Diverse strategic approaches en route to Taxol total synthesis
Zexian Li,Jianfeng Zheng,Wei-Dong Z.Li
Sichuan Engineering Research Center for Biomimetic Synthesis of Natural Drugs,School of Life Science and Engineering,Southwest Jiaotong University,Chengdu 610031,China
Keywords:Taxol Total synthesis Biomimetic synthesis Linear strategy Convergent strategy
ABSTRACT Taxol is one of the most famous diterpenoid natural products used in clinical cancer therapy.Taxol and its analogues are popular synthetic targets and have attracted worldwide attention over the past decades.Tremendous research efforts have already been made and ten groups have achieved the total synthesis of Taxol since 1994.This mini-review summarized the recent highlights of divergent strategic approaches towards the chemical synthesis of Taxol’s carbocyclic framework bearing a bridged eight-membered ring.
1.Introduction
Taxol(1)is a complex polyoxygenated diterpenoid natural product which was originally isolated from the bark ofTaxus brevifoliaby Wall and co-workers[1].Taxol is a well-known anticancer drug with a unique molecular mechanism for various human cancers[2–4],which has promoted significant interests from chemical and biological communities.The source of Taxol has always been a primary concern since its discovery due to the extremely low content in the natural plant[5].Considerable efforts have been devoted to find alternative sources to natural harvesting of Taxol,including:(1)total synthesis,(2)semi-synthesis,(3)microbial systems,and(4)plant cell fermentation.Among them,the chemical semi-synthesis and plant cell fermentation of theTaxusspecies are documented as the clinical supply of Taxol.
Accordingly,no natural product discovered in the last decades has stimulated as much public interests as Taxol.Structurally,Taxol has a highly strained 6−8−6 tricyclic carbocyclic core with two all-carbon quaternary stereocenters and a bridgehead double bond(Fig.1).The potent bioactivity and intriguing chemical complexity of this oxygenated polycyclic diterpenoid has attracted considerable interests from the synthetic communities.Indeed,more than hundreds of reports presenting synthetic studies have been documented since the landmark total synthesis of Taxol achieved by Holton and Nicolaou groups in 1994,including ten total syntheses of Taxol(Fig.2)[6–20].It has been noted that the nature of substituents plays an important role in the construction of conformationally flexible central eight-membered ring.Various synthetic strategies including coupling reaction,ring-closing metathesis,oxy-Cope rearrangement,Diels−Alder reaction,radical cyclization,and ring expansion have been well developed to construct the eightmembered core ring.To maintain the coherence and focus of this review,the well-known success total syntheses of Taxol(Fig.2)will not discuss further herein.Therefore,this mini-review mainly highlights on the diverse strategic approachesen routeto Taxol’s total synthesis,covering mainly the literature reports since 1995[21–24].
2.Biomimetic approaches to Taxol
Early in 1966,Lythgoe and co-workers proposed a biosynthetic pathway,which suggested that the tricyclic carbon framework of the taxoids was envisioned to derive from geranylgeranyl pyrophosphate(GGPP)through intramolecular cyclization[25,26].Based on feeding studies by Croteau and co-workers,the proton at C11 of intermediate 3 shifted to C7 to generate carbocation intermediate 4,followed by transannular cyclization to afford the taxa-4(5),11-(12)-diene 5 as the final product of the cyclase phase[27–29].Notably,several research groups including Croteau,Coates,Floss,Pattenden and Williams have also proposed that biosynthetic pathway of Taxol can be conceptually divided into several discrete stages,namely:cyclization,stereospecific oxidation,acylation,benzoylation,and assembly of C13-side chain(Scheme 1)[30–35].
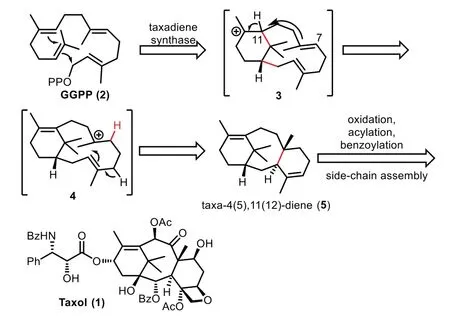
Scheme 1.Proposed biosynthetic pathway of Taxol.
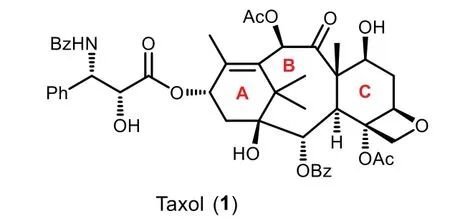
Fig.1.The structure of Taxol.
In 1985,Pattenden and co-workers reported the total synthesis of verticillene(8),an assumed biosynthetic precursor of Taxol,by an intramolecular reductive carbonyl coupling as key step[34,36].Treatment of dialdehyde 6 with titanium trichloride and Zn−Cu couple,followed by 1,4-reduction gave verticillene 8.However,various Lewis acid-catalyzed biomimetic transannular cyclization of 8 or its isomers failed to yield the desired tricyclic products 9(Scheme 2a)[37].In 2009,the same group developed a cascade radical cyclization strategy leading to the 6−8−6 tricyclic core of Taxol[38–40].Treatment of the radical precursor 10 with Bu3SnH and AIBN in PhH at 80°C afforded the desired tricycle 12viapresumably the intermediate 11 through the radical transannular cyclization.It is worthy to note that 12 contains oxidative substitutions at C1,C13 with desired stereochemistry and an unsaturated bond at C3−C4(Scheme 2b).
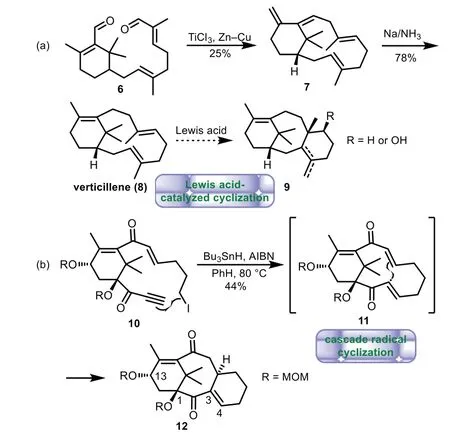
Scheme 2.Pattenden’s biomimetic approaches through Lewis acid-catalyzed and cascade radical cyclization(1985−2009).
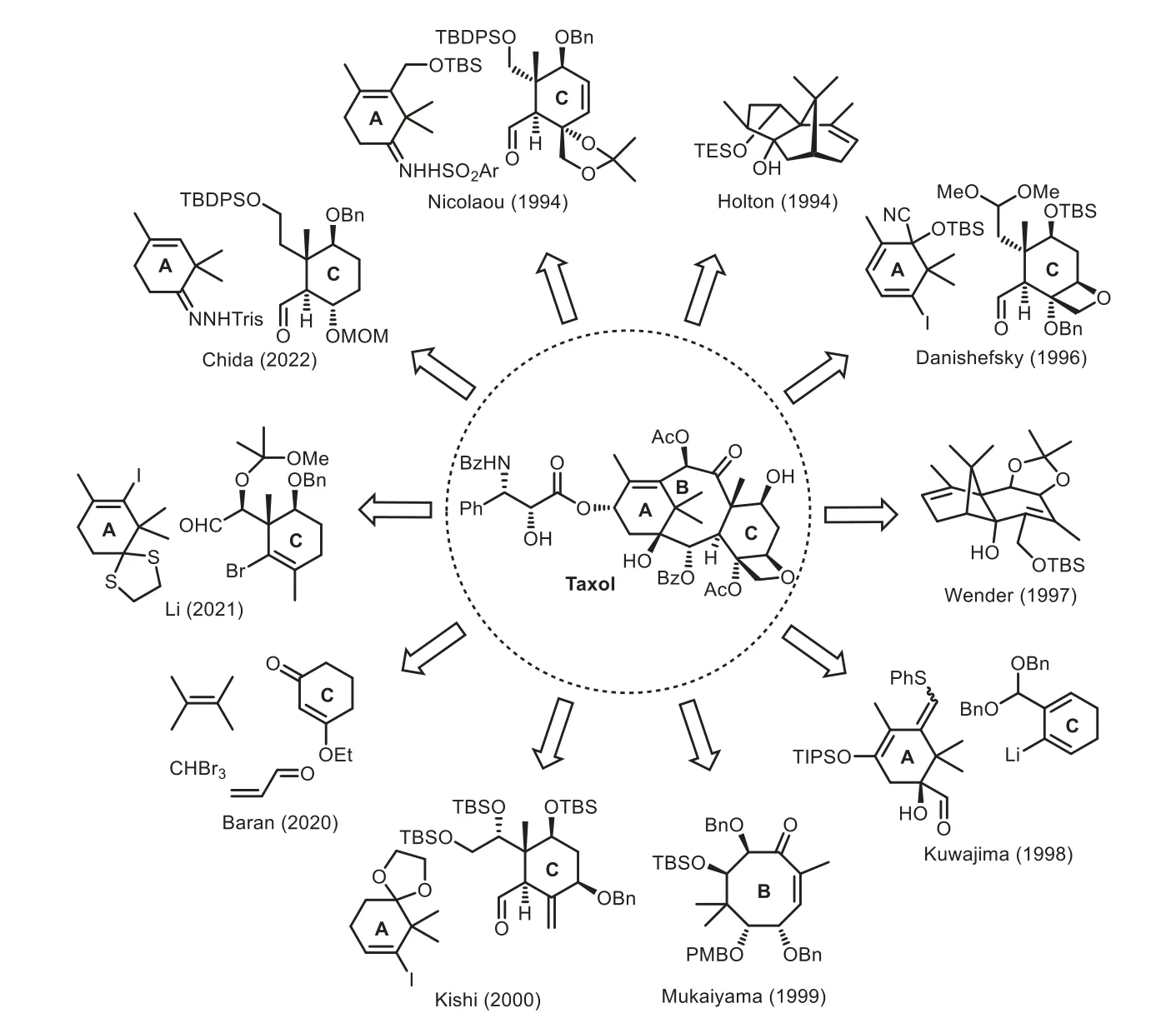
Fig.2.The ten-total synthesis of Taxol.
Scheme 3.Takahashi’s biomimetic approach through Lewis acid-catalyzed cyclization(1997).
