Universal 4-qualifiable fluorene-based building blocks for potential optoelectronic applications
Xing An,Jingho Yng ,Mn Xu,Lili Sun,Luing Bi ,Ki Wng ,Zhiqing Zhuo ,Yingying Zheng ,Jinyi Lin,*,Xuehu Ding ,Yuyu Liu,Linghi Xie ,Chengrong Yin,Wei Hung ,,c,*
a Key Laboratory of Flexible Electronics(KLOFE)&Institute of Advanced Materials(IAM),Nanjing Tech University(NanjingTech),Nanjing 211816,China
b State Key Laboratory of Organic Electronics and Information Displays&Institute of Advanced Materials(IAM),Nanjing University of Posts&Telecommunications,Nanjing 210023,China
c Frontiers Science Center for Flexible Electronics(FSCFE),MIIT Key Laboratory of Fle xible Electronics(KLoFE),Northwestern Polytechnical University,Xi’a n 710072,China
d Electrical Engineering College,Nanjing Vocational University of Industry Technology,Nanjing 210 023,China
Keywords:Side-chain coupling Small molecular emitter Morphological stability Deep-blue emission Optoelectronic device
ABSTRACT In the design of conjugated molecules,modular production enables materials to easily realize structure modification and precisely tune their photoelectrical property.Construction of a novel and universal building block is crucial to design and manufacture high performance and stable conjugated molecules for optoelectronic application.Herein,we originally demonstrated a universal 4-qualifiable fluorene-based building block,which is a fundamental molecular segment to functionalize and obtain novel conjugated materials.Compared to the traditional modification at 9-site,additional 4-position functionalization provided an exciting blueprint to not only tune electronic structure and excited state via p-n molecular design engineering and space charge-transfer strategy,but also allow for optimizing intermolecular arrangement and obtaining solution-processing ability.The introduction of the 4-site substituent in fluorene based semiconductors may endow materials with unique properties.Finally,we successfully prepared two stable deep-blue light-emitting conjugated polymer,PODOPF and PODOF,by utilizing the 4-substituent fluorene based building block.It is believable that the performance,stability and processibility of reported outstanding fluorene-based conjugated molecules can be further optimized based on this universal building block.
In last decades,conjugated materials are widely applied in organic light-emitting diodes(OLEDs),organic solar cell and organic field-effect transistor(OFET)and bio-chem sensor,associated with their easily structural modification,intrinsic low-cost processing and flexible ability[1–12].Similar to the industrial manufacturing,modular production of conjugated materials enables them to present a precise energy-level tunability,controllable structural modification,and low-cost large-scale preparation,analogous to Lego(Fig.1a).As an effective strategy to precisely tune electronic structures,p(D)-n(A)molecular design principle provide an universal tool to control the intramolecular electron delocalizationviacontrolling the fraction of p-or n-type segment,and bondedπbridge(Fig.1b)[13–18].Compared to directly chemical-bonded engineering,steric hindranceπ-bridge(included rigid and soft type)between two p-or n-type units is an alternative to not only modulate electronic structure and photophysical behavior,but also dominate the intermolecular arrangement and film morphological stability.Therefore,outstanding building block is an effective platform to design high-performance conjugated molecules,including both enhancement of optoelectronic property and device operation stability.In this regard,construction of novel building block is crucial to obtain novel and high-performance conjugated materials for optoelectronic applications.
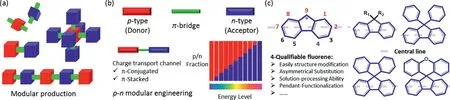
Fig.1.Modular production of conjugated materials.(a)Variable modular synthons for conjugated molecules.(b)Tuning electronic structure of conjugated materials via p-n design principle.(c)Chemical structure of conventional fluorene building blocks.
