes like PVDF and PES membranes.Unfortunately,low chlorine tolerance,pores blocking,limited water flux and low separation efficiency are the major drawbacks of conventional polymer membranes[110].
Membrane technology for wastewater treatment is generally used in conjunction with biological treatment technology or advanced treatment technology to remove organics and inorganics salts from water[111,112].Membrane bioreactor(MBR)is the representative technology of membrane separation combined with biological technologies.Due to its high efficiency in removing contaminants and high quality of effluent water,MBR is widely used in water treatment plants.At present,the market share of MBR is about 40%,and the application of MBR technology has extended from the field of urban sewage to food processing,landfill,medical wastewater treatment and many other industries[113].However,membrane fouling is a critical problem in MBR systems.In the long-term filtration process,small molecular flocs adhere on membrane surfaces,like extracellular polymeric substance(EPS)metabolized by bacteria,soluble microbial products(SMP),inorganic salt crystals,solid particles,etc.These substances will block the membrane pores and cause membrane fouling quickly,leading to regular cleaning during the operation process,and resulting in comparatively higher energy consumption compared with other biotechnologies.Thus,the mitigation of membrane fouling and developing anti-fouling materials are urgent for membrane researchers[112].Moreover,with the vigorous development of economy and technology nowadays,the complex micropollutants(like pharmaceuticals and personal care products)and harmful inorganics like heavy metal elements bring more challenges for high-efficiency membrane materials development[114,115].
4.1.Mechanism of the removing of contaminants
GO has abundant oxygen-containing functional groups and negative charges which make GO has a strong adsorption effect on positively charged contaminants[116].Using GO to modify membrane material can improve its removal efficiency towards organic contaminants or inorganic metals,achieving the aim of wastewater purification.
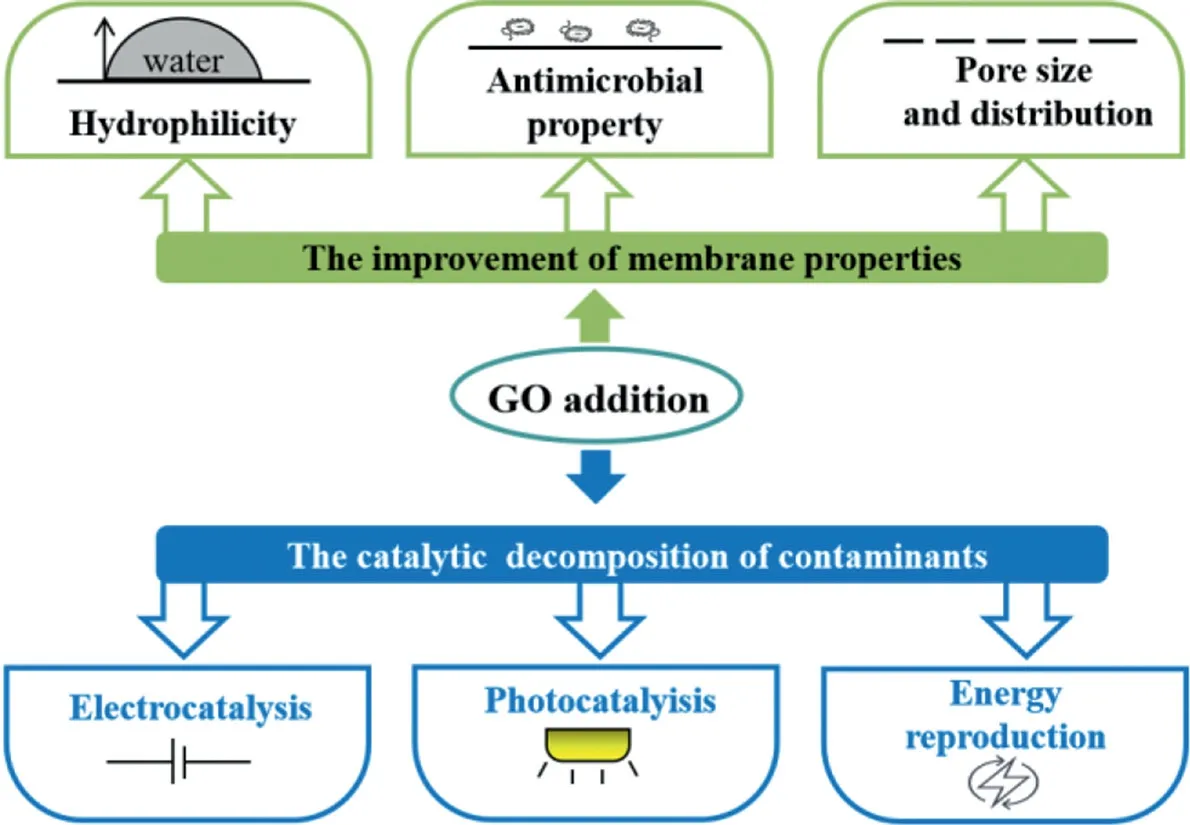
Fig.5.Benefits of GO composite membranes in wastewater treatment.
