Photodimerization of azaanthracene derivatives mediated by cucurbit[10]uril
Huxing Li ,Xinchen Hu,Fengbo Liu,Dongdong Sun,Yong Wu,Simin Liu,b,*
a School of Chemistry and Chemical Engineering,Wuhan University of Science and Technology,Wuhan 430081,C hina
b The State Key Laboratory of Refractories and Metallurgy,Institute of Advanced Materials and Nanotechnology,Wuhan University of Science and Technology,Wuhan 430081,China
Keywords:Supramolecular nanoreactor Cucurbit[10]uril Azaanthracene Photodimerization Host-guest interaction
ABSTRACT Challenges of achieving efficient photodimerization of azaanthracene derivatives remain due to the low selectivity and slow reaction rate.In this paper,cucurbit[10]uril(CB[10]),with the largest rigid and hydrophobic cavity among CB[n]s,was used to affect the photodimerization reaction of four water-soluble 1-(2-)substituted azaanthracene derivatives(1-4).It revealed that 1-4 could form 1:2 host-guest complexes with CB[10]in aqueous solution.Irradiation of 1 in the presence of 0.5 equiv.of CB[10]selectively produced a head-to-tail(anti-HT)photodimer product.As for 2-4,CB[10]acted as a nanoreactor accelerating their photodimerization reaction in water.Our results suggest that photodimerization of azaanthracene derivatives could be promoted by the CB[10]-based host-guest strategy with high efficiency and selectivity.
Featured with strong rigidity and fantastic chirality of products,the reversible dimerization of(aza)anthracene derivatives had been an intriguing building block for various functional material[1,2].Strategies have been proposed to realize photodimerization of(aza)anthracene derivatives,including solid-phase reaction,cation-πpromoted reaction and catalysis by macrocyclic hosts.By pre-orientating substrates in cavity of hosts,supramolecular catalysis by artificial macrocycles has been one of efficient strategies to promote reactions with high yield and selectivity[3–14].For example,Yang and coworkers improved the selectivity of photodimerization of anthracene formic acid by preorientating the substrate dimer in the cavity of cyclodextrins and pillar[n]arenes[15–19].Cucurbit[n]urils(CB[n]s,n=5-8,10),with hydrophobic cavities and electron-rich carbonyl rims[20–22],have been attractive supramolecular nanoreactors/catalysts for reactions in aqueous solutions[23–28].Intriguingly,with cavities large enough to simultaneously accommodate two anthracene derivatives,CB[8]and CB[10]have been used to efficiently catalyze photodimerization of anthracene derivatives.For example,CB[8]has been used by Inoue and coworkers to catalyze inter-and intramolecular photodimerization of anthracene pendant anchored onα-cyclodextrin[15].Scherman and coworkers demonstrated polymer ligation and network formation based on CB[8]-catalyzed dimerization of anthracene pendant on poly(ethylene glycol)(PEG)and hydroxyethyl cellulose(HEC)[29].Our group also found that photodimerization of 9-substituted anthracene derivatives could be catalyzed in the presence of CB[10]with high efficiency and selectivity[30].Compared to photodimerization of anthracene dimer,there has been few reports on photodimerization of azaanthracene derivatives,which was mainly based on cation-πinteractions in the solid phase[31–36].However,due to the low selectivity and slow reaction rate,challenges of achieving efficient photodimerization of azaanthracene derivatives remain.
According to our previous research on the CB[10]-catalyzed photodimerization of anthracene derivatives[30],in this paper,we proposed an efficient strategy to promote photodimerization of four azaanthracene derivatives(1-4 in Scheme 1)by their preorganization in the cavity of CB[10].Experiments by1H NMR,UV-vis,fluorescence spectra and ESI-MS analysis revealed that all the azaanthracene derivatives formed 1:2 host-guest complexes(CB[10]·G2)with CB[10]in aqueous solution.It was also found that the photochemical reactivity of the azaanthracene derivatives could be modulated by complexation with CB[10].Specifically,upon irradiation by UV light,photodimerization of CB[10]·12produced a head-to-tail(anti-HT)photodimer with high yield and purity;while the photodimerization of 2-4 with faster reaction rates were observed in the presence of CB[10].