As one of the important p-type andπ-bridge aromatic unit,fluorene,which has large bang-gap,multi-functional sites and diverse topological structure,is a mostly building block to construct functional materials,including red-green-blue light-emitting,host or guest,charge-transport and interface modified materials,to meet the variable requirement of optoelectronic devices[10,13,14,18-26].Up to date,most fluorene based building blocks are obtainedviaintroducing various functional group at 9-sites,such as 9,9′-spirobifluorene(SBF)[13,26-28],9,9-diphenylfluorene(DBF)[19,22,29,30]and spiro[fluorene-9,9′-xanthene](SFX)(Fig.1c)[23,24,31,32].Generally speaking,2-and 7-sites of fluorene are used as active center to bond with other aromatic units,to tune intramolecular electronic structure and obtain multifunctional photoelectrical property(Fig.1c).For example,n-type units are introduced at 2-and 7-sites to reduce band-gap and improve charge transport ability[21],and heteroatomic aromatic group at 9-and 1-site always controlled the triplet energy levelviacontrolling space charge-transfer for constructing host and thermally activated delayed fluorescence(TADF)materials[18].More interestingly,unique multi-dimensional structure of the chiral sp3functional position at 9-site also provided a platform to control energy level,topological structure and conformation behavior,which are the key factors to improve photoelectrical property and morphological stability for optoelectronic applications.However,compared to widely functionalize at 1-,2-,7-and 9-site of fluorene,there is rare attention paid on designing fluorene-based moleculesviaintroducing the aromatic units at 4-site,attributed to the lack fast and efficient synthesis methods.In fact,beyond substituted at the other positions,exploration of additional 4-functionalization for fluorene-based materials present some advantages as follow:i)New substituted position provided more possible and space to tune electronic structure;ii)Asymmetrical substitute of fluorene at two side(backbone structure is the center line)can easily control hierarchical structure and enhance photophysical property,such as effectively suppress residual green-band emission(Fig.1c);iii)Flexible pendant side-chain at 4-site enable all reported small molecule to obtain solution-processing ability and simultaneous slightly influenced on the electronic structures.Herein,we demonstrated a novel 4-qualifiable fluorene-based building block,which is a fundamental synthon to obtain all fluorene-based conjugated materials for optoelectronic devices.
Up to date,most of fluorene-based materials are obtained from original products,such as fluorene(obtained from coke tar)or fluorenone building blocks.The common synthetic route of 9-substituted fluorene has been shown in Schemes 1a-c.These general methods can effectively extend the number of substituents at 9-site and endow polymers with unique properties,such as theβ-conformation of PFO[33–35].However,only the substituents at 9-site exposes the other side that may create an intermolecular electronic coupling in the aggregation state.The formation of the residual aggregation defects is harmful to the emission spectral stability and property of deep-blue light-emitting films and devices.Fortunately,the modifiable 4-substituted fluorene was broadened by Baeyer-Villeger oxidative rearrangement(Scheme 2).The synergistic effect of 4-and 9-substituents may endow materials unique photophysical properties.A typical polyfluorene,PODPF,with an octyloxy substituent at 4-site,has been explored and proved to have a uniqueβ-conformation and good stability[36–38].The further investigation also suggested that 4-substituted fluorene is of great significance to the basic research and development of optoelectronics and flexible electronics[22,29,30,39,40].Nevertheless,after extensive experiments we also found that this synthetic route has its intrinsic defects that the type of 9-site substituents is extremely limited.Therefore,it is of great importance for developing optoelectronics and flexible electronics to explore a new synthetic route to construct a building block that can keep the number of 9-site substituents while introducing the modifiable substituents at 4-site.
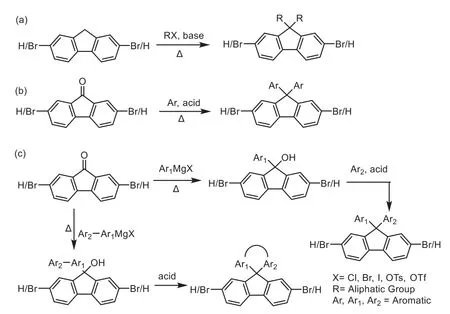
Scheme 1.The known common synthetic route of 9-substituted fluorene.