The contaminants removal of GO composite membranes is based on three mechanisms.Firstly,the interception and blocking effect of the GO composite membranes could separate the macromolecular organics from wastewater.Secondly,GO could catalyze the process of oxidation/reduction reactions to remove organics or inorganics.The catalytic decomposition processes of GO towards matters includes photocatalysis and electrocatalysis.The abundant functional groups on the surface of GO can combine with microcurrent action,thus accelerating the processes of organics decomposition or heavy metals reduction,or mitigating membrane fouling.Thirdly,the functional groups on the surface of GO membrane are negatively charged,which are easily to attract positive charged matters in wastewater,so the retention rate of membrane are improved.The improved properties of GO on the GO composite membranes were summarized in Fig.5.
4.2.The improvement of membrane properties
4.2.1.Hydrophilicity
The addition of GO was verified to improve the hydrophilicity of membranes.Wanget al.[117]used a facile and eco-friendly process to prepare a novel superhydrophilic and superolephobic isotactic polypropylene(iPP)/PDA/GO membrane.The membrane presented outstanding superhydrophilicity(the water contact angle decreased from 120°to 0°).The as-prepared iPP/PDA/GO membrane also had excellent recyclability and antifouling ability.In the membrane,GO layer contains a large number of oxygen-containing functional groups(including hydroxyl and carboxyl groups),which greatly improved the membrane hydrophilicity.Zinadiniet al.[118]prepared a PES nanofiltration membrane containing GO by the phase inversion method.The increased addition of GO from 0,0.1 and 0.5 wt%GO could effectively reduced the water contact angles of membranes from 65.2°to 58.61°and 53.21°,respectively.And the results showed that 0.5 wt%GO in the composite membrane had the highest mean pore radius,porosity and water flux.Zambareet al.[119]synthesized PSF/GO MMMs with enhanced hydrophilicity,permeability and antifouling property than virgin membranes.The addition of GO and ethylenediamine reduced the contact angle of PSF membrane from 71.1°to 53.1°,while the water flux of the GO composite membranes increased from 5.1 L m−2h−1to 170.5 L m−2h−1.Abadikhahet al.[120]fabricated a rGO/TiO2/Ag composite membrane.It found that the water contact angels of the TFC membranes decreased from 46°to 21°,which was contributed by the abundant oxygen functional groups(hydroxyl and carboxyl functional groups)of GO in the PA selective layer.