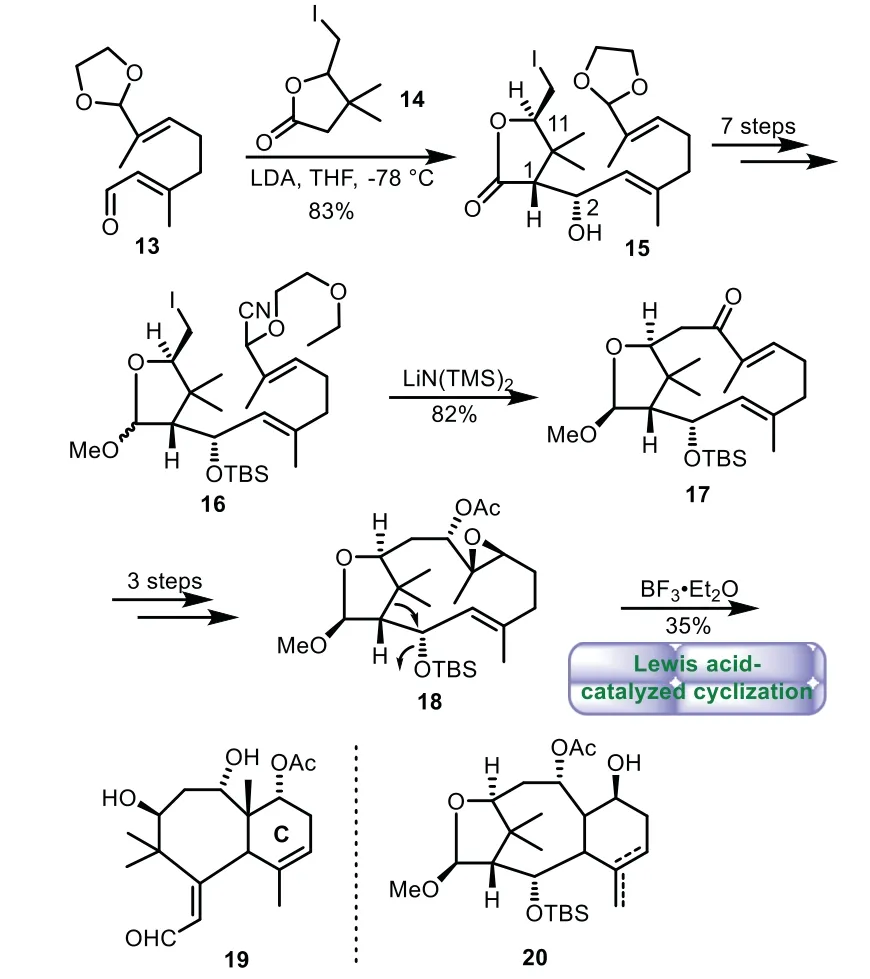
In 1997,Takahashi and co-workers reported their biomimetic approach[41].The aldol condensation of citral derivative 13 withγ-butyrolactone 14 generated compound 15 with three stereogenic centers at positions C1,C2 and C11 respectively,which corresponds to Taxol.A seven-step sequence of reduction of lactone,cyanoaddition,and protection of hydroxyl groups afforded cyanohydrin ether 16.Then intramolecular alkylation of 16 efficiently formed the 12-membered ring intermediate 17 in 82%yield.A three-step sequence of reduction,epoxidation,and acetylation gave acetate 18 in 87%yield.However,all attempts of acid-catalyzed cyclization to the formation of B/C ring of Taxol failed,instead rearrangement product bicyclo[5.4.0]undecene 19 bearing the desired six membered C-ring was obtained(Scheme 3).
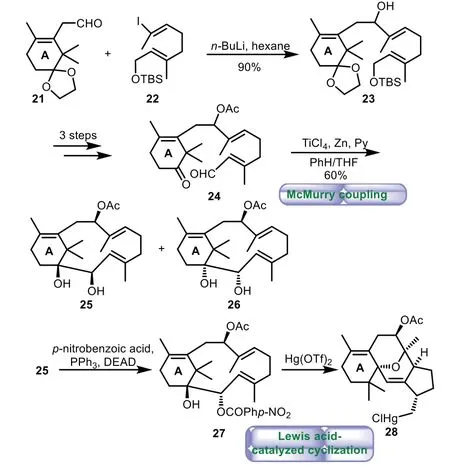
In 1998,Nishizawa and co-workers reported synthetic efforts toward biomimetic construction of the taxane skeletonviatransannular cyclization[42,43].The aldol reaction of acetal aldehyde 21 with vinyl iodide 22 gave alcohol 23 in 90%yield.A three-step sequence of protection of hydroxyl group,cleavage of acetal and TBS groups,and Swern oxidation provided ketoaldehyde 24.Ti Cl4−Zn promoted McMurry coupling yielded diols 25 and 26 with bicyclo[9.3.1]pentadecatriene skeleton in 60%yield.However,all attempts to achieve the biomimetic transannular cyclization leading to the B/C ring system of Taxol failed and an unexpected rearrangement product 28 was obtained(Scheme 4).Although the biomimetic synthesis of Taxol has not yet achieved to date,the studies along this direction have enriched our understanding of the unique role of Taxol synthases in the plant biosynthesis assembly.Future research would be much needed to simulate the delicate conformational control of the GGPP precursor for a smooth tandem cationic C−C bond-forming and transannular cyclization to the core ring system with proper stereochemical control.
Scheme 4.Nishizawa’s biomimetic approach through McMurry coupling and Lewis acid catalyzed cyclization(1998).
Scheme 5.Malacria’s approach to the tricyclic ring system through cobalt(I)-mediated[2+2+2]cyclotrimerization and intramolecular Diels−Alder reaction(2002).
3.Linear strategies toward A/B/C ring core
Among the strategies applied to the construction of the A/B/C ring core of Taxol,linear strategy is widely developed to the synthesis of challenging taxane framework,including Diels−Alder strategy,A to A/B/C strategy,and C to A/B/C strategy.
3.1.Diels−Alder strategy
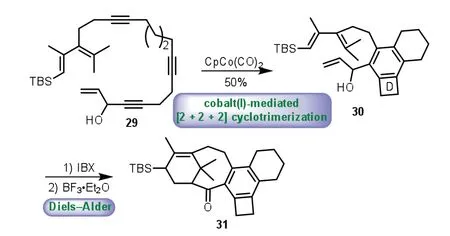
In 2002,Malacria and co-workers described the combination of cobalt(I)-mediated[2+2+2]cyclotrimerization and intramolecular Diels−Alder reaction to construct the 6−8−6 tricyclic core of Taxol[44].Exposure of polyunsaturated precursor 29 to CpCo(CO)2afforded alcohol 30 with all carbon D ring in 50%yield.Next,oxidation of the secondary alcohol with IBX,followed by BF3·Et2O mediated intramolecular Diels−Alder reaction afforded the desired pentacyclic framework 31 as only one diastereomer(Scheme 5).
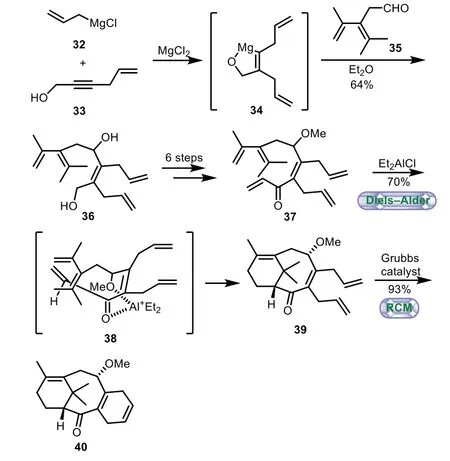
In 2003,Fallis and co-workers also applied intramolecular Diels−Alder reaction coupled with the ring-closing metathesis to the synthesis of 6−8−6 tricyclic core of Taxol[45,46].The sequential combination of Grignard reagent 32 with alcohol 33 generated the magnesium chelate 34in situ,followed by condensation with aldehyde 35 to afford the diol 36 in 64%yield.A six-step sequence of selective protection the secondary alcohol,oxidation the primary alcohol,Grignard addition,and oxidation of the resulting secondary alcohol was preformed,thus converting 36 to the Diels–Alder precursor 37.With the key intermediate 37 in hand,the desired Diels−Alder cycloaddition was smoothly performed,thus affording the adduct 39 as a single diastereomerviachelation control model 38.Lastly,the bicycle 39 annulated to give 6−8−6 tricyclic ring-system 40 by ring-closing metathesis(Scheme 6).
In 2018,Li and co-workers documented a highly concise and elegant enantioselective synthesis of the 6−8−6 tricyclic core of taxezopidine A and B[47].The aldehyde 42 was prepared from 41 based on Shea’s work[48,49],which includes glycolate Ireland−Claisen rearrangement,reduction,and Swern oxidation.Next,treatment of 42 with bromofuran and subsequent transformations of functional groups gave 43.Then the type II intramolecular Diels−Alder furan reaction of 43 was carried out,thus giving 6−8−6 tricyclic core of Taxol 44 as a single diastereomer in 55%yield.It is worthy note that the acetoxy group at the allylic position of 43 is crucial for high diastereoselectivity(Scheme 7).
Scheme 6.Fallis’s approach to the tricyclic ring system through Diels−Alder reaction and ring-closing metathesis(2003).
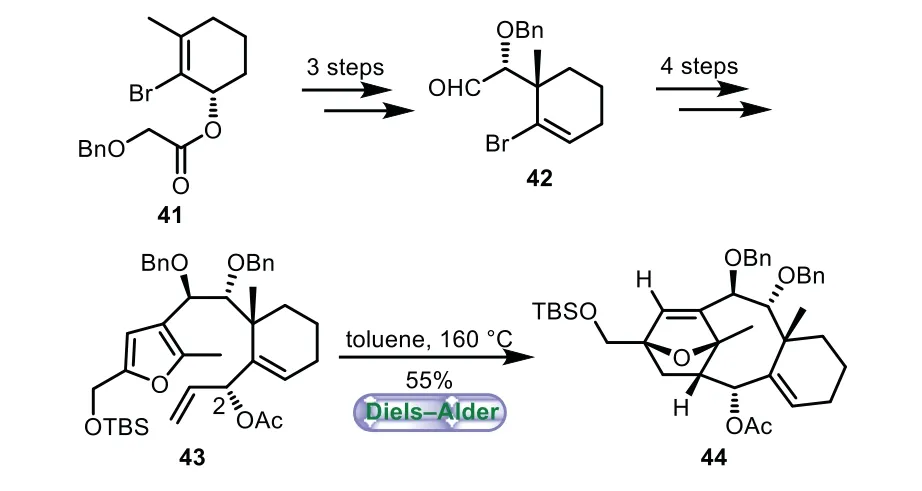
Scheme 7.Li’s approach to the tricyclic ring system through type II intramolecular Diels−Alder furan reaction(2018).