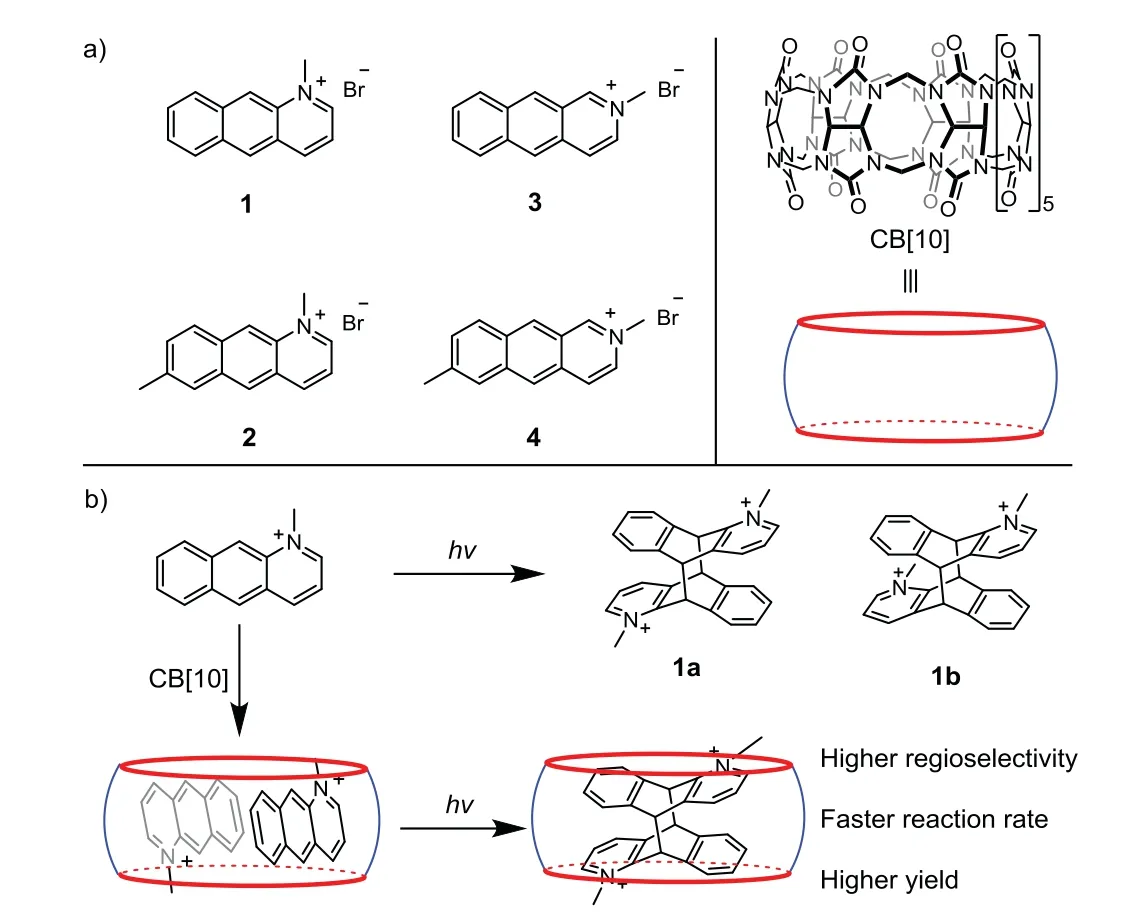
Scheme 1.(a)Structures of guests 1-4 and host CB[10];(b)schematic photodimerization of 1 in the absence and presence of CB[10].