4.2.2.Antimicrobial property
Studies have found that GO had good antimicrobial properties[121–123].Faridet al.[124].employed laser-induced breakdown spectroscopy(LIBS)as a rapid,inexpensive spectroscopic technique to reveal the antimicrobial mechanism of GO composite membranes.They found that GO could degrade bacterial intracellular components by mediating oxidative stress inducing degradation of the cell membrane,thus making it more susceptible to puncture by the sharp edges of GO(due to loss of adenosine-triphosphates(ATPs)),leading to the loss of intracellular molecules and reducing cell viability.Thus,the results indicated that the antimicrobial effect of GO is the result of a combination of membrane and oxidative stress mechanisms.Liuet al.[125]systematically compared the antimicrobial activity of graphite,graphite oxide,GO and rGO towardE.coli.It was proved that GO showed the highest antimicrobial activity,followed by rGO,graphite and graphite oxide under similar conditions.Based on this,Liuet al.[126]synthesized quaternized GO(QGO)membrane modifier by in-situ growth of quaternary ammonium salt on the surface of GO.Compared with the unmodified PVDF membrane,the hydrophilic,antimicrobial and mechanical properties of the modified membrane were significantly enhanced.The results stated GO,as a carrier of quaternary ammonium salt,could significantly reduce the loss of antimicrobial substances on the surface of membrane.Yanget al.[127]prepared chitosan/poly(vinyl alcohol)/GO composite nanofibrous membrane by electro-spinning.The resulted membrane showed enhanced antimicrobial activity againstE.coliandStaphylococcus aureus.Huanget al.[128]modified PA layerviasurface grafting of GO.The GOReverse osmosis membrane exhibited a 17-fold reduction in biofouling after contact for 24 h withE.coli.The results showed that by anchoring GO molecules on the surface of PA membrane,membrane with more hydrophilic and antibacterial properties can be prepared.
4.2.3.Pore size and distribution
By adjusting the pore sizes and distribution of GO composite membranes,efficient sieving of different diameter particles can be achieved.Chenet al.[129]designed a ultra-thin GO composite membrane with precisely controlled sub-nanoporesviaco-assembling of GO nanosheets and polymers on porous ceramic substrates,followed by subsequent reduction and carbonization.The as-prepared GO composite membrane showed excellent molecular-sieving water evaporation properties with an intercalated structure,the water evaporation flux reached 49.8±1.5–472.3±14.2 L m−2h−1.The above study provided a new strategy for membrane design in wastewater treatment.Panget al.[130]used Universitetet i Oslo-66(abbreviated as UiO-66,a kind of MOFs)as the microporous fillers to intercalated it into the GO layers to form a ultrathin"sandwich"membrane,which can effectively allow water to permeate through,while hinder the solutes of Na+ions.Liet al.[131]developed a facile,low-cost method to synthesize porous GO membraneviapartial combustion of GO which was imperfectly covered by hydrotalcite.First,GO solution was added to aqueous solution with Zn(NO3)2high-concentrated.After ultrasonic dispersion,the mixture was filtered so that a certain amount of GO and Zn(NO3)2would be trapped on the filter paper.After dried,a layer of Zn salt template with defective pores would covered on the surface of GO.After igniting,the bare GO laid in the defective pores was burned,finally the porous GO membrane was obtained after washed with HCl solution.The asprepared porous GO membrane exhibited high selectivity towardsK+and Na+separation.The above method can control the pore size of membrane precisely,with less energy and time consumption.Xuet al.[132]synthesized polyacrylonitrile(PAN)/GO hollow fiber membrane.The results indicated as the amount of GO increased from 0 to 0.15%,the pore sizes increased from 156.4 nm to 590.8 nm,and the porosity increased from 37.0%to 81.4%,and it also resulted in a narrower pore size distribution.