In 2020,Fletcher and co-workers described the concise procedure to prepare the Taxol core by type II intramolecular Diels−Alder reaction and Cu(I)-catalyzed asymmetric conjugate addition[50].The addition reaction of cyclohexanone 45 and alkyl zirconocene(generated from bromodiene 46 and Cp2ZrHCl)with copper-phosphoramidite complexes as catalyst followed by Vilsmeier−Haack reaction generatedβ-chloroaldehyde 47 in 69%yield with 92%ee.A three-step sequence of Grignard addition,Suzuki−Miyaura reaction,and oxidation of the resulting secondary alcohol afforded the intermediate 48.Finally,treatment of 48 with TiCl4in CH2Cl2,the Diels−Alder reaction proceeded smoothly to give tricyclic ring core 49 in 35%yield with 1:1drand 92%ee(Scheme 8).
3.2.A to A/B/C strategy
The A/B ring system of Taxol contains bicyclo[5.3.1]undecane,which has attracted considerable attention since Taxol’s discovery.Significant efforts have been made regarding to synthesis of A/B ring,such as ring-expansion of cyclopropane[51–53],oxy-Cope rearrangement[54–57],ring-closing metathesis[58–60],transition metal-catalyzed carbocyclization[61,62]and so on(Scheme 9).
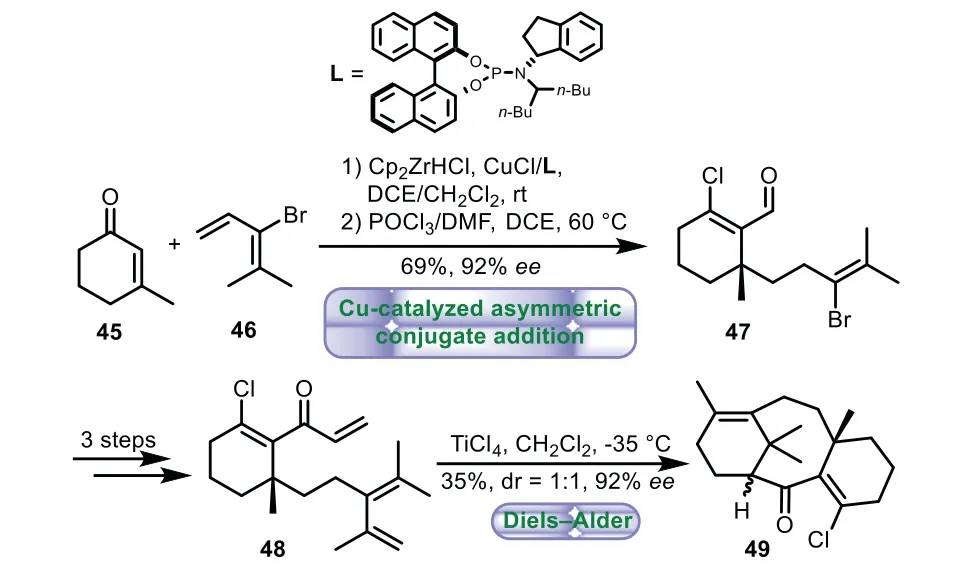
Scheme 8.Fletcher’s approach to the tricyclic ring system through type II intramolecular Diels−Alder reaction and Cu(I)-catalyzed asymmetric conjugate addition(2020).
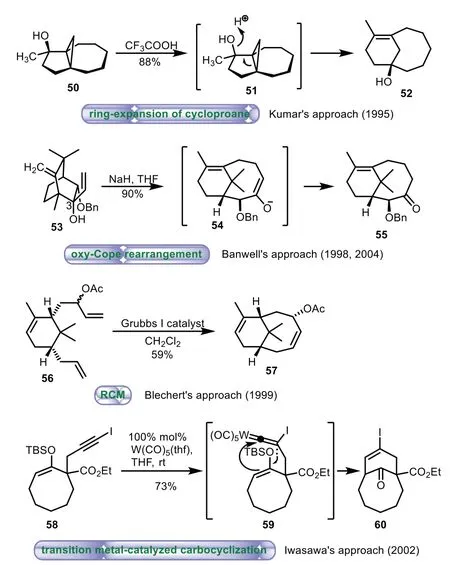
Scheme 9.Selected strategies to the A/B ring system.
During the past decades,Paquette and co-workers made remarkable efforts to pursue Taxol by using an anionic oxy-Cope rearrangement as the key strategic reaction[63–65].Treatment of 61 with KHMDS and 18-crown-6viathe crucial anionic oxy-Cope rearrangement smoothly generated nine-membered ring,followed by methylationin situ,thus affording 62 in 81%overall yield.A four-step sequence of dihydroxylation,protection,desilylation,and Swern oxidation afforded keto aldehyde 63.Intramolecular aldol reaction of 63 generated the C-ring stereoselectively,then transannular hydride shift of the resulting alcohol with KOt-Bu gave 64.A seven-step sequence was carried out to generate 65.Finally,65 underwentα-ketol rearrangement with Al(Ot-Bu)3to provide highly functionalized taxane system 66(Scheme 10).
In 1995,Martin and co-workers achieved the 6−8−6 tricyclic ring of taxaneviaan anionic oxy-Cope rearrangement protocol[66].The crucial anionic oxy-Cope rearrangement reaction of alcohol 67,followed by alkylationin situ,furnished the bicyclo[5.3.1]undecenone 68 in 76%yield with 2:1 ratio of diastereoselectivity.Finally,exposure of excess MeLi by a vinyllithium reagent generated tricyclic compound 69 in 58%yield(Scheme 11).
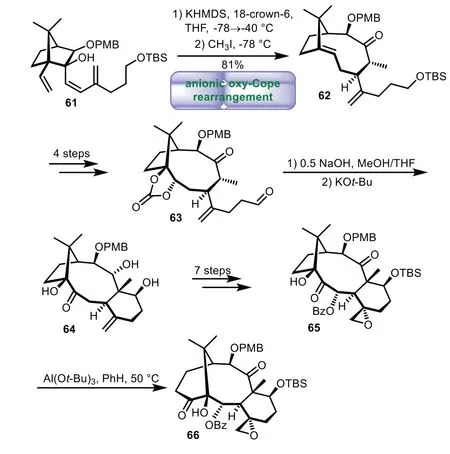
Scheme 10.Paquette’s approach to the tricyclic ring system through anionic oxy-Cope rearrangement(1989−2003).
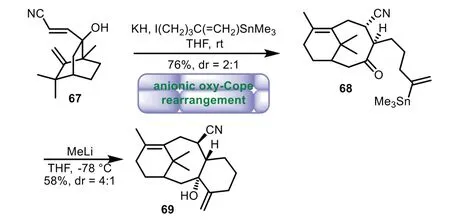
Scheme 11.Martin’s approach to the tricyclic ring system through anionic oxy-Cope rearrangement(1995).
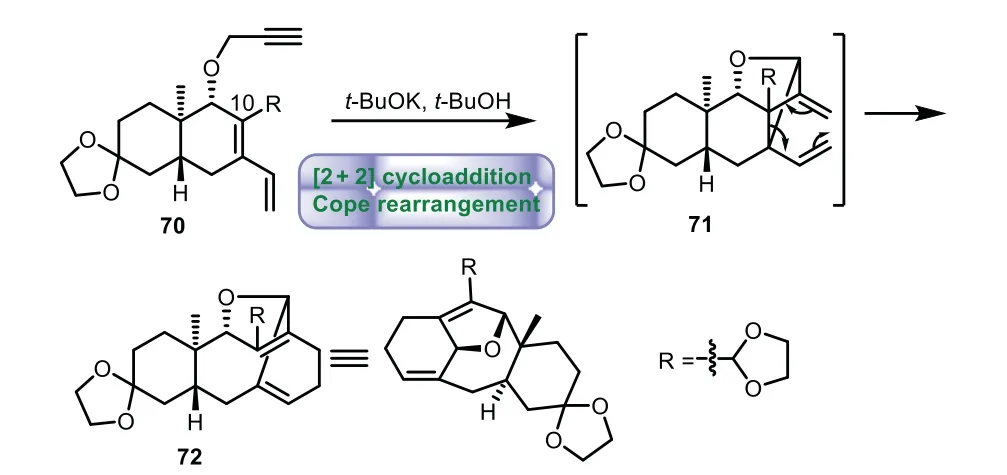
Scheme 12.Kanematsu’s approach to the tricyclic ring system through tandem intramolecular[2+2]cycloaddition and Cope rearrangement reaction(1995).
In 1995,Kanematsu and co-workers applied tandem intramolecular[2+2]cycloaddition and Cope rearrangement strategy to the enantioselective synthesis of 6−8−6 tricyclic core of Taxol[67].Treatment of enyne 70 witht-BuOK int-BuOH,generated intermediate 71in situ viaa[2+2]cycloaddition,followed by Cope rearrangement affording product 72 bearing 6−8−6 tricyclic skeleton of taxane(Scheme 12).
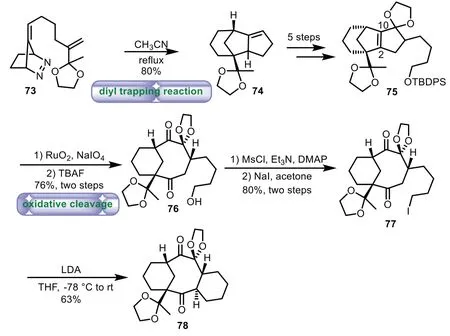
Scheme 13.Little’s approach to the tricyclic ring system through intramolecular diyl trapping and oxidation of cleavage(1997).