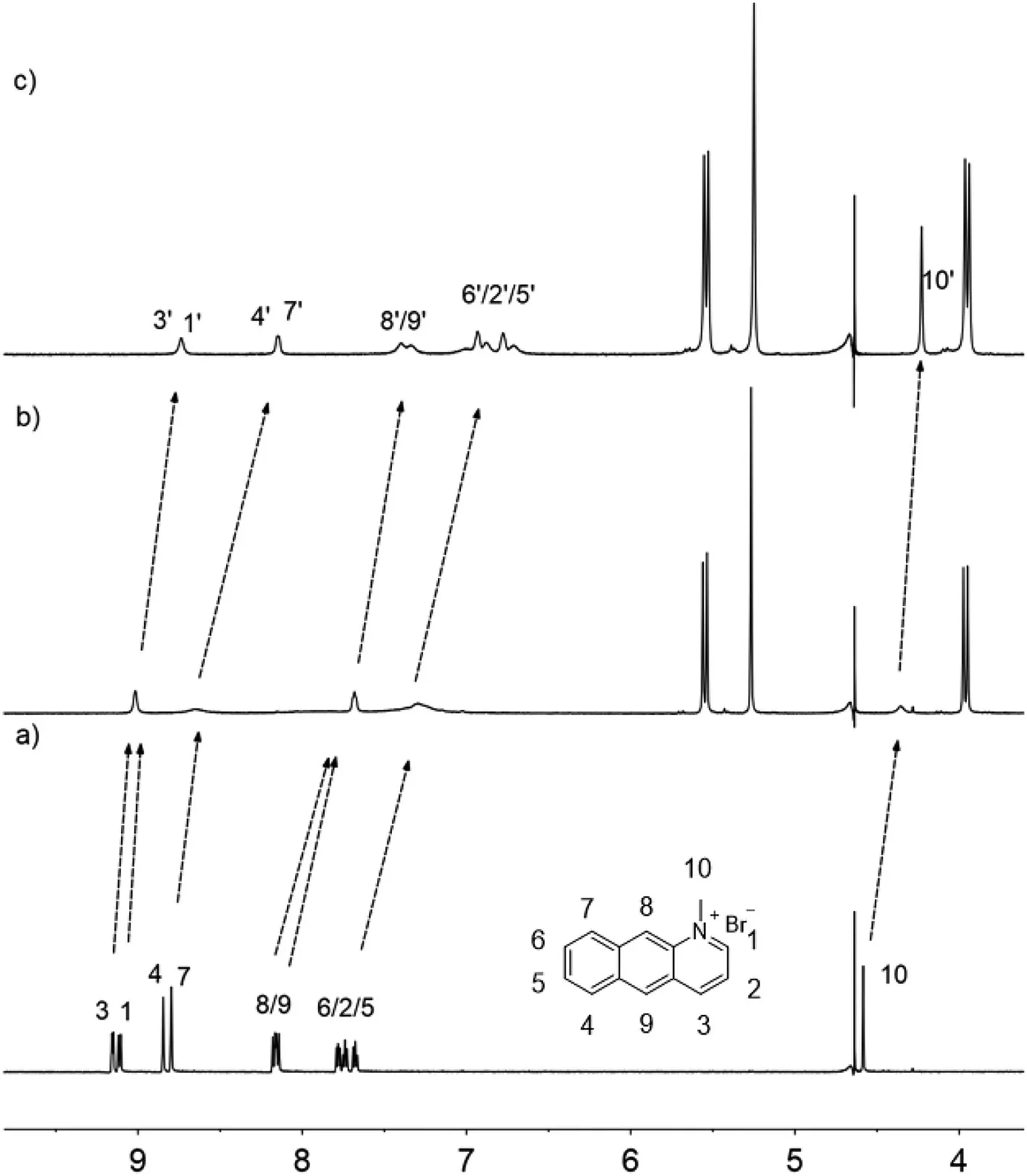
Fig.1.1H NMR(600 MHz,D2O,298 K)of 2.0 mmol/L 1 in the presence of 0(a),0.25 equiv.(b),and 0.5 equiv.of CB[10](c).
The syntheses four water-soluble azaanthracene derivatives(1-4)with different nitrogen positions and substituents were performed according to reported methods(Scheme S1 in Supporting information).The guest molecules were characterized by1H NMR(Figs.S1–S4 in Supporting information),13C NMR(Figs.S5–S8 in Supporting information)and ESI-MS analyses(Figs.S9–S12 in Supporting information).
As shown in Fig.1,complexation between CB[10]and 1 exhibited fast exchange kinetics on1H NMR time scale.Upon addition of CB[10],the signals of all the chemical shifts of 1 were upfield shifted,indicating that the whole 1 was encapsulated in the cavity of CB[10].UV-vis and fluorescence spectra(Fig.S13 in Supporting information)were also recorded to unravel the hostguest interaction between CB[10]and 1.And there were two intense absorption peaks at 270 nm and 360 nm and a broad absorption peak at 420 nm.All the absorption intensity decreased upon increasing equivalents of CB[10],indicating the enhancedππstacking of 1 in cavity of CB[10].Correspondingly,the fluorescence emission intensity of 1 was weakened upon complexation with CB[10].Electrospray mass spectrometry(ESI-MS)test further evidenced the formation of CB[10]·12complex in aqueous solution withm/z1024.8427([CB[10]·12]2+)(Fig.S14 in Supporting information).