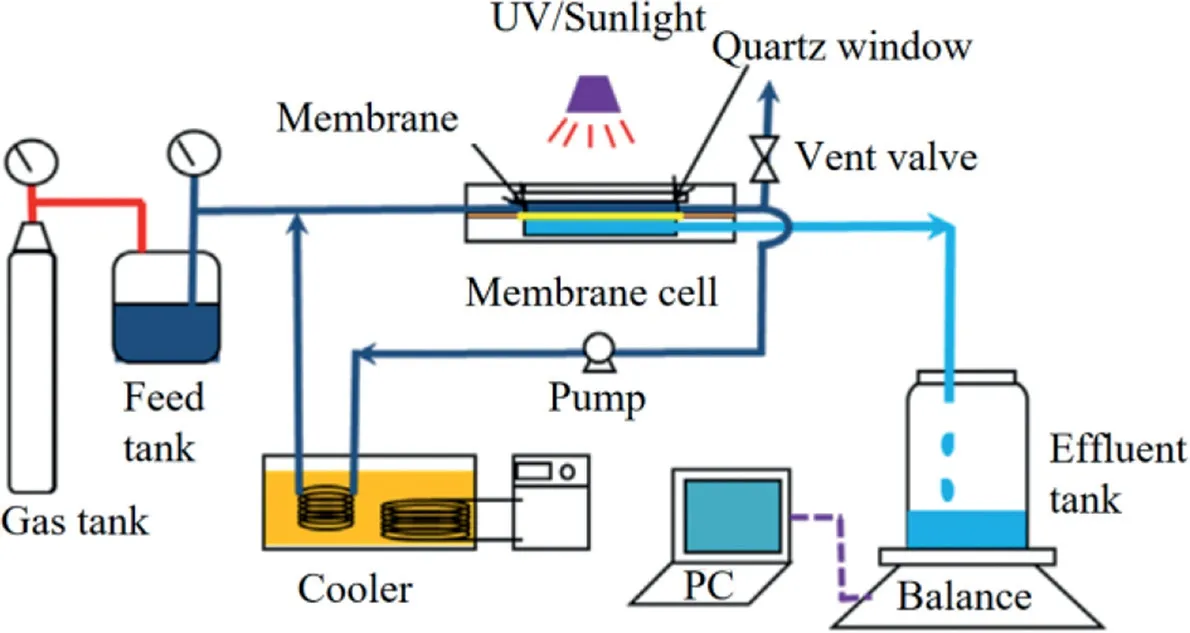
Fig.6.Schematic diagrams a photocatalytic membrane reactor in lab-scale.Reproduced with permission[134].Copyright 2014,Elsevier.
4.3.The catalytic decomposition of contaminants
4.3.1.Photocatalysis
GO and its functionalized products are widely used as energy storage materials and catalytic materials due to their good dielectric properties,thermal stabilities,huge surface areas and excellent electrochemical activities.In environmental treatment,the excellent electrical properties of GO and its functionalized products make them generally used as additives in catalytic membranes.The photocatalysis mechanism is that utilizing the catalytic property,GO was used to transmit micro-current to the surface of the membrane.O2or potassium ferricyanide was used as electron acceptor to form reactive oxygen species to completely decompose organics deposited on the surface of fouled membranesviacatalytic reactions.Alternatively,under light irradiation,the holes with oxidizing ability were generated.After attached with electron acceptors(i.e.,adsorbed O2),the radicals was produced.Finally,the foulants on the surface of membranes was oxidized and decomposed.The process can not only alleviate membranes fouling,but also assist to improve the overall treatment efficiencies of contaminants,increasing the operational stability of membranes.Therefore,the composite membranes prepared by GO can achieve the aims of membrane fouling mitigation and high-efficiency of organics removal simultaneously.
Almeidaet al.[133]prepared a composite membrane using TiO2,PVDF and GO composite membrane(abbreviated as TiO2/GO/PVDF composite membrane).The TiO2/GO/PVDF composite membrane had a removal rate of nearly 100%towards MB in aqueous solution under light.The holes(h+)generated by light was reacted with water molecules to form•OH,and the electrons(e−)transferred onto the surface of GO were reacted with GO to form HO2•.The two above oxidative radicals jointly degraded MB in the final.Miet al.[134]modified the surface of membranes with TiO2–GO to enhance their photocatalytic activities under both Ultraviolet(abbreviated as UV)and sunlight irradiation(Fig.6).Compared with PSF membranes surface-modified with TiO2or GO,respectively,the TiO2-GO modified membrane exhibited significantly improved photodegradation kinetics behaviors under UV(about 60%–80%faster)and sunlight(about 3–4 times faster)(Fig.7)irradiation.Therefore,the modification of TiO2-GO grafting provided a very promising route to the fabrication of high-performance photocatalytic membranes for sustainable water treatment.