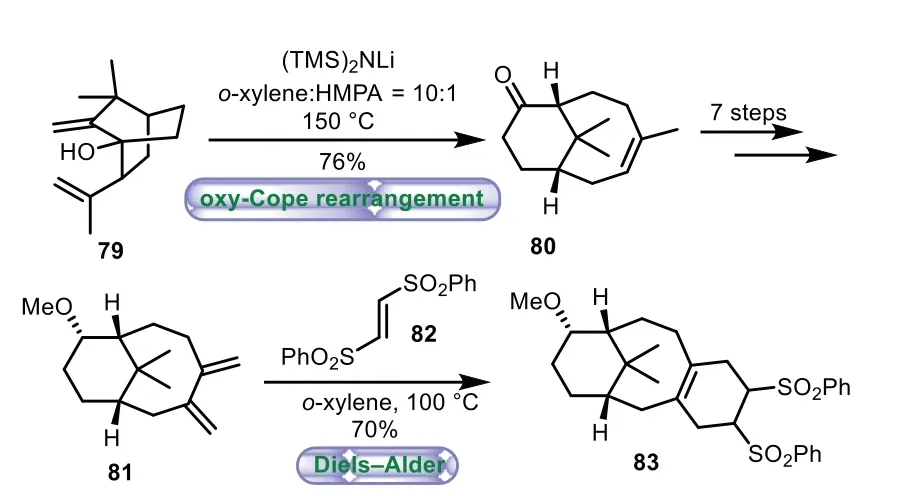
Scheme 14.Nagaoka’s approach to the tricyclic ring system through oxy-Cope rearrangement and intermolecular Diels−Alder cycloaddition(1998).
In 1997,Little and co-workers reported the synthesis of 6−8−6 tricyclic ring of the taxaneviaintramolecular diyl trapping and oxidation of cleavage of olefin strategy[68].The intramolecular diyl trapping reaction of bicyclic diazene 73 formed compound 74 in 80%yield.A five-step sequence of the addition of phenylselenyl trifluoroacetate,Swern oxidation,elimation,alkylation,and ketalization was carried out,thus affording 75.Oxidation of cleavage of olefin by using sodium periodate and ruthenium dioxide,followed by removing the silyl ether gave 76 with eight-membered ring in 76%yield over two steps.Then mesylation and iodization of 76 afforded 77.Lastly,treatment of 77 with excess of LDA afford 6−8−6 tricyclic scaffold 78 with highly functionalized eight membered ring in 63%yield(Scheme 13).
In 1998,Nagaoka and co-workers described the synthesis of 6−8−6 tricyclic core of Taxolviaoxy-Cope rearrangement and intermolecular Diels−Alder cycloaddition[69].Treatment of 79 with(TMS)2NLi in a mixture ofo-xylene and HMPA(10:1)at 150°C gave bicyclo[5.3.1]undecane 80 in 76%yield through oxy-Cope rearrangement.Then a seven-step sequence of stereoselective reduction,methyl etherification,epoxidation,ring-opening,oxidation,and olefination was carried out to give diene 81.Lastly,intermolecular Diels−Alder reaction of diene 81 withtrans-1,2-bis(phenylsulfony)ethylene 82 was carried out ino-xylene at 100°C to afford the tricyclic product 83 in 70%yield(Scheme 14).
In 1998,Stork and co-workers applied cyanohydrin cyclization and aldol reaction to the synthesis of the 6−8−6 tricyclic core of Taxol[70,71].The compound 85 containing A ring was prepared from stannylactone 84.Then the cyanohydrin cyclization of 85 was easily conducted with NaHMDS as base,thus resulting in the desired closure of the cyclooctane ring B to afford 86 in 72%yield.Next,a five-step sequence of desilylation,hydrolysis of cyanohydrin,Johnson−Claisen elongation,reduction,and oxidation was carried out to give dicarbonyl compound 87.Finally,87 underwent a smoothly intramolecular facile aldol reaction with K2CO3in MeOH and 18-crown-6 to provide the desired 6−8−6 tricyclic core 88(Scheme 15).
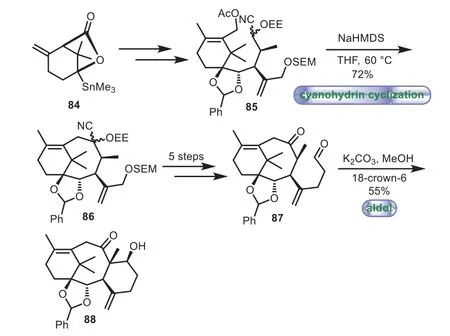
Scheme 15.Stork’s approach to the tricyclic ring system through cyanohydrin cyclization and aldol reaction(1998).
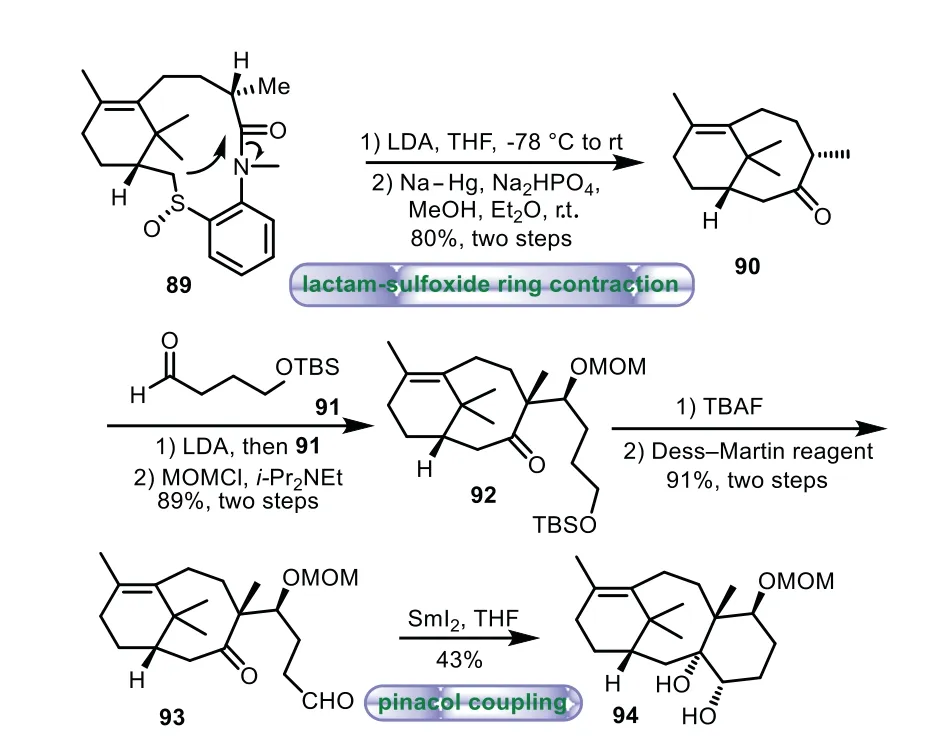
Scheme 16.Kajiwara’s approach to the tricyclic ring system through lactamsulfoxide ring contraction and pinacol coupling(1999).
In 1999,Kajiwara and co-workers described the synthesis of the 6−8−6 tricyclic core of Taxol by lactam-sulfoxide ring contraction and intramolecular pinacol coupling[72–76].Ring contraction of lactam-sulfoxide 89 was achieved with LDA,followed by reductive removal of the spacer moiety with Na−Hg to afford A/B ring system 90.Treatment of 90 with aldehyde 91,followed by MOM protection afforded ether 92 in 89%yield.Removal of the TBS group of 92 with TBAF and Dess−Martin oxidation of the resulting primary alcohol gave aldehyde 93 in 91%yield.Lastly,upon treatment of aldehyde with SmI2in THF,the pinacol-type reaction proceeded smoothly to yield 6−8−6 tricyclic ring system 94 in 43%yield(Scheme 16).
In 2011,Suffert and co-workers completed the synthesis of 6−8−6 tricyclic core of Taxol through a palladium catalyzed domino reaction[77].Reaction of alkenylbromide 95 with a catalytic amount of Pd(PPh3)4produced the desired tetracyclic ring 100,in which a tandem cyclization process including 4-exo-dig,6-exo-trigcyclizations and a disrotatory 6πelectrocyclization were performed.Subsequent oxidation of the secondary alcohol with Dess–Martin periodinane generated enone 101.Finally,regioselective oxidation cleavage of 101 delivered taxane skeleton 102(Scheme 17).
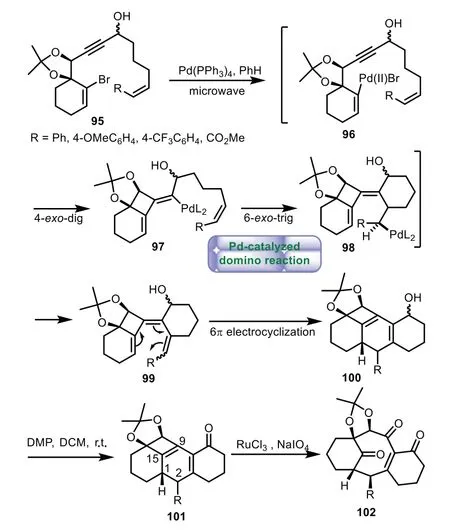
Scheme 17.Suffert’s approach to the tricyclic ring system through palladium catalyzed domino reaction(2011).
3.3.C to A/B/C strategy
In 1995,Magnus and co-workers provided the synthesis of 6−8−6 tricyclic core of Taxolvia[5+2]-pyrylium ylide-alkene cyclization and ring expansion[78,79].Treatment of pyrylium-ylide precursor 103 with DBU generated pyrylium-ylide intermate 104in situ,followed by stereoselective[5+2]cycloaddition to give bicyclo[5.4.0]undecenone 105 in 77%yield with 10:1dr.Then a threestep sequence of bromination,cyanation,and asymmetric cyclopropanation was performed,giving cyclopropane 106 in 91%yield over three steps.Subsequent,taxane 107 with B/C ring of Taxol was obtained in 95%yield with 2:1drthrough the cleavage of the internal cyclopropane bond.A six-step sequence of addition and reduction was carried out to give methyl ester 108.Lastly,intramolecular nucleophilic addition and desulfonylation were carried out to obtain the desired tricyclic product 109 in 95%yield(Scheme 18).
In 1998,d’Angelo and co-workers described the synthesis of 6−8−6 tricyclic core of Taxolviaa Mukaiyama-type condensation[80,81].Regioselective annulation diketone 110 with piperidine and acetic acid provided cyclohexanone 111 in 83%yield.Then a threestep sequence of oxidation,Wittig olefination,methylation,and silylation was carried out,thus affording silyl enol 112.Then intramolecular Mukaiyama-type condensation of 112 was performed with TiCl4as catalyst,affording the expected tricyclic product 113 in 61%yield with 2:1dr(Scheme 19).