2-4 exhibited similar host-guest recognition behavior with CB[10].Specifically,all chemical shift signals for 2 and 3 underwent upfield shift upon addition of CB[10](Figs.S15 and S17 in Supporting information),indicating the encapsulation of azaanthracene core in cavity of CB[10].Broadened chemical shift signals were observed for 4 in the presence of CB[10]and the downfield shift of singlet signal H9for the pyridinium methyl group suggested that protons H9were positioned near the carbonyl rims of CB[10](Fig.S19 in Supporting information).All of 2-4 exhibited fast exchange kinetics on1H NMR time scale.The complexation between 2-4 and CB[10]also induced decrease in both UV-vis absorption and fluorescence intensity.The results above supported the enhancedπ-πstacking of the azaanthracene derivatives in cavity of CB[10].
With the 1:2 host-guest recognition between CB[10]and 1 in mind,the photodimerization of 1 in the absence and presence of CB[10]were investigated.All samples in D2O or water were degassed with nitrogen for 10 min before irradiation under UV light from a 500 W high-pressure mercury lamp at room temperature.Irradiation solution of 1(2.0 mmol/L)decrease the intensity of chemical shift signals for unreacted 1 along with the formation of photodimerization products(Fig.S21 in Supporting information).A set of symmetrical doublets could be observed at 5.5 ppm and 5.8 ppm,respectively,which were the characteristic proton signals for the bridge junctions of symmetrical double-bridged photodimer products of azaanthracene derivatives[31].There was also another distinguishable set of symmetrical double peaks at 5.4 ppm and 5.6 ppm,respectively,which suggested that the photodimerization of 1 producedanti-HT(1a)andanti-HH(1b)photodimers due to the cation-πand electrostatic repulsion interactions between pyridinium and aromatic rings.Beside,two distinct singlets for methyl group at 4.43 ppm and 