4.3.2.Electrocatalysis
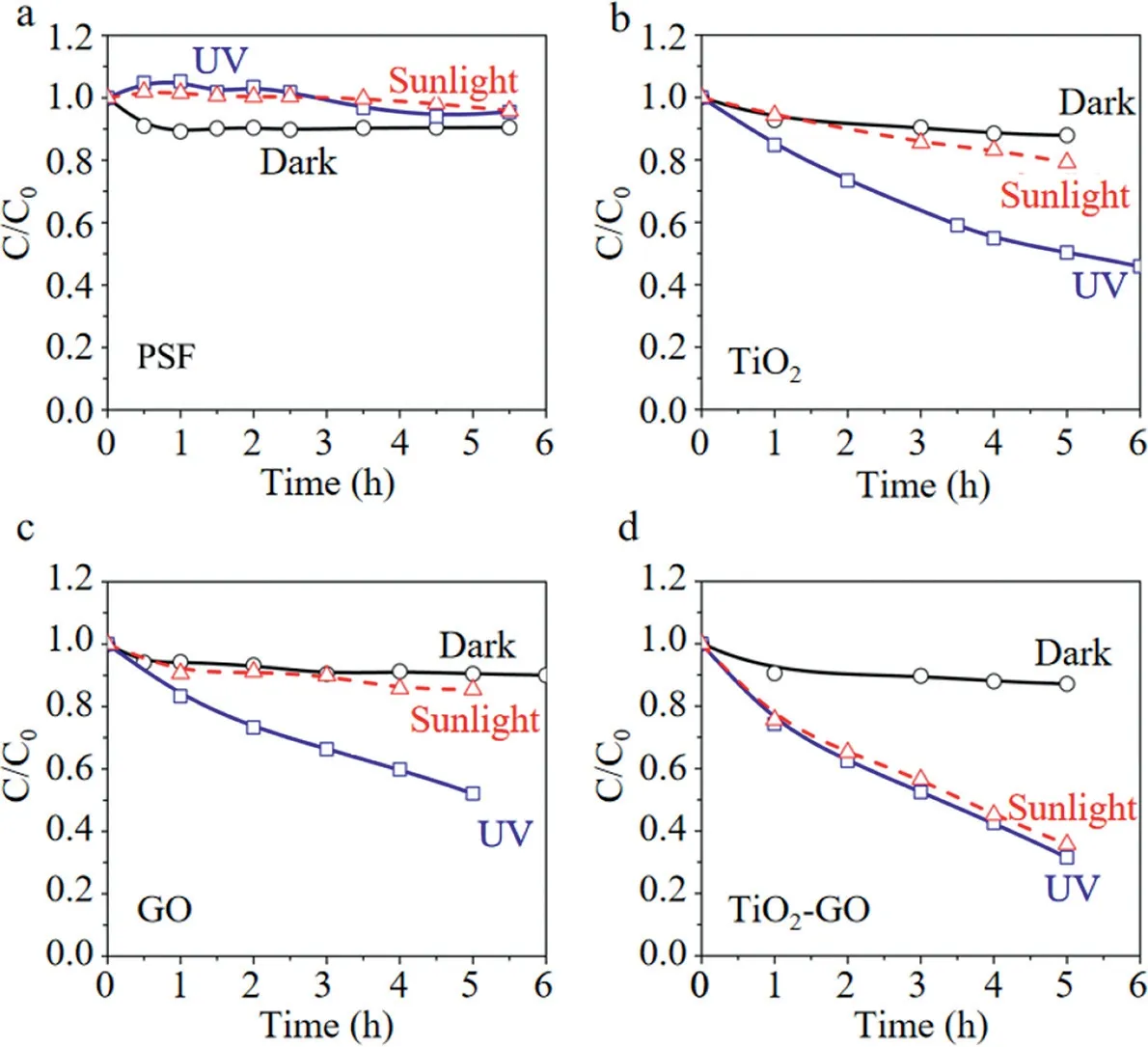
Fig.7.MB removal by:(a)the virgin PSF membrane,(b)surface modified with TiO2,(c)surface modified with GO and(d)surface modified with TiO2-GO,respectively.Reproduced with permission[134].Copyright 2014,Elsevier.