In the previous synthetic route(Scheme 2),the limited variety of Grignard’s reagents may be the major obstacle to obtain the number of 9-site substituents while introducing the modifiable substitution to 4-site.As listed in Scheme 2,several monomers with different substitutions at 4-and 9-site were prepared by the traditional synthetic route.Although organolithium reagents can expand the selectivity of the 9-site substituents,strong electron donating of 9-site units may lead to a dehydration product.The target product 2 cannot be obtained by the previous synthetic route.As presented in Scheme 3a,product 2e’(88%yield)was gained by adding(4-methoxyphenyl)magnesium bromide to compound 1 at 85oC.For compound 2,the strong electron-donated substituents on the tertiary carbon may increase the leaving ability of the tertiary hydroxyl group,thus the dehydration products are formed.To verify our assumption,2a was put in an acidic condition to leave the tertiary hydroxyl group.As displayed in Scheme 3b,2a was solved by dichloromethane and stirred with an aqueous hydrochloric acid solution with pH 3,and 2a was completely reacted to form 2a’after 1 h(characterized by1H NMR,Fig.S12 in Supporting information).This result suggested that while the leaving ability of the tertiary hydroxyl group is enhanced,it is easy to dehydrate with the hydroxyl group at 2-site and obtain the dehydration product.For the double 9-substituted of fluorene,the dehydration reaction caused by strong electron donating groups is the greatest difficulty in the previous synthetic route.Beyond that,asymmetric substitutions at 9-site are also difficult to achieveviathe previous synthetic route(Scheme 2).Therefore,a new synthetic method to obtain a novel 4-aualifiable building block is necessary to explore for constructing a series of fluorene-based conjugated molecules.
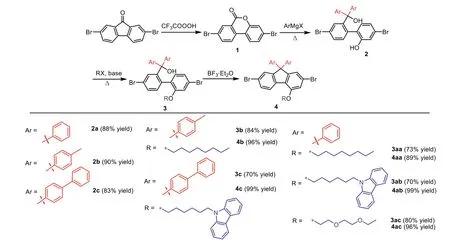
Scheme 2.Scope of the previous synthetic route of 4-substituted fluorene by traditional Baeyer-Villeger oxidative rearrangement.
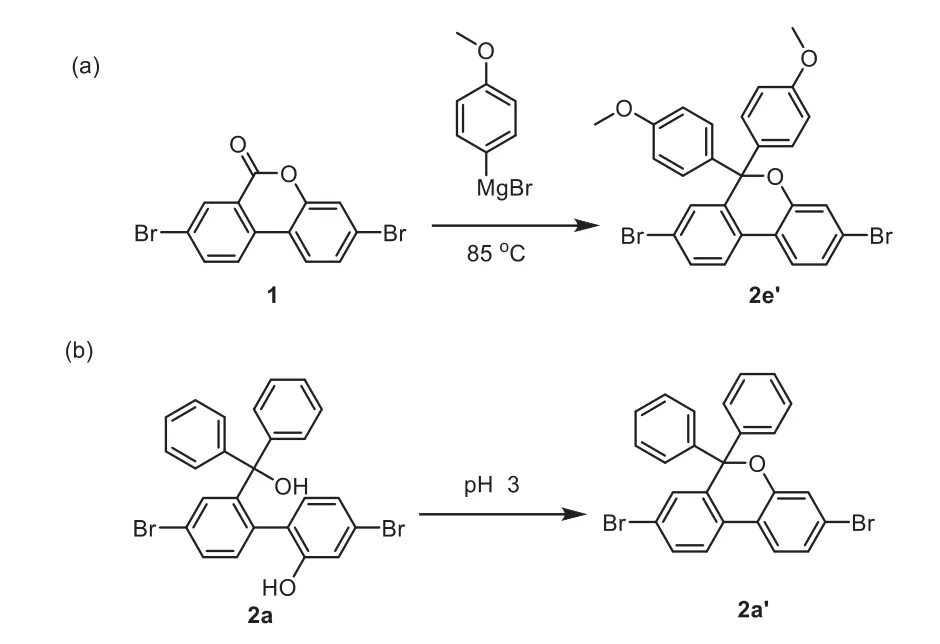
Scheme 3.(a)The dehydration product 2e’obtained via the previous synthetic route.(b)The dehydration product 2a’obtained from 2a in acidic condition.