4.48 ppm also indicated the limited reaction selectivity for photodimerization of free 1.The uncompleted conversion of 1 after irradiation even for 180 min suggested the slow reaction rate for the photodimerization of 1(Fig.2b),and two photodimer products 1a and 1b with an integral ratio of∼6:1 in1H NMR spectrum(Table 1,Fig.S21).
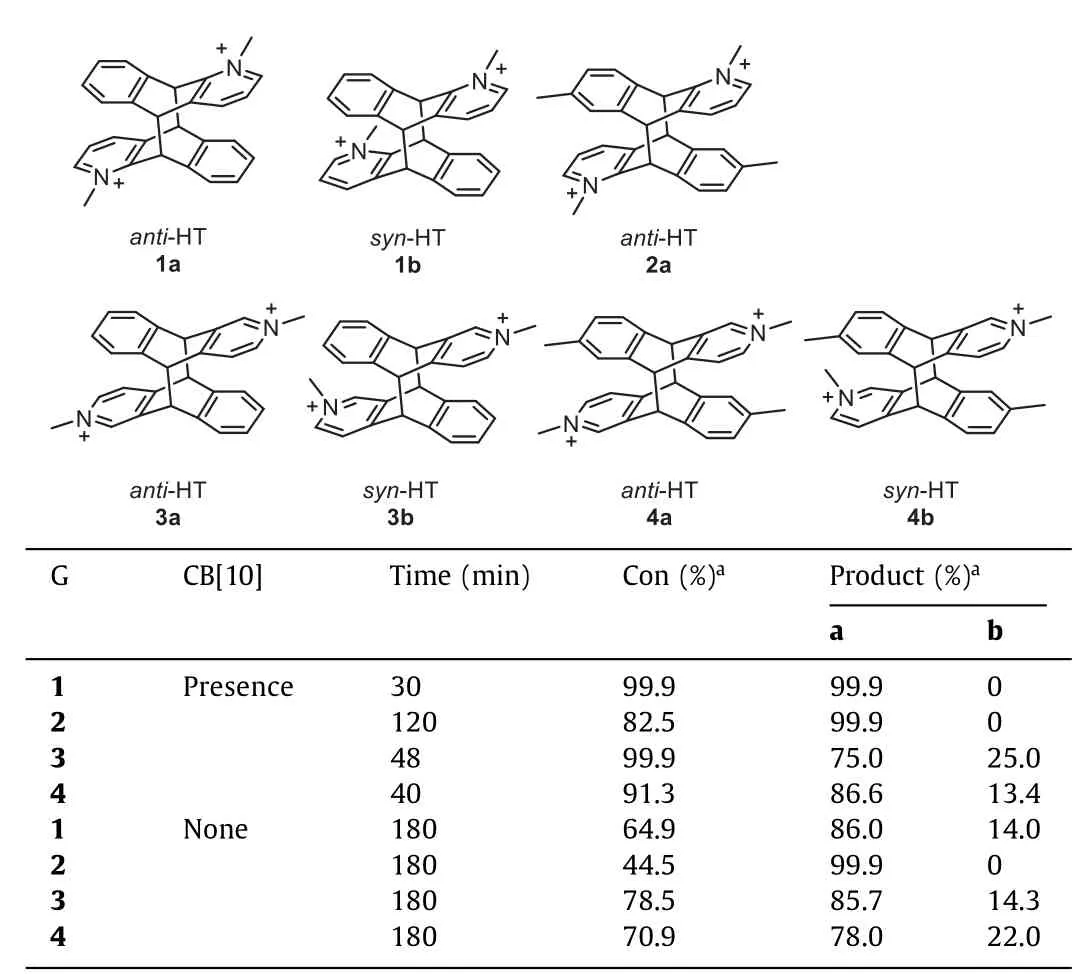
Table 1 Photodimerization of guests with/without CB[10]in D2O at 298 K.
The photodimerization of the CB[10]·12complex was tracked by1H NMR under the same conditions(Fig.3).In the presence of 0.5 equiv.of CB[10],white precipitate was observed and the proton signals of CB[10]host kept decreasing when the solution was irradiated with UV light.The bound peaks of 1 fully disappeared after 35 min and only one set of CB[10]host and bound product peaks remained(Fig.3h).The appropriate amount of 3,5-dimethylamantadine hydrochloride(3,5-DMADA)as a competitor was then added to the suspension to displace the photodimer out of the cavity of CB[10](CB[10]·(3,5-DMADA)2is extremely insoluble in water)[21].The photodimer was characterized as pureanti-HT 1a by1H NMR(Fig.3i)and13C NMR(Figs.S22 and S23 in Supporting information).The photodimerization process was also monitored by UV-vis and fluorescence spectra (Fig.4),which demonstrated that the reaction completed in 35 min.1H NMR,13C NMR and ESI-MS spectra were recorded to further determine the structures of the photodimer product.In addition,ESI-MS(Fig.S24 in Supporting information)also revealed single peak of the photodimer atm/z194.0965([1a]2+).
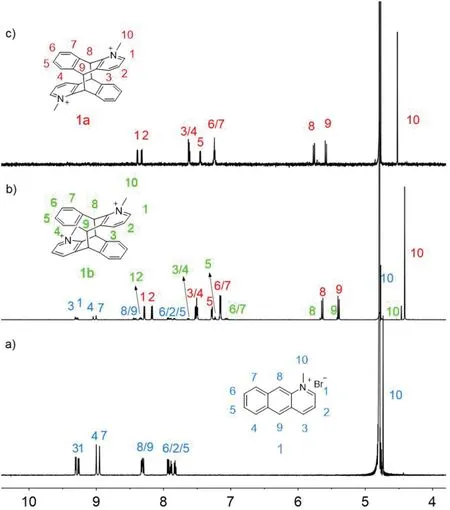
Fig.2.1 H NMR spectra(600 MHz,D2O,298 K)of(a)1;(b)photoreaction product of 1 after UV light irradiation for 180 min;(c)photoreaction product of the CB[10]·12 complex after UV light irradiation for 35 min(CB[10]was precipitated upon addition of excess 3,5-DMADA as competing guest after complete conversion).