The electrocatalytic technology is always coupled with the membrane separation process to improve the comprehensive performance of wastewater treatment.In the process of electrocatalytic degradation of contaminants,utilizing the large specific surface area and excellent electron transport properties of GO,high catalytic decomposition of contaminants can be achieved.Functional groups such as N,P,S,Fe and noble metals are often grafted on the surface of GO to enhance the catalytic activity.Similar to the photocatalysis mechanism,the separated electrons(e−)can be transferred rapidly to the conduction band of GO and propagated on the surface of GO,which may react with the electrocatalytically generated H2O to further produce reactive oxygen species(i.e.,•OH)[135].Zhouet al.[136]used a two-step electrochemical reduction method to prepare an anthraquinone/GO modified membrane electrode.The electrochemical test results showed in a weakly acidic or neutral condition(when the pH is 5–7),the reduction reaction of oxygen on the surface of anthraquinone/GO modified membrane electrode was mainly carried out in a two-electron transmission path.In the experiment,the anthraquinone/GO modified membrane electrode was used as cathode,and FeOOH/Al2O3was added as a solid-phase catalyst to construct a heterogeneous electro-Fenton system to degrade Rhodamine B.The results exhibited that the anthraquinone/GO modified membrane electrode increased the conversion rate of the Fe3+/Fe2+pair significantly,which will accelerate the Fenton chain reactions and further improve the degradation efficiency of Rhodamine B.Wanget al.[135]fabricated a novel 3D structured GO composite membrane with high water flux.The prepared rGOTiO2/oxidized CNT membrane showed a removal rate of up to 95%at a high liquid velocity,which was 2 times more than that of the same condition without the electrocatalyst.The results indicated GO composite membrane had a high water flux(44.14 L m−2h−1)and excellent dye removal performanceviacombining membrane process with electrocatalysis.
4.3.3.Energy reproduction
In the process of wastewater treatment,a lot of energy was consumed.Forcing with serious energy crisis,how to recover energy effectively from environment or how to achieve efficient environmental treatment while saving energy are the breakthrough directions for environmental researchers.The emergence of Microbial Fuel Cell(abbreviated as MFC)has brought electrochemical wastewater treatment technology to a new level.The emergence of MFC began in 1911[137],when British scientists Potteret al.[137]discovered that bacteria can deliver electrical current.Subsequently,a large number of experiments about the use of bacteria in batteries were conducted.By 1990s,scientists tried to use bacteria to treat domestic wastewater,discovering that electrons can continuously transmit to the cathode even there was no electron mediator[138–140].Since then,research on the application of MFC in wastewater treatment has become a hot topic in decades.Compared with conventional water treatment technologies,MFC has the obvious advantage of saving energy consumption in wastewater treatment,saying it can convert organic energy to electricsviathe action of extracellular electron-transferred bacteria,i.e.,Shewanellaspp.[141,142].However,the low quality of effluent is a defect of MFC used in wastewater treatment,which can be solved by combination with membrane technologies.
In most cases,the electrons generated by the degradation of organics of extracellular electron-transferred bacteria in anode were transmitted to cathode to the electron acceptor(i.e.,O2),forming the electrochemical potential.In the catalytic reduction process,O2,as a common electron acceptor,was always reduced to H2O through a four-electron transmission path(say an O2molecule would accept four electron in the reduction process).In the meanwhile,organics were also degraded under the action of catalytic oxidation by the medium products.The wastewater treatment process is more energy-saving and efficient.Moreover,due to the good properties of GO,many researchers used GO for electrode modifications in order to improve the performance of electrical production and contaminant removal of MFCs[143,144].Based on this concept,Zhanget al.[143]added GO powder into the polyvinylidene fluoride(PVDF)membrane casting solution and scraped the membrane on the surface of carbon fiber film.After the membrane was formed by phase inversion,the GO/PVDF/carbon fiber composite membrane was prepared byin-situthermal reduction using hydroiodic acid.The catalytic membrane material was used as the combination of MFC with MBR.In the device,the catalytic membrane achieved the dual functions at the same time:catalytic decomposition of contaminants and membrane filtration.Figs.8a-f showed the scanning electron microscope(SEM)images of the prepared membranes.The results showed that GO(0.1 wt%)increased the viscosity of casting solution,entailed a slower phase inversion,which led to smaller pores.The membrane fouling test results showed that the GO/PVDF/carbon fiber composite membrane had good anti-fouling performance,and the contact angle of water decreased from 83°of the PVDF membrane to 79°(Figs.8g and h).In the process of wastewater treatment by the MFC/MBR combined system,the removal rates of chemical oxygen demand(COD)and ammonia nitrogen reached 97%and 98%,respectively,after 20 days of continuous operation.
