Supramolecular interaction controlled and calix[4]arene ligand assisted Pd-catalyzed C(sp3)−H arylation of aliphatic aldehydes
Yao Wu,Zhiyan Ma,Jing Shi,Xiaoqiang Sun,Ke Yang,Zheng-Yi Li
Jiangsu Key Laboratory of Adva nced Catalytic Materials and Technology,School of Petrochemical Engineering,Changzhou University,Changzhou 213164,China
Keywords:Supramolecular interaction Palladium-catalyzed C−H activation Calixarene Aliphatic aldehyde
ABSTRACT A calix[4]arene ligand assisted directβ-C−H arylation of tertiary aliphatic aldehydes has been developed via a Pd-catalyzed C(sp3)−H functionalization process.This strategy exhibited good functional group compatibility and C−H bond site-selectivity.Mechanism studies have shown that both synergistic effect and cationic-πsupramolecular interaction between calixarene cavity and transition-metal catalytic center may play an important role in this catalytic cycle.This complementary method would be used in organic and medical chemistry due to the importance of tertiary aliphatic aldehydes.
In the last decade,directing-group-assisted transition-metalcatalyzed C−H bond functionalization has become one of most efficient approaches for the selective C−C and C−heteroatom bond constructions in organic chemistry[1–7].In spite of being an effective method,some inevitable steps are still required for the preinstallation and release of the covalently directing group,which diminishing the overall efficiency of the target molecule synthesis.
Recently,the functionalization of C−H bonds catalyzed by transition-metals through supramolecular interactions has attracted considerable attention[8–16].Among in this filed,some pioneering strategies for the Rh-catalyzed C(sp2)−H functionalization by utilizing reversible covalent bonds have proven successfully[17–22].After that,Yu group firstly developed the Pd-catalyzed C(sp3)−H arylation ofo-alkyl benzaldehydes and ketones in the presence of catalytic amounts of amino acids by utilizing reversible imine bonds[23].Moreover,some other important reports have also been developed in transition-metal-catalyzed C(sp3)−H functionalization of aldehydes,ketones and amines[24–31].In addition to reversible covalent bonding force,some other supramolecular interactions,including hydrogen and ionic bonding force,are also used in the C−H bond functionalization[32–36].Although extensive efforts have been attempted in this filed,the development of a novel supramolecular catalysis strategy would be of great significance.
The upper-or lower-rim functionalized calixarenes,as ideal supramolecular catalysts,have been widely used in organic reactions[37–41].Furthermore,they are also important transition metal ligands in C−H bond functionalization[42].It should be noted that upper-rim functionalized calixarenes are much more valuable than the lower-rim functionalized calixarenes,because the synergistic effect between calixarene cavity and catalytic center provides the better catalytic performance[43–46].In our continuing efforts for developing novel supramolecular catalysis reactions[47–49],the designed upper-rim functionalized calix[4]arenes L1–L5 have been synthesized as transient directing ligands for the Pd-catalyzedβ-C(sp3)−H arylation of aliphatic aldehydes(Fig.1).Moreover,this strategy provides an important complementary method to access arylated aliphatic aldehydes,important skeletal backbones of many natural products,pharmaceuticals and organic materials[50–53].
Our investigation began with the Pd(II)-catalyzed cross coupling of pivalaldehyde(1a)and methyl 4-iodobenzoate(2a)in the cosolvent of HFIP and AcOH at the volume ratio of 1:1 under a nitrogen atmosphere for 24 h in the presence of AgTFA as additive and upper-rim functionalized calix[4]arene L1 as the transient ligand(Table 1,entries 1–4).The desired arylated product 3a was obtained in 60%NMR yield by using 80 mol%of L1.The subsequent examination on ligands revealed that none of other ligands L2-L5 provided the better results than L1(Table 1,entries 5–10).Next,the solvent screening indicated that the co-solvent of HFIP and AcOH at the volume ratio of 1:1 was optimal(Table 1,entries 11–14).Following the above investigations,the screening of palladium catalysts was then conducted,and a moderate yield was observed in PdCl2,PdBr2and Pd(TFA)2,(Table 1,entries 15–17).Finally,no desired product 3a was isolated,when either AgTFA or ligand was absent(Table 1,entries 18 and 19).