Subsequently,novel 4-modifiable fluorene building blocks are developed by new preparative route,as shown in Scheme 4.This synthetic route contains three main steps,Baeyer-Villeger oxidative rearrangement,reduction ring-open reaction and oxidation cyclization reaction.A potential 4-site can be generated by the Baeyer-Villeger oxidative rearrangement.The reduction ring-open reaction is positive to release the 4-site for subsequent modification,and the oxidation cyclization reaction will finally form the building block with 4-site substituents.In the ring-open reaction(Scheme 4),1(1.06 g,3 mmol)was solved in THF(15 mL)in nitrogen,then 6 mL THF solution of LiAlH4(1 mol/L,2 equiv.)was added into the mixture.After stirred for 30 min,the mixture was quenched by 100 mL hydrochloric acid aqueous solution(0.25 mol/L)to give 5 in 98%yield.In this reaction,long reaction time under a high temperature may result into the formation of excessive byproducts,that the bromine atoms may be reduced to hydrogen.In the subsequent 4-site substitution reaction,the yield of 6a-6c was 86%∼92%.Besides,we also explored two kinds of oxidation cyclization,one-step[41]and two-step oxidation cyclization[42].As shown in Scheme 5,in one-step cyclization reaction,3 equiv.ofn-BuOOH and 0.1 equiv.of TBAI were stirred with 6 in DCE at 100oC.However,the yield of 8 was only 20%∼25%.In the twostep cyclization,first 0.1 equiv.of TEMPO and 1.2 equiv.of NaOCl were used to oxidize 6 to 7.The yield of 7 was more than 90%.In the subsequent cyclization reaction,0.1 equiv.of TBAB and 1.2 equiv.of K2S2O8were stirred with 7,providing 8 in 54%∼62%yield.The total yield of 8 from two-step oxidation and cyclization reaction was 51%∼56%,which is more than twice the yield of onestep oxidation cyclization.Therefore,the building block with a 4-site substituent was successfully prepared by oxidation-reductionoxidation method,and the total yield can be 38%∼46%from 2,7-dibromo-9H-fluoren-9-one to the building block with 4-site substituent.
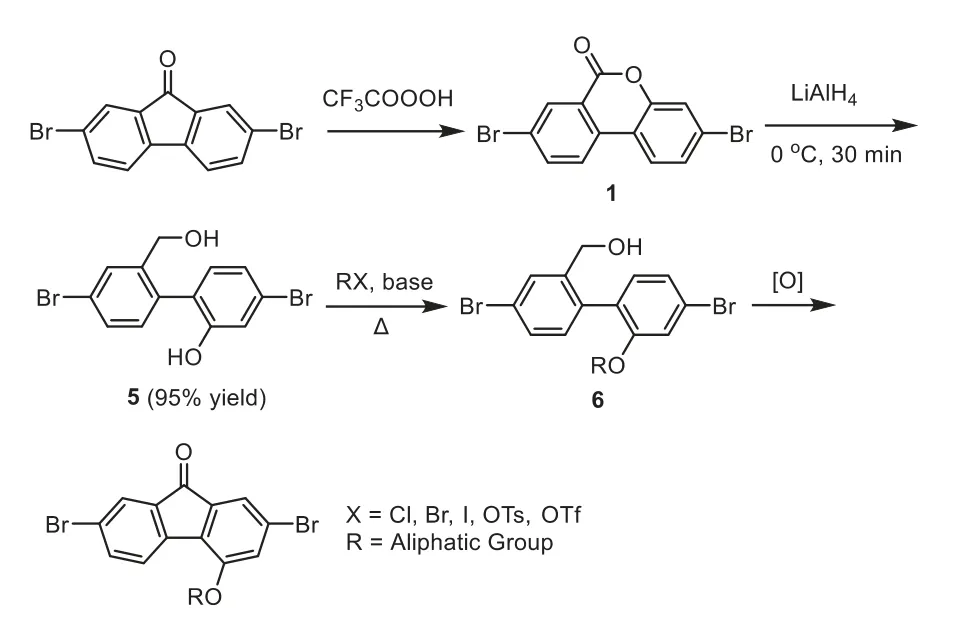
Scheme 4.The novel synthetic method for 4-substituted fluorene-based building block.