In 2000,Shair and co-workers disclosed an elegant synthesis of 6−8−6 tricyclic core of Taxol by a tandem alkylation,oxy-Cope rearrangement,and transannular Dieckmann cyclization procedure[82].The triple-domino cyclization was initiated by treating of 114 with the vinyl Grignard reagent,affording the C-aromatic taxane framework 117 directly in 63%yield.The elegant transformation underwent a tandem anion-accelerated oxy-Cope rearrangement(115)followed by spontaneous transannular Dieckmann cyclization(116)(Scheme 20).
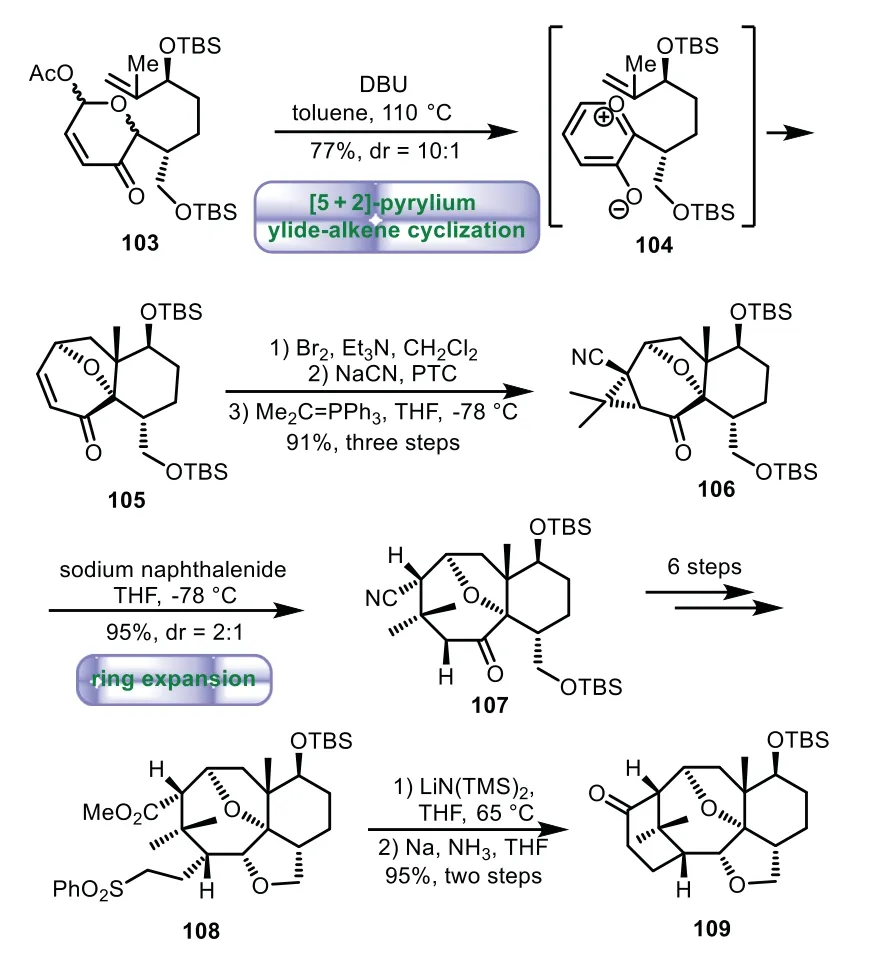
Scheme 18.Magnus’s approach to the tricyclic ring system through[5+2]-pyrylium ylide-alkene cyclization and ring expansion(1995).
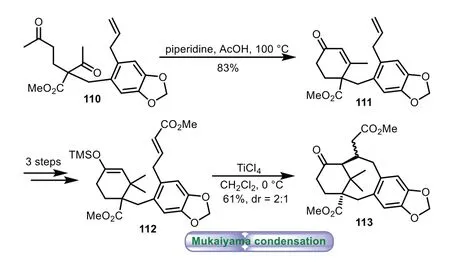
Scheme 19.d’Angelo’s approach to the tricyclic ring system through Mukaiyama condensation(1998).
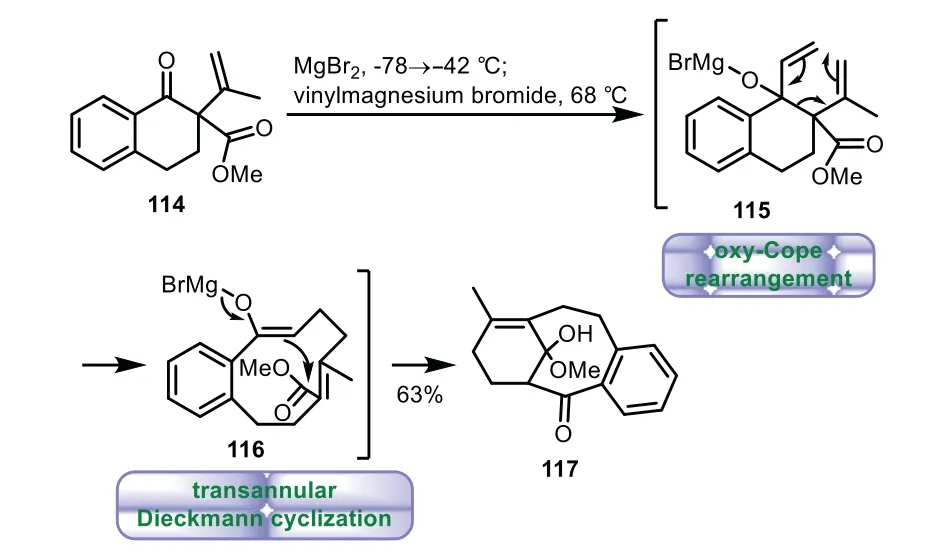
Scheme 20.Shair’s approach to the tricyclic ring system through a tandem alkylation,oxy-Cope rearrangement,and transannular Dieckmann cyclization(2000).
In 2005,Arseniyadis and co-workers applied Grob fragmentation and SmI2-mediated intramolecular aldol reaction to the synthesis of 6−8−6 tricyclic core of Taxol[83–85].Methylsulfonylation of acetonide 118 set the stage for the crucial B-ring formation through a Grob-type fragmentation,thus affording bicyclo[6.4.0]-system 119 in 82%yield over two steps,which corresponded to the taxoid B/C subunit.An eleven-step sequence including epoxideopening,methylation,reduction,and oxidation was carried out,thus affording seco-taxane 120.Finally,SmI2-mediated intramolecular aldol reaction of 120 resulted in the desired product 121 in 74%yield(Scheme 21).
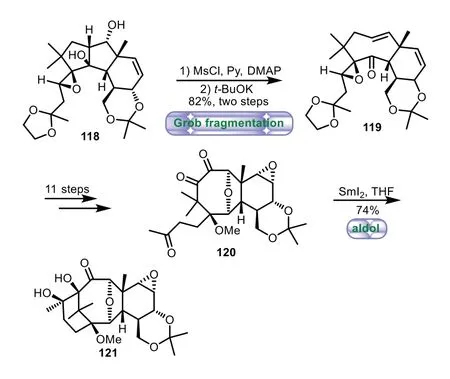
Scheme 21.Arseniyadis’s approach to the tricyclic ring system through Grob fragmentation and SmI2-mediated intramolecular aldol reaction(2005).
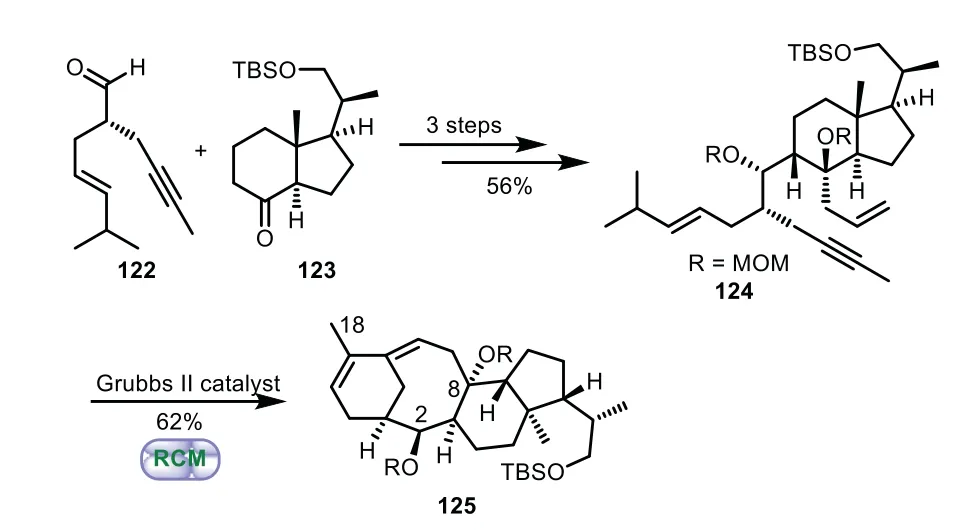
Scheme 22.Granja’s approach to the tricyclic ring system through cascade dienyne ring-closing metathesis(2007).
In 2007,Granja and co-workers documented a cascade dienyne ring-closing metathesis approach to the construction of 6−8−6 tricyclic core of Taxol[86–89].The RCM precursor 124 was obtained from aldehyde 122 and ketone 123 through a three-step sequence of addition,carbonyl allylation and protection.Lastly,in the presence of Grubbs II catalyst,the tandem ring-closing reaction of readily available enyne 124 was performed,thus affording tricycle 125 in 62%yield bearing with C2,C8-hydroxy groups and the methyl of C18 in a single-step(Scheme 22).
In 2014,Tanino and co-workers applied[6+2]cycloaddition reaction to the synthesis of the 6−8−6 tricyclic core of Taxol[90].Initially,the coupling reaction of dicobalt acetylene complex 126 with enol ether 127 was induced with EtAlCl2as Lewis acid,affording B/C ring intermediate 128.Then a five-step sequence of transformations was carried out,affording nitrile 129 as a mixture of diastereomers.Finally,treatment of nitrile 129 with LiNEt2afforded the tricyclic skeleton 130 in 84%yield with 10:1dr viaepoxy nitrile cyclization(Scheme 23).