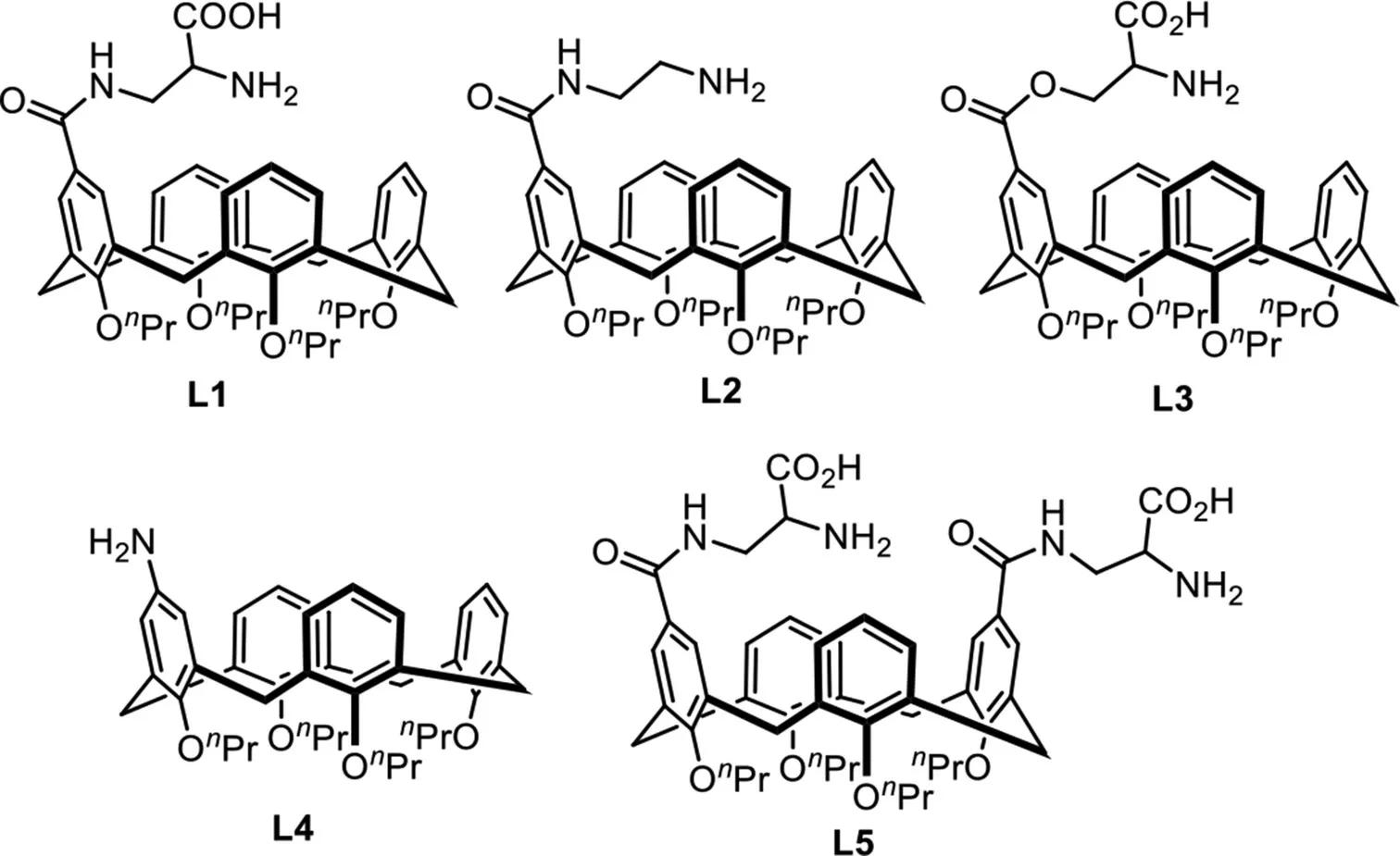
Fig.1.Designed upper-rim functionalized calix[4]arene ligands.