After obtaining the building block,4 and 9-substituted fluorenes can be preparedviathe common synthetic route in Scheme 1.Finally,two deep-blue light-emitting polymers,PODOPF and PODOF,were constructed by using 8a.As shown in Scheme 6a,9 was firstly prepared by solving 8a and phenol(10 equiv.)in chloroform(CHCl3)and reacting at 55oC for 24 h after adding 5 mL methanesulfonic acid.The yield of 9 can achieve 98%.The monomer of PODOPF was obtainedviathe nucleophilic substitution in EtOH,giving 10 in 91%yield.PODOPF was finally obtainedviaYamamoto coupling with a yield of 71%.To prepare PODOF,11 was first reduced from 8a by Et3SiH(6 equiv.)and BF3•Et2O(2 equiv.),as presented in Scheme 6b.The yield of 11 was 95%.Then the nucleophilic substitution reaction was initiated by the 9-site carbon of 11 in THF with 1-bromooctane,providing 12 in 88%yield.PODOF was also obtainedviaYamamoto coupling and had a yield of 73%.As displayed in Fig.S46(Supporting information),both the two polymers have a high decomposition temperature(Td)that more than 400oC,and the glass-transition temperature(Tg)of PODOF and PODOPF was at about 50oC and 150oC,respectively.
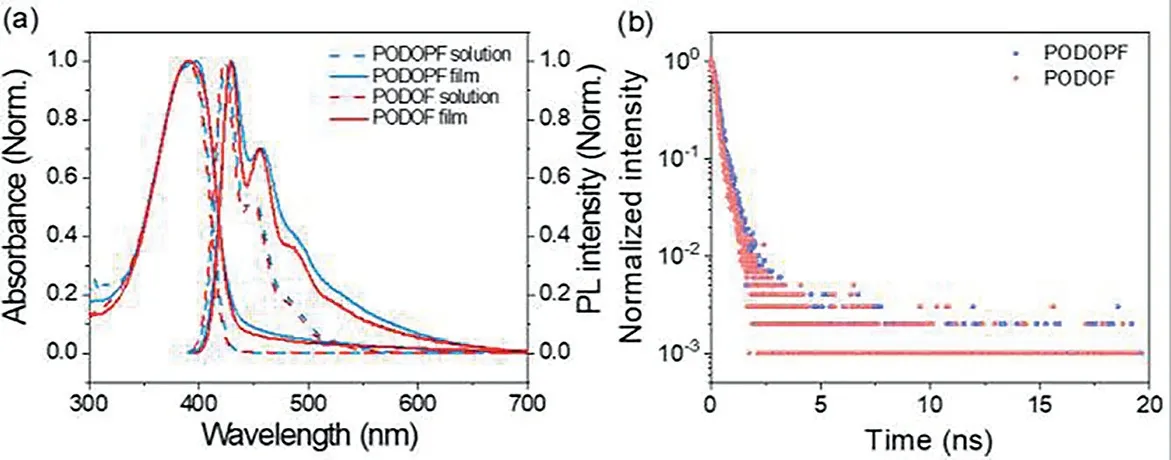
Fig.2.(a)The absorption and PL spectra of PODOPF and PODOF film and diluted toluene solution(10-3 g/L).(b)The fluorescent lifetime of PODOPF and PODOF.