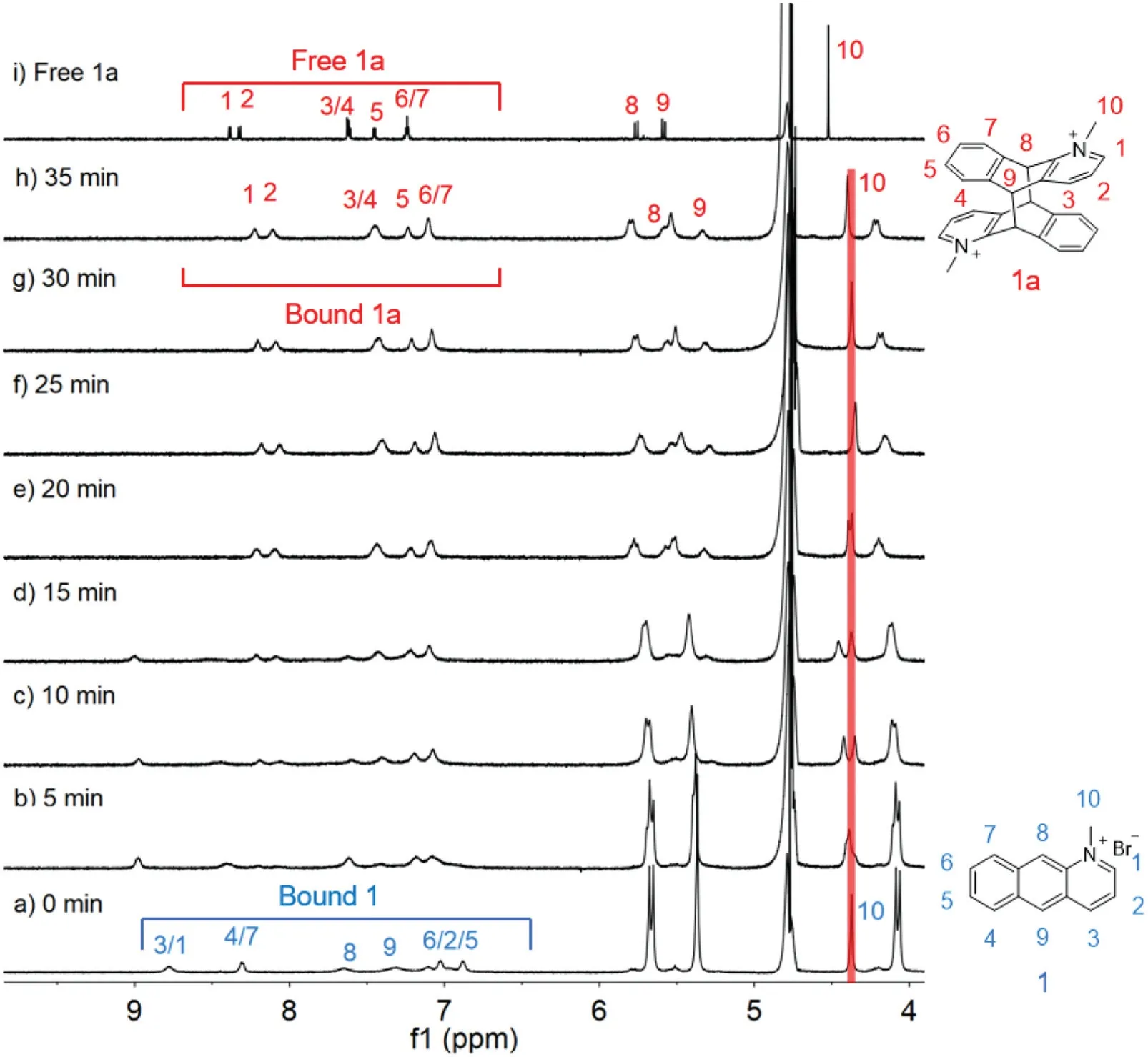
Fig.3.1H NMR spectra(600 MHz,D2O,298 K)of CB[10]·12([1]=2.0 mmol/L)with various UV irradiation time:(a)0,(b)5,(c)10,(d)15,(e)20,(f)25,(g)30,(h)35 min,and(i)free photodimer product 1a.