Liet al.[144]combined MFC with photocatalysis and catalytic membrane technology.By preparing a CoFe2O4-GO/PVDF/carbon fiber composite photocatalytic membrane,a MFC/MBR coupled reactor was constructed.The extracellular electron-transferred bacteria in MFC was used as the anode,and the CoFe2O4-GO/PVDF/carbon fiber composite membrane was used as the cathode membrane,performing the triple functions simultaneously in wastewater treatment:biodegradation,electrocatalysis and membrane filtration.The results showed that the addition of CoFe2O4-GO in the catalytic membrane improved the catalytic activity and hydrophilicity of the catalytic membrane,which finally resulted in 30%increase of the stable flux of the membrane Moreover,the addition of CoFe2O4-GO in the catalytic cathode membrane improved the electronic output efficiency of the MFC/MBR coupled reactor.
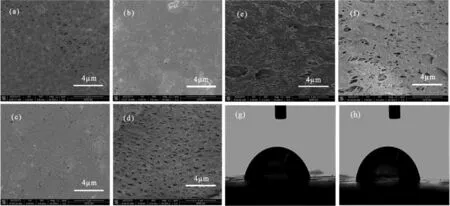
Fig.8.SEM images and water contact angles of membranes.Surface and cross-section morphologies:(a,d)PVDF;(b,e)GO/PVDF;(c,f)rGO/PVDF membranes.Water contact angles of the tested membranes:(g)GO/PVDF with the contact angle of 79°±8°;(h)RGO/PVDF with the contact angle of 83°±18°.Reproduced with permission[143].Copyright 2016,Wiley Online Library.
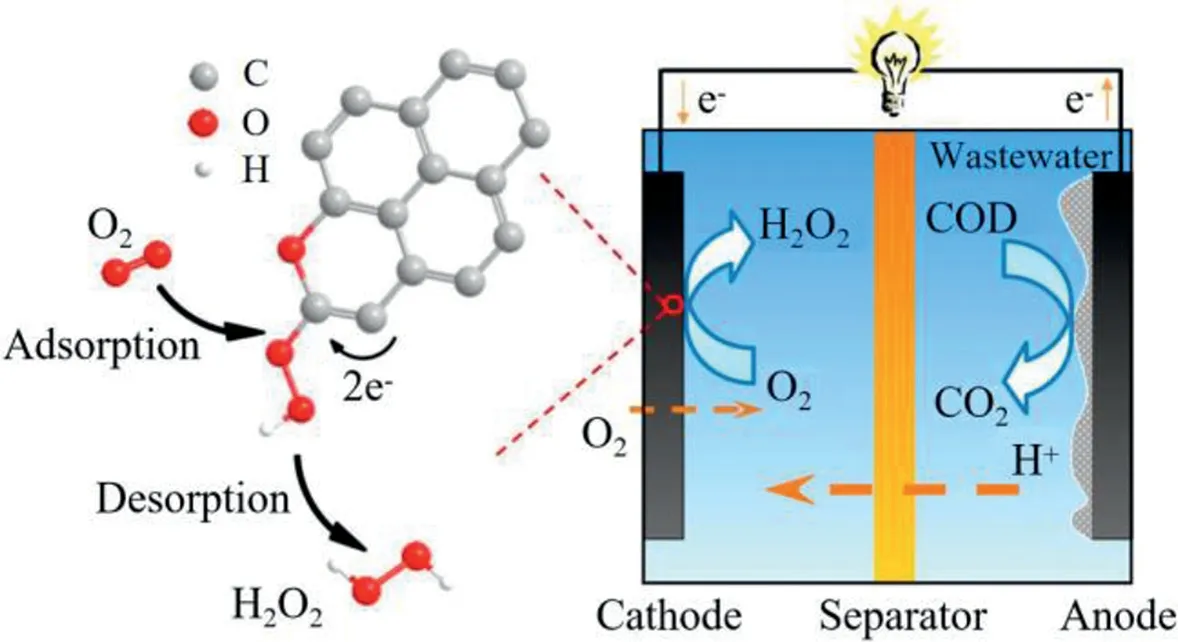
Fig.9.Possible mechanisms of ORR on the oxidized graphene air-cathode in the MFC.Reproduced with permission[144].Copyright 2018,Elsevier.