In 2016,Prunet and co-workers applied a ring-closing dienyne metathesis reaction to the synthesis of 6−8−6 tricyclic core of Taxol[91–98].The Shapiro coupling reaction of aldehyde 131 with trisylhydrazone 132,hydrolysis of the trimethylsilyl ether and protection of the diols gave the carbonate 133.Then a five-step sequence of hydrolyzation,reduction,and elimination using the Grieco protocol was carried out to furnish the metathesis precursor 134.With enyne 134 as cyclic precursor,the desired tricycle product 135 with A/B/C ring was obtained in 47%yield through tandem metathesis reaction(Scheme 24).
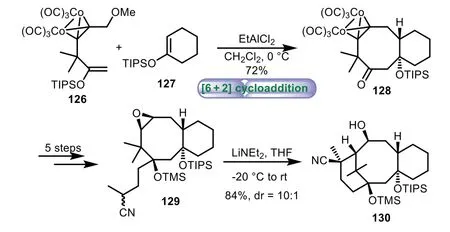
Scheme 23.Tanino’s approach to the tricyclic ring system through[6+2]cycloaddition reaction(2014).

Scheme 24.Prunet’s approach to the tricyclic ring system through ring-closing dienyne metathesis(2016).
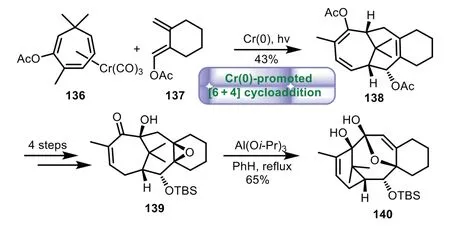
Scheme 25.Rigby’s approach to the tricyclic ring system through Cr(0)-promoted[6+4]cycloaddition(1995).
4.Convergent strategies toward A/B/C ring core
The construction of taxane frameworkviaconvergent strategies has also attracted great attention from synthetic communities with different strategies,including pericyclic reaction,transition metalcatalyzed cyclization and miscellaneous cyclization.
4.1.Pericyclic reaction
In 1995,Rigby and co-workers reported Cr(0)-promoted[6+4]cycloaddition for the synthesis of 6−8−6 tricyclic core of Taxol[99].The tricyclic product 138 was prepared from Cr(0)-promoted[6+4]cycloaddition of 136 with diene 137.Then a four-step sequence of transformations of protecting group,epoxidation,and enolate oxidation was carried out,thus giving epoxide 139.Finally,α-ketol rearrangement of 139 with Al(Oi-Pr)3gave the required tricyclic product 140 in 65%yield(Scheme 25).
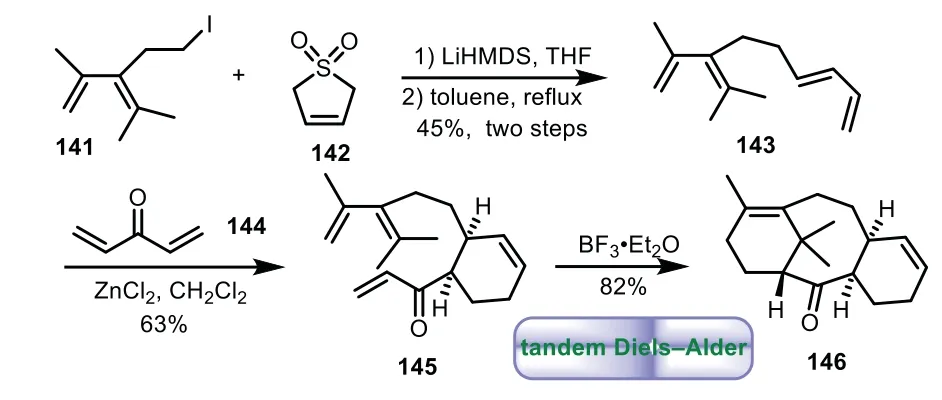
Scheme 26.Winkler’s approach to the tricyclic ring system through tandem Diels−Alder reaction(1995).
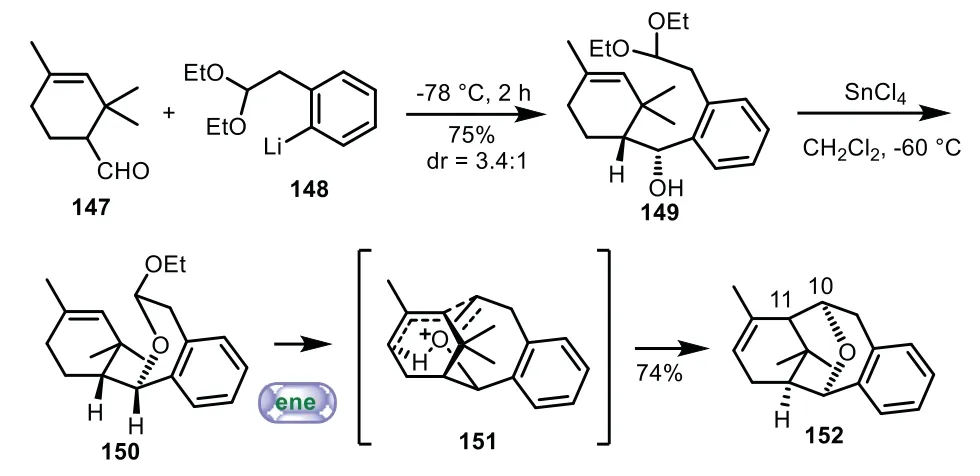
Scheme 27.Sonawane’s approach to the tricyclic ring system through sequential transacetalation oxonium ene reaction(1998).
In 1995,Winkler and co-workers reported the synthesis of the 6−8−6 tricyclic core of Taxol by tandem Diels−Alder strategy[100].The condensation of iodide 141 with butadiene sulfone 142,followed by extrusion of SO2,resulted in the formation of tetraene compound 143.The ZnCl2catalytic intermolecular Diels−Alder reaction of 143 with 144 give cyclohexene 145 bearing C-ring core in 63%yield.Lastly,the desired tricyclic enone 146 was obtained as a single diastereomerviaBF3·Et2O mediated intramolecular Diels−Alder reaction(Scheme 26).Later then,Winkler and co-workers disclosed the synthesis of tricyclic ring system through the intramolecular Diels−Alder reaction starting from Aring synthon[101,102].
In 1998,Sonawane and co-workers applied novel sequential transacetalation oxonium ene reaction to the synthesis of 6−8−6 tricyclic core of Taxol[103].The B-seco-taxane 149 was prepared from the coupling reaction of 147 with aryllithium 148 in 75%yield with 3.4:1dr.In the presence of SnCl4,the intramolecular nucleophilic substituted reaction and ene reaction were carried out to the assemble of the C-aromatic taxane skeleton 152 in 74%yield(Scheme 27).
4.2.Transition metal-catalyzed cyclization
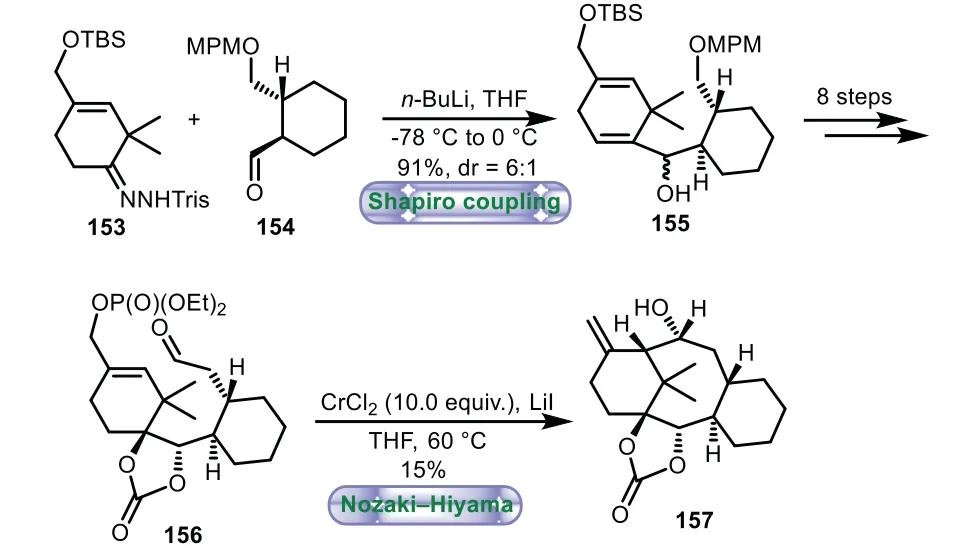
In 2004,Nakada and co-workers described the synthesis of 6−8−6 tricyclic core of Taxol by using Nozaki–Hiyama reaction[104].Trisyl hydrazone 153 was converted to the corresponding alkenyllithium withn-butyllithium,followed by reaction of aldehyde 154 to produce 155 in 91%yield with 6:1dr.Then,an eight-step sequence of oxidation,reduction,Witting reaction,and hydrolysis was carried out,producing the allylic phosphate 156.Pleasingly,the cyclization reaction could be achieved under the condition of excessive CrCl2and LiI in THF,which gave the secure 6−8−6 tricyclic target 157 in only 15%yield(Scheme 28).
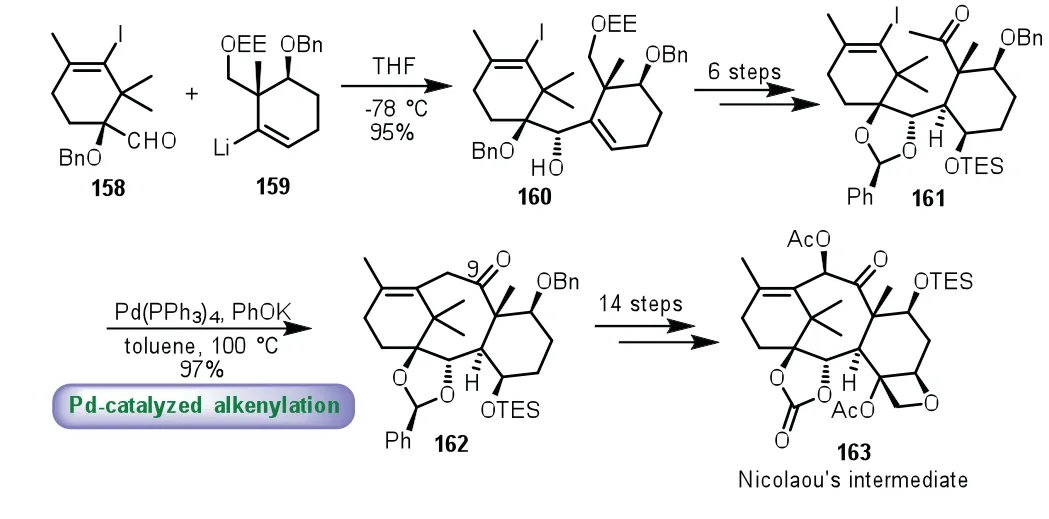
In 2015,the same group described the formal synthesis of Taxolviaa palladium-catalyzed alkenylation of methyl ketone to furnish the eight-membered ring[105,106].Firstly,treatment of the A-ring precursor 158 with alkenyllithium 159 in THF at−78°C afforded 160 as a single isomer in 95%yield.Then the key precursor 161 was obtained through a six-step sequence of transformations.Palladium-catalyzed alkenylation of methyl ketone 161 was smoothly taken place,thus producing the tricyclic compound 162 with the oxygen atom at C9 in 97%yield.Finally,162 transformed to Nicolaou’s intermediate 163viaa fourteen-step sequence to complete the challenging work(Scheme 29).