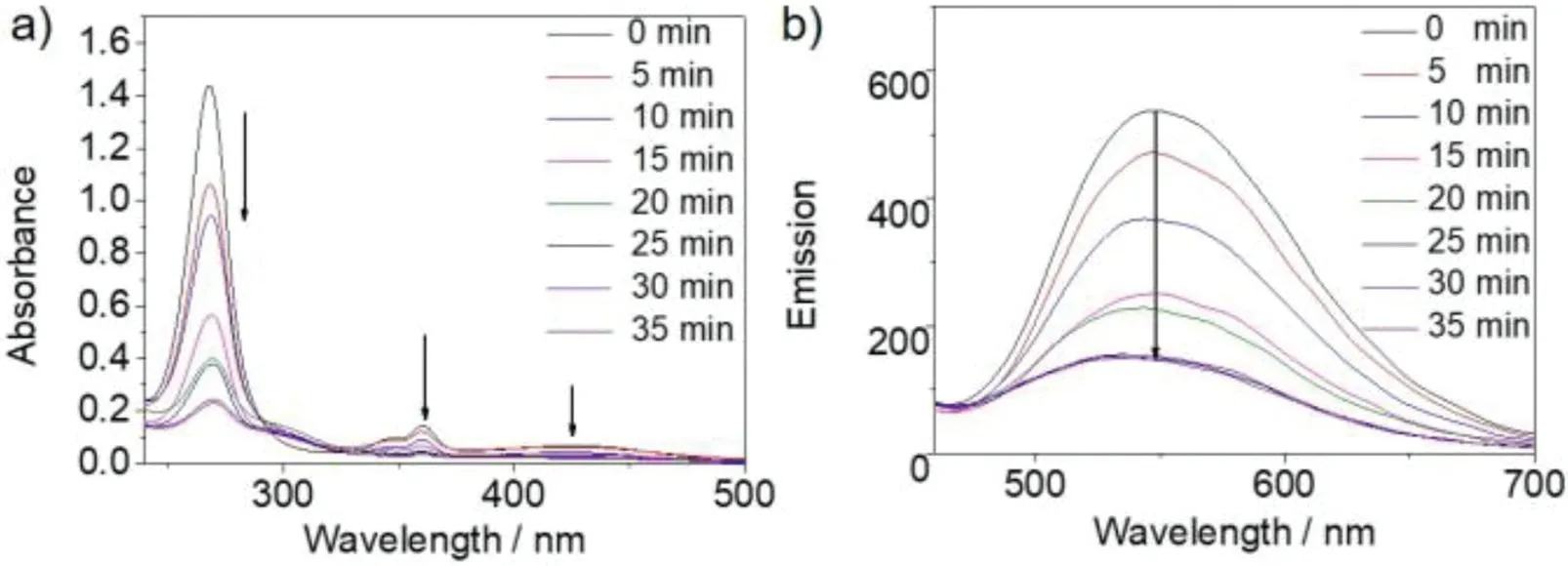
Fig.4.(a)UV-vis and(b)fluorescence spectra of the CB[10]·12 complex([1]=20 μmol/L)upon UV light irradiation.λex=420 nm,slit width:8/10.
Photodimerization of 2-4 in the absence/presence of CB[10]was also investigated(Figs.S25–S36 in Supporting information).It was found that irradiation of 2.0 mmol/L aqueous solution of 2 for 180 min gave a conversion ratio of∼44.5%with a single product of 2a(Table 1,Fig.S25).As Yamada reported in 2012[31],we believe that a cation-πinteraction and cation-cation repulsion play a key role in controlling the orientation of 2,resulting in producing a single photodimeranti-HT 2a.In the presence of CB[10],the complete conversion for the photodimerization reaction of 2 was reduced to 120 min with the same product,and the conversion ratio was∼82.5%(Fig.S27).The result indicated that CB[10]acted as a supramolecular nanoreactor accelerating the photodimerization reaction of 2.Afterwards,irradiation of 3 for 180 min by UV light produced two photodimer products 3a and 3b with an integral ratio of∼6:1 in1H NMR spectrum(Table 1,Fig.S29).However,in the presence of CB[10],photodimerization of 3 was completed in∼48 min,and the ratio of the two photodimer products became∼3:1(Fig.S31).These results verified that CB[10]not only accelerated the photodimerization of 3,but also affected the reaction selectivity.Similarly,the photodimerization of 4 was also monitored by1H NMR.In the absence of CB[10],the photoreaction of 4 allowed for the production of photodimer 4a and 4b with an integral ratio of∼3.5:1 in slow reaction rate(Fig.S33).Irradiation of the CB[10]·42complex produced the same photodimer product,but the ratio of the two photodimer products became∼6.5:1 in∼40 min(Fig.S35).