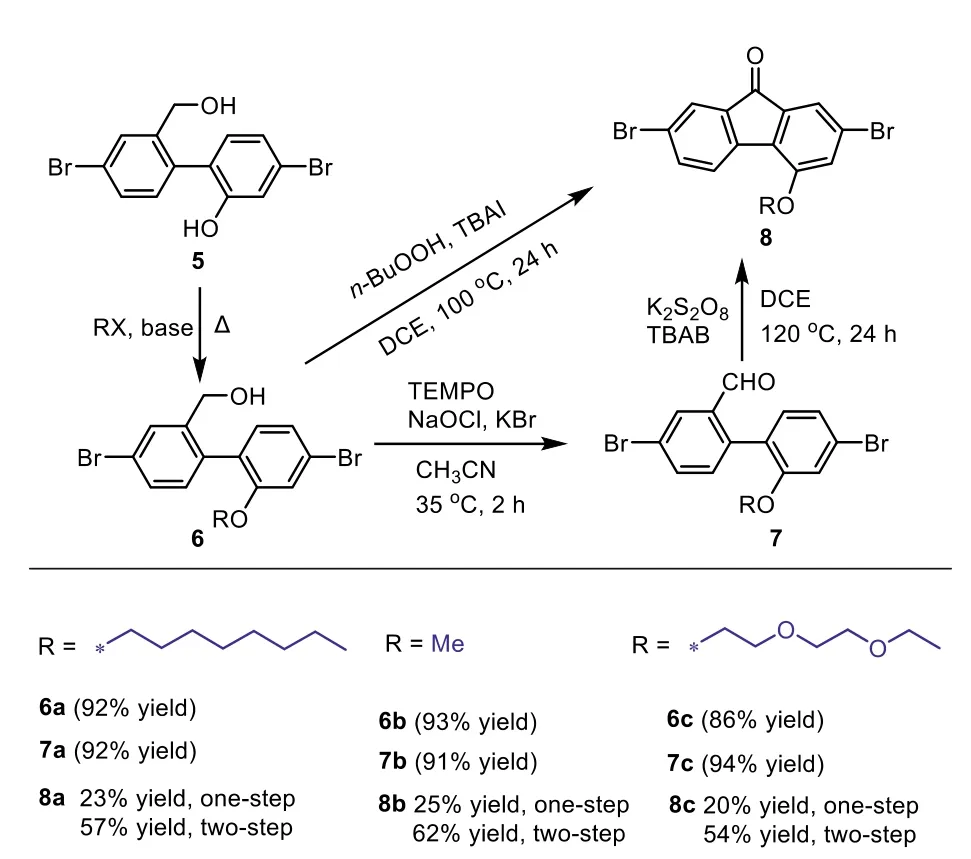
Scheme 5.Scope of the new synthetic method.
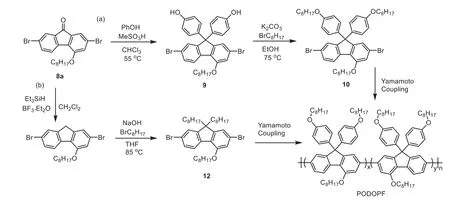
Scheme 6.The synthetic route of(a)PODOPF and(b)PODOF.
The optical property of PODOPF and PODOF was shown in Fig.2.Absorption(Abs)and photoluminescence(PL)spectra of PODOPF and PODOF toluene solution were similar,owing to the same backbone structure.The Abs peak of PODOPF and PODOF in diluted toluene solution was both estimated at 391 nm.Corresponding PL spectra of PODOPF and PODOF solution both consisted of three emission peaks at 423 nm,450 nm and 484 nm,associated to the 0–0,0–1 and 0–2 vibronic transitions of a single polymer chain.Besides,the three vibronic transition emission peaks of PODOPF film were estimated at 430 nm,457 nm and 492 nm,respectively.The corresponding emission peaks of PODOF film were at 429 nm,456 nm and 487 nm,respectively.The Abs peak of PODOF was still at 391 nm,but PODOPF present a maximum Abs peak at 397 nm together with a shoulder peak at 386 nm,associated to the 0-0 and 0-1 vibronic transitions.As shown in Fig.2b,the fluorescent lifetime of PODOPF and PODPF was 319 ps and 289 ps,respectively.As listed in Table S1(Supporting information),the PLQY of PODOPF and PODOF spin-coated film was 36.8%and 26.2%,respectively.According to the fluorescent lifetime and PLQY,we calculated their radiative transition rate(kr)and non-radiative transition rate(knr).As listed in Table S1,thekrandknrof PODOPF was 1.15×109s-1and 1.98×109s-1,respectively.By contrast,PODOF has a lowerkr(9.07×108s-1)and higherknr(2.55×109s-1),which revealed the reason for the lower PLQY of PODOF.