Scheme 28.Nakada’s approach to the tricyclic ring system through Nozaki–Hiyama reaction(2004).
Scheme 29.Nakada’s approach to the tricyclic ring system through Pd-catalyzed alkenylation(2015).
Scheme 30.Inoue’s approach to the tricyclic ring system through decarbonylative radical coupling and Pd-catalyzed alkenylation(2018).
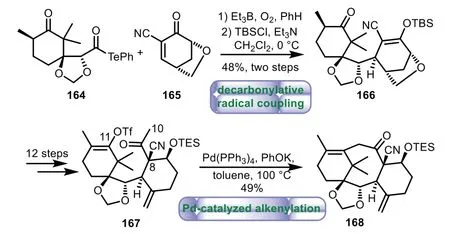
In 2018,Inoue and co-workers applied decarbonylative radical coupling and Pd-catalyzed intramolecular alkenylation to assemble the eight-member ring[107].Treatment of 164 and 165 with Et3B and O2in benzene at room temperature,followed by regioselectively protecting with TBSCl,afforded 166 in 48%yield over two steps.Then C10−C11 seco-taxane 167,bearing the C8-quaternary carbon was achievedviathe linear twelve-step sequence of transformations from 166.Finally,subjecting with Pd(PPh3)4and PhOK at 100°C in toluene,the eight-member ring was cyclized to deliver the tricyclic product 168 in 49%yield(Scheme 30).
4.3.Miscellaneous cyclization
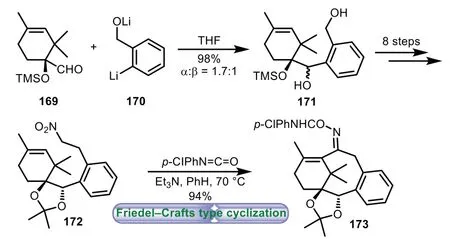
In 1997,Nagaoka and co-workers described the synthesis of 6−8−6 tricyclic core of TaxolviaFriedel−Crafts type cyclization[108].Initially,the coupling of ring A precursor 169 with aryllithium reagent 170 gave alcohol 171 as a mixture.Then the major isomer of 171 was transformed into nitro compound 172 through an eight-step sequence.Treatment of precursor 172 with excesspchlorophenyl isocyanate afforded tricyclic product 173 in 94%yield(Scheme 31).
Scheme 31.Nagaoka’s approach to the tricyclic ring system through Friedel−Crafts type cyclization(1997).
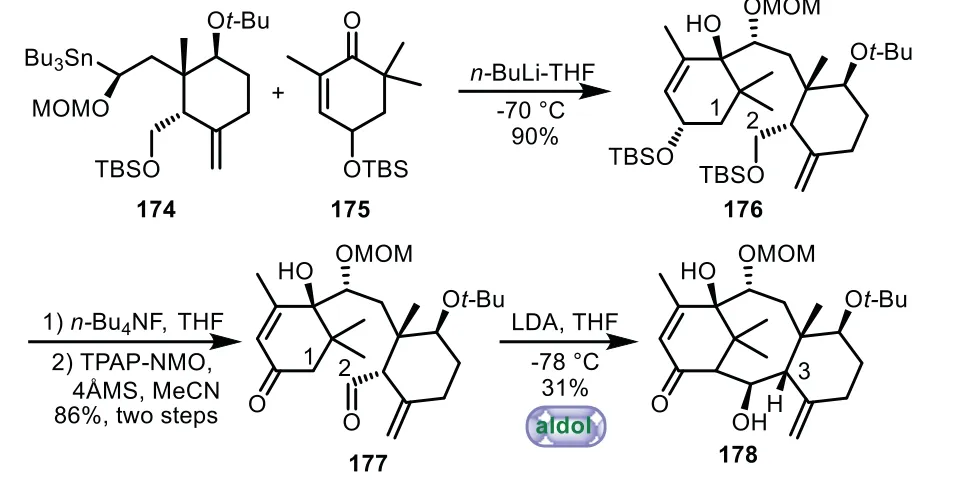
Scheme 32.Arseniyadis’s approach to the tricyclic ring system through intramolecular Aldol reaction(1999).
In 1999,Arseniyadis and co-workers applied intramolecular aldol reaction to assemble of taxoid A/B/C ring system[109–111].The addition of organostannane 174 to A-ring building block 175 generated 176.Then desilylation and oxidation afforded B-secotaxoids 177.Finally,the A/B/C tricycle 178 was accomplished by intramolecular aldol reaction in 31%yield,in which only the C3-αsubstituted precursor could be cyclized smoothly(Scheme 32).
In 2006,Takahashi and co-workers documented their efforts toward the formal synthesis of Taxol by using microwave-assisted alkylation reaction as a novel step[112,113].The Shapiro coupling reaction of A ring hydrazone 179 with aldehyde 180 provided the desired product 181 in 54%yield with 2α/2β=12:1.Then they achieved the ideal intermediate 182viaan eight-step sequence of transformations from 181.Upon treatment of 182 with LiN(TMS)2in refluxing dioxane under microwave irradiation triggered the intramolecular alkylation reaction,thus affording cyclization product 183 in 49%yield.Further transformations were carried out to complete the synthesis of Danishefsky’s intermediate 184(Scheme 33).
In 2007,Chavan and co-workers employed the strategy of Pummerer reaction and oxidative cleavage to the synthesis of 6−8−6 tricyclic core of Taxol[114].The bicyclic system 186 was generated fromβ-oxo sulfoxide 185 through Pummerer reaction,followed by coupling with bromo-2-(bromomethyl)cyclohex-1-ene 187 to obtain 188 in 70%yield.The addition ofs-BuLi to 188 delightfully afforded the tetracyclic compound 189 in 60%yield.Upon treatment of 189 with Pb(OAc)4broke C2−C10 bond,thus affording the A/B/C ring system 190 in 75%yield.Lastly,190 was isomerized to 191 with catalytic amount of rhodium trichloride(Scheme 34).
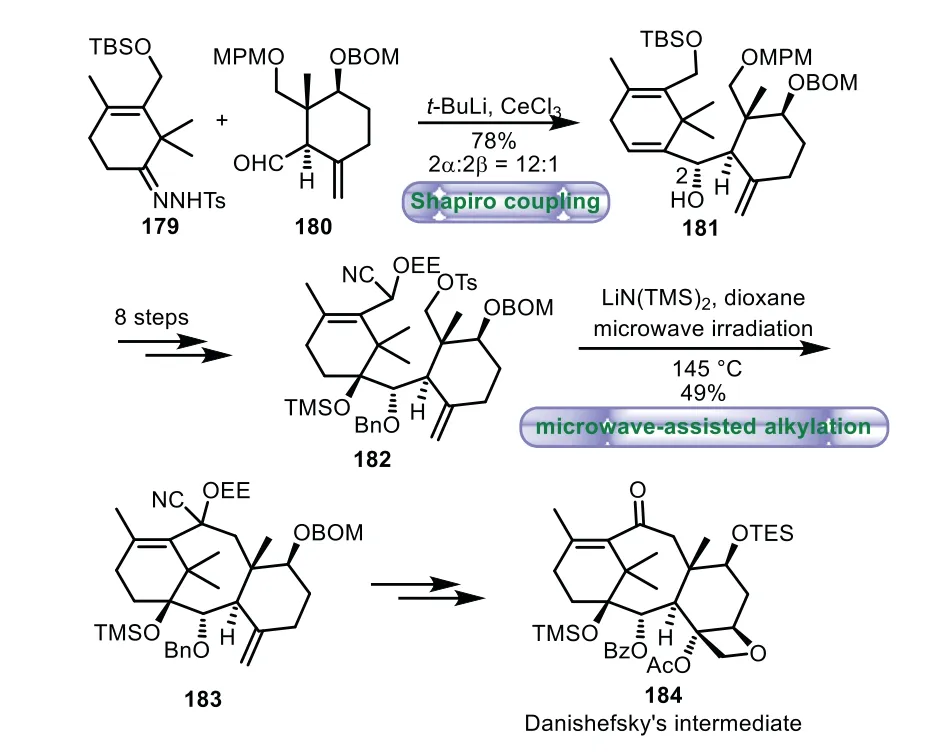
Scheme 33.Takahashi’s approach to the tricyclic ring system through microwaveassisted alkylation(2006).