Donget al.[145]analyzed the catalytic performance of GObased materials for 2-electron oxygen reduction reaction(ORR)and the influence of oxygen-containing functional groups(Fig.9).The results showed that the MFC with GO cathode could achieve the purpose of wastewater treatment,power output and H2O2generation simultaneously.Oxygen-containing functional groups,especially epoxide groups,were found to enhance the 2-electron ORR performance.Thus,the MFC with GO cathode exhibited excellent performance of power output and organics removal.
In conclusion,with the properties of antimicrobial,hydrophilic,super-fast electron transmission and various functional groups,GO composite membranes showed excellent performance in wastewater treatment.
5.Perspective
Membrane technology has occupied increasing share in the area of environmental remediation in decades[146].Significant progress has been made to introduce GO to membranes to improve the comprehensive performance of numerous environmental processes since the fascinating properties of GO.In present stage,GO composite membranes have already showed excellent performance in salt rejection,CO2capture and wastewater treatment including the organics decomposition,energy recovery and membrane fouling mitigation.
However,despite tremendous progress,there are still challenges for GO composite membranes in commercial application.For instance,due to the difference in operating conditions between the laboratory and the plant,the mass-scale production of GO composite membranes has not yet been achieved.Thus,more in-depth researches in regard to the green and cost-effective membrane synthetic techniques are expected to make GO composite membranes as next-generation,cost-effective and sustainable alternative to the long-operated thin-film composite polyamide membranes for industrial environmental treatment.
Furthermore,to achieve satisfactory rejection rate of saline ions,potential improvements of designing the spacing between GO layers can be taken by incorporating different-sized cross-linkers,modifying membrane charges by functionalizing GO with different functional groups,optimizing membrane thickness by varying the number of GO layers,etc.
Besides,although GO composite membranes have competitive separation efficiency,it is less stable under harsh conditions.In most solvents,GO will spontaneously aggregate and swelling,which makes it difficult to get expected results.Researchers need to study deeply and overcome these defects.
Nevertheless,researches on GO composite membranes will still be at the forefront of research,and GO composite membranes have the potential to be further developed and gain wide acceptance.Therefore,GO composite membranes will continue to play an important role in environmental aspects.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China(No.42107090),the Science and Technology Project of Sichuan Province,China(No.2019YJ0262)and the financial support of the Basic Scientific Research Special Fund Project of Southwest Minzu University,China(No.2020NQN13).
 Chinese Chemical Letters2022年12期
Chinese Chemical Letters2022年12期
- Chinese Chemical Letters的其它文章
- Diverse strategic approaches en route to Taxol total synthesis
- Recent advances in gold-complex and chiral organocatalyst cooperative catalysis for asymmetric alkyne functionalization
- Unmodified methodologies in target discovery for small molecule drugs:A rising star
- Recent advances in single-crystalline two-dimensional polymers:Synthesis,characterization and challenges
- Recent advances in the application of metal organic frameworks using in advanced oxidation progresses for pollutants degradation
- Recent progress on two-dimensional materials confining single atoms for CO2 photoreduction