Finally,we prepared the polymer light-emitting device(PLED)by using PODOPF and PODOF as the emitter layer(EML).We first measured the energy levelsviacyclic voltammetry(CV).As shown in Fig.S47(Supporting information),the HOMO of PODOPF and PODOF was calculated as−5.762 eV and−5.713 eV,respectively,and the corresponding LUMO was about−2.007 eV and−1.938 eV,respectively.According to the HOMO and LUMO of the EMLs,devices with following structure were fabricated:ITO(50 nm)/PEDOT:PSS(30 nm)/EML(40 nm)/TPBi(25 nm)/LiF(1 nm)/Al(100 nm).The electroluminescence(EL)spectra of PODOPF and PODOF were shown Figs.3a and c.Both the two devices exhibited a deep-blue emission and had a tune-on voltage of 4.5 V.As shown in Figs.3b and d,the maximum luminance of PODOPF and PODOF was 753 cd/m2and 773 cd/m2.The external quantum efficiency(EQE),current efficiency(CE)and power efficiency(PE)curvesversuscurrent density of the two devices were shown in Fig.S48(Supporting information).For PODOPF device,the maximum EQE was 0.53%as the current density reaches 14.5 mA/cm2,associated with the CEmax=0.63 cd/A and PEmax=0.40 lm/W.For PODOF device,the maximum EQE was 0.62%,CEmax=0.57 cd/A and PEmax=0.35 lm/W.The CIE 1931 chromaticity coordinate of PODOPF and PODOF device was(0.17,0.11)and(0.16,0.09),respectively.The PLED results showed that polyfluorenes with 4-site modifiable substituent had exploring potential in optoelectronics.
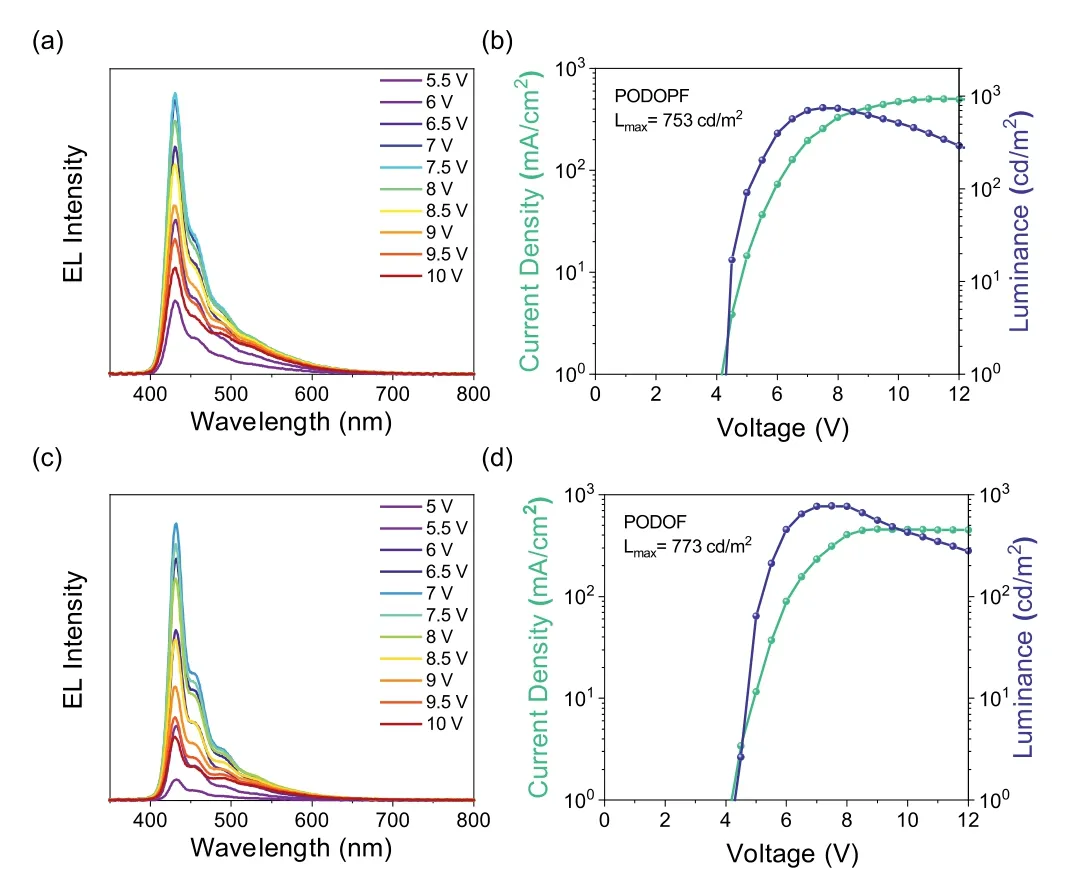
Fig.3.(a)EL spectrum of PODOPF.(b)Current density and luminance versus driving voltage characteristics of PODOPF.(c)EL spectrum of PODOF.(d)Current density and luminance versus driving voltage characteristics of PODOF.
In summary,we demonstrated a universal synthetic method to construct 4-qualifiable fluorene-based building blocks for optoelectronic applications.Compared to previous units,variable substitutes at 4-site enable the reported fluorene-based materials to obtain easily structural modification and solution-processing ability but slightly influenced on the electronic structures.The new synthetic route contains