The density functional theory(DFT)calculations at the B3LYP/6-31G*level were carried out to analyze the total energy of four probable photodimer products(anti-HT,syn-HT,anti-HH,syn-HH)of 1 and four probable geometries for the accommodation of two 1 guests inside CB[10](anti-HT-[CB[10]·12]2+,syn-HT-[CB[10]·12]2+,anti-HH-[CB[10]·12]2+,syn-HH-[CB[10]·12]2+).The calculation results showed that the total energy of 1a and 1b are lower among probable photodimers(Table S1 and Figs.S37–S40 in Supporting information).Beside,theanti-HT-[CB[10]·12]2+conformation requires the lowest energy among all the possible inclusion complexes(Fig.5,Table 2,Figs.S41–S43 in Supporting information).This calculated structure is in good agreement with the NMR result.It could be concluded that,before photodimerization upon UV light irradiation,two 1 molecules were pre-orientated in a headto-tail manner in CB[10]through host-guest recognition.In this way,CB[10]acted as an efficient nanoreactor and provided a confined micro-environment to promote the formation of 1a with fast reaction rate and high regioselectivity.
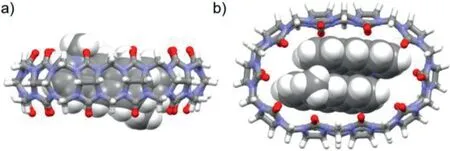
Fig.5.(a)Side view and(b)top view of DFT minimized structure of the anti-HT-[CB[10]·12]2+complex(B3LYP/6-31G*).
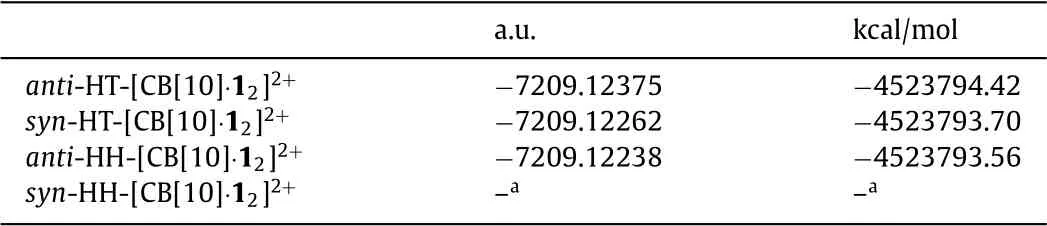
Table 2 The total energy of anti-HT-[CB[10]·12]2+, syn-HT-[CB[10]·12]2+, anti-HH-[CB[10]·12]2+,syn-HH-[CB[10]·12]2+optimized at the B3LYP/6-31G*level.
In summary,macrocyclic CB[10]host was used as a supramolecular nanoreactor to promote the photodimerization of four azaanthracene derivatives.In particular,irradiation of the CB[10]·12complex by UV light produced single photodimer with high reaction rate and regioselectivity.Our results show that host-guest interaction can enhance theπ-π/cation-πinteractions between two azaanthracene molecules,thus providing an efficient way to realize the highly regioselective photodimerization of azaanthracene derivatives.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was financially supported by the National Natural Science Foundation of China(No.21871216).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.04.030.
 Chinese Chemical Letters2022年12期
Chinese Chemical Letters2022年12期
- Chinese Chemical Letters的其它文章
- Diverse strategic approaches en route to Taxol total synthesis
- Recent advances in gold-complex and chiral organocatalyst cooperative catalysis for asymmetric alkyne functionalization
- Unmodified methodologies in target discovery for small molecule drugs:A rising star
- Recent advances in single-crystalline two-dimensional polymers:Synthesis,characterization and challenges
- Environmental applications of graphene oxide composite membranes
- Recent advances in the application of metal organic frameworks using in advanced oxidation progresses for pollutants degradation