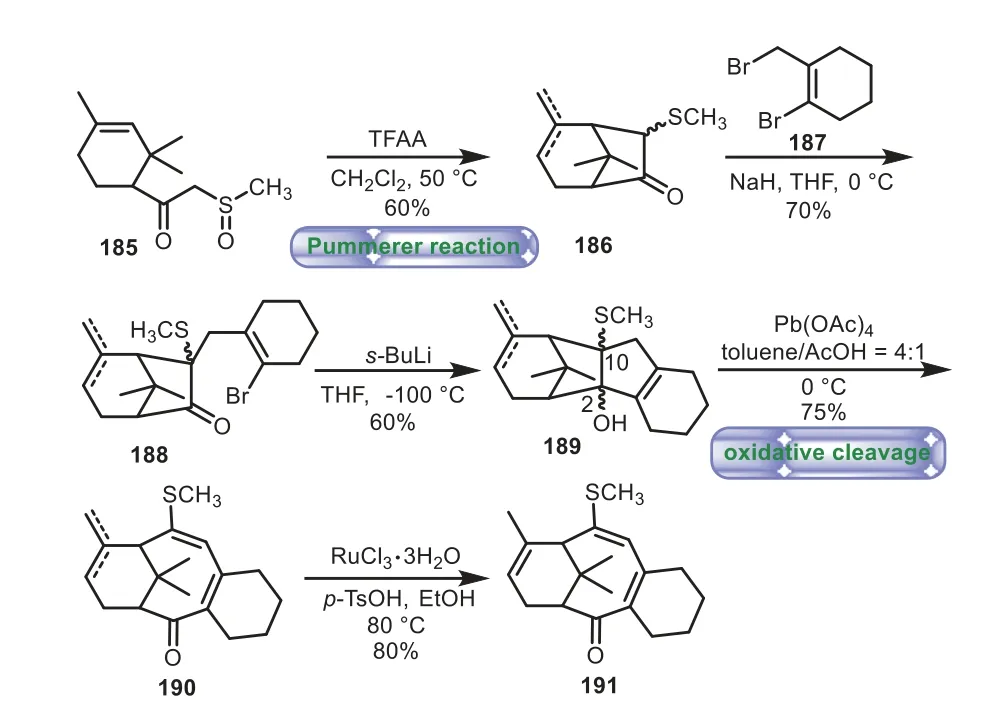
Scheme 34.Chavan’s approach to the tricyclic ring system through sulfur-assisted synthetic protocol(2007).
In 2019,Inoue and co-workers applied decarbonylative radical coupling protocol and pinacol coupling reaction to build tricyclic core[115].Vinyl iodide 192 bearing A ring framework was transformed into telluride 193viaa five-step sequence.Then coupling of 193 with C ring fragment 194 was carried out under the previous decarbonylative radical condition[107],followed by oxidation of the resulting borane enolate by DDQ,thus affording the adduct 195.Then 195 was converted to the key precursor ketoaldehyde 196viaa three-step sequence of methylation,reduction,elimination,reduction of the nitrile,and deprotection.Treatment of 196 with TiCl4,Zn,and pyridine in THF at 50°C,followed by acetylation furnished the tricyclic compound 197 in 45%yield over two steps.Finally,1-hydroxytaxinine 198 was synthesizedviaan eightstep sequence of transformations(Scheme 35).
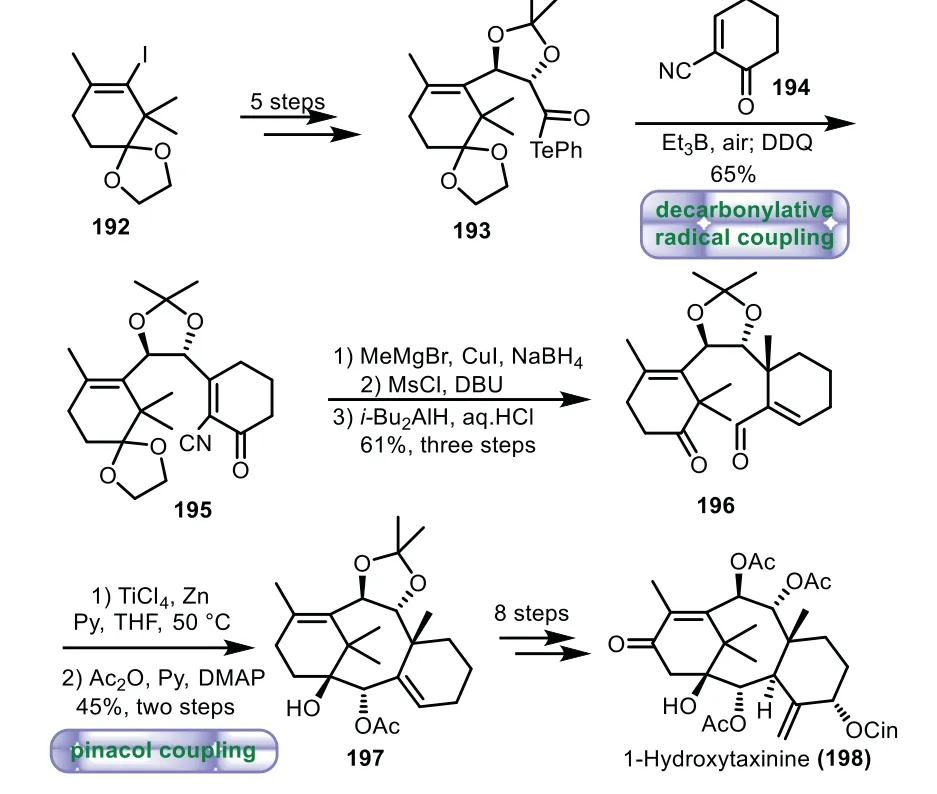
Scheme 35.Inoue’s approach to the tricyclic ring system through decarbonylative radical coupling and pinacol coupling reaction(2019).
5.Summary and outlook
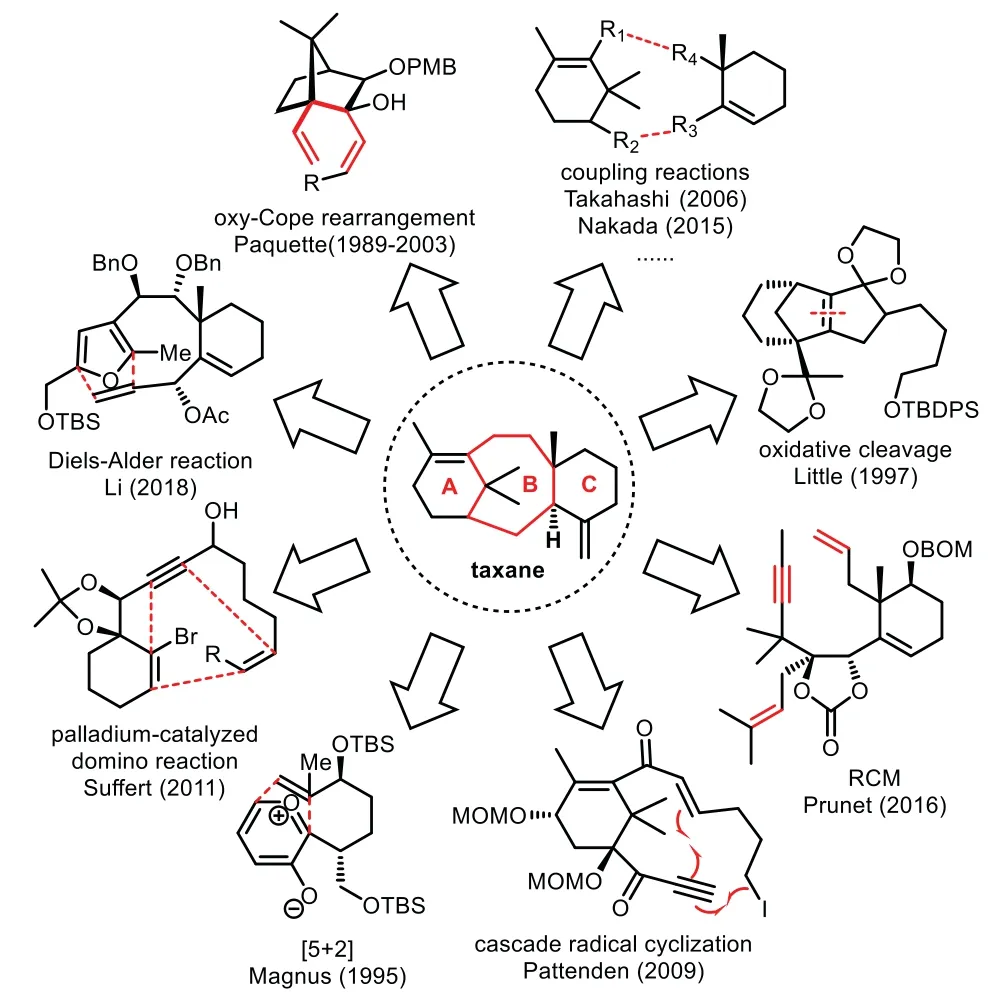
Fig.3.Highlights of diverse approaches en route to the taxane core ring.
Taxol has attracted considerable attention from the synthetic communities due to its intriguing structure and important anticancer bioactivity.Since the pioneering total synthesis of Holton and Nicolaou groups,ten total syntheses of Taxol have been achieved up to the present.Nevertheless,development of efficient synthetic methods to construct the eight-membered Bring remains a challenging task for organic synthesis in general.This mini-review has summarized the synthetic approaches toward eight-membered B-ring formation.Tremendous efforts made by a number of research groups worldwide have resulted in a range of diverse creative and elegant strategic approaches and methodologies.For example,oxy-Cope rearrangement,Diels–Alder reaction,Pd-catalyzed domino reaction,[5+2]-pyrylium ylide-alkene cyclization,cascade radical cyclization,ring-closing metathesis,oxidative cleavage,and diverse coupling reactions(Pdcatalyzed intramolecular alkenylation,decarbonylative radical coupling,acid/base/SmI2-mediated aldol condensation,intramolecular Michael addition of sulfonyl carbanion,Nozaki–Hiyama reaction,and so on)have been successfully employed to construct these eight-membered B-rings(Fig.3).These novel approaches could provide inspiration to synthetic communities.The further possible approach may be the combination of synthetic convergency and biomimetic conciseness,which could address the overall efficiency of total synthesis.Taxol will still stand for an ideal target molecule in total synthesis for many years to come.We believe that novel synthetic strategies and tactics would be well developed further and could be successfully applied to the total synthesis of Taxol in future.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We acknowledge financial support from the National Natural Science Foundation of China(Nos.21901215,21672030),and the Fundamental Research Funds for the Central Universities(No.2682021CG020).
 Chinese Chemical Letters2022年12期
Chinese Chemical Letters2022年12期
- Chinese Chemical Letters的其它文章
- Recent advances in gold-complex and chiral organocatalyst cooperative catalysis for asymmetric alkyne functionalization
- Unmodified methodologies in target discovery for small molecule drugs:A rising star
- Recent advances in single-crystalline two-dimensional polymers:Synthesis,characterization and challenges
- Environmental applications of graphene oxide composite membranes
- Recent advances in the application of metal organic frameworks using in advanced oxidation progresses for pollutants degradation
- Recent progress on two-dimensional materials confining single atoms for CO2 photoreduction