three main steps with a high yield consisted of Baeyer-Villeger oxidative rearrangement,ring-open reaction(reduction)and cyclization reaction(oxidation).The total yield of the building blockviathe new synthetic method can be∼50%.Finally,two efficient blue-light-emitting polymers,PODOPF and PODOF,are prepared by utilizing the building block 8a,and the PLEDs of the two polymers were successfully fabricated.This result also implied the significance and effectiveness of the fluorene based building block with 4-site modifiable substituent for optoelectronic application.
Declaration of competing interest
There is no conflict of interest of this work
Acknowledgments
The work was supported by the Natural Science Foundation of Jiangsu Province(No.BK20200700),National Natural Science Foundation of China(Nos.22075136,61874053),Natural Science Funds of the Education Committee of Jiangsu Province(No.18KJA430009),“High-Level Talents in Six Industries”of Jiangsu Province(No.XYDXX-019),Postgraduate Research&Practice Innovation Program of Jiangsu Province(Nos.KYCX21_1097,KYCX21_0771),Nanjing Vocational University of Industry Technology Start-up Fund(No.YK21-02-07),the open research fund from Anhui Province Key Laboratory of Environment-friendly Polymer Materials and Anhui Province Key Laboratory of Optoelectronic Materials Science and Technology.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.03.102.
 Chinese Chemical Letters2022年12期
Chinese Chemical Letters2022年12期
- Chinese Chemical Letters的其它文章
- Diverse strategic approaches en route to Taxol total synthesis
- Recent advances in gold-complex and chiral organocatalyst cooperative catalysis for asymmetric alkyne functionalization
- Unmodified methodologies in target discovery for small molecule drugs:A rising star
- Recent advances in single-crystalline two-dimensional polymers:Synthesis,characterization and challenges
- Environmental applications of graphene oxide composite membranes
- Recent advances in the application of metal organic frameworks using in advanced oxidation progresses for pollutants degradation