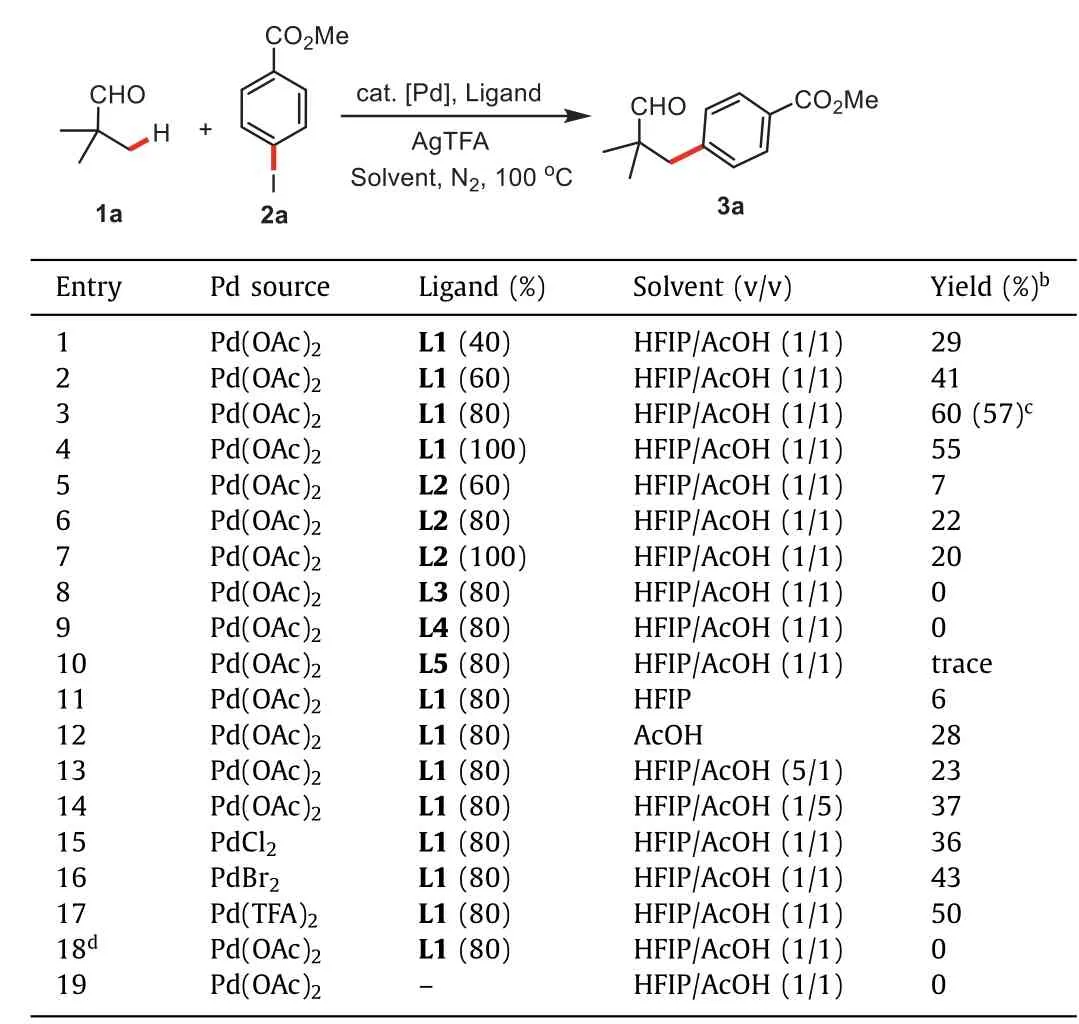
Table 1 Optimization of reaction conditions.a
With the optimized reaction conditions in hand,the substrate scope study of aliphatic aldehydes was carried out in Scheme 1.α-Methyl-α,α-dialkyl substituted acetaldehyde derivatives provided the correspondingβ-arylated products in moderate yields with excellent site-selectivity(3a–3c).Next,whenα,α-dimethylα-(4-chlorophenyl)substituted acetaldehyde was employed,only 21%yield of product 3d was isolated.Furthermore,phenoxy-,4-methylphenoxy-,or 4-chlorophenoxy-substituted alkylaldehydes were also effective substrates to react with methyl 4-iodobenzoate(3e–3g).After that,this catalytic reaction was also suitable for the aliphatic aldehyde bearing a cyclic alkyl group,affording the desired product 3h in moderate yield.Unfortunately,nonα-quaternary aldehydes failed in our current reaction conditions(3i,3j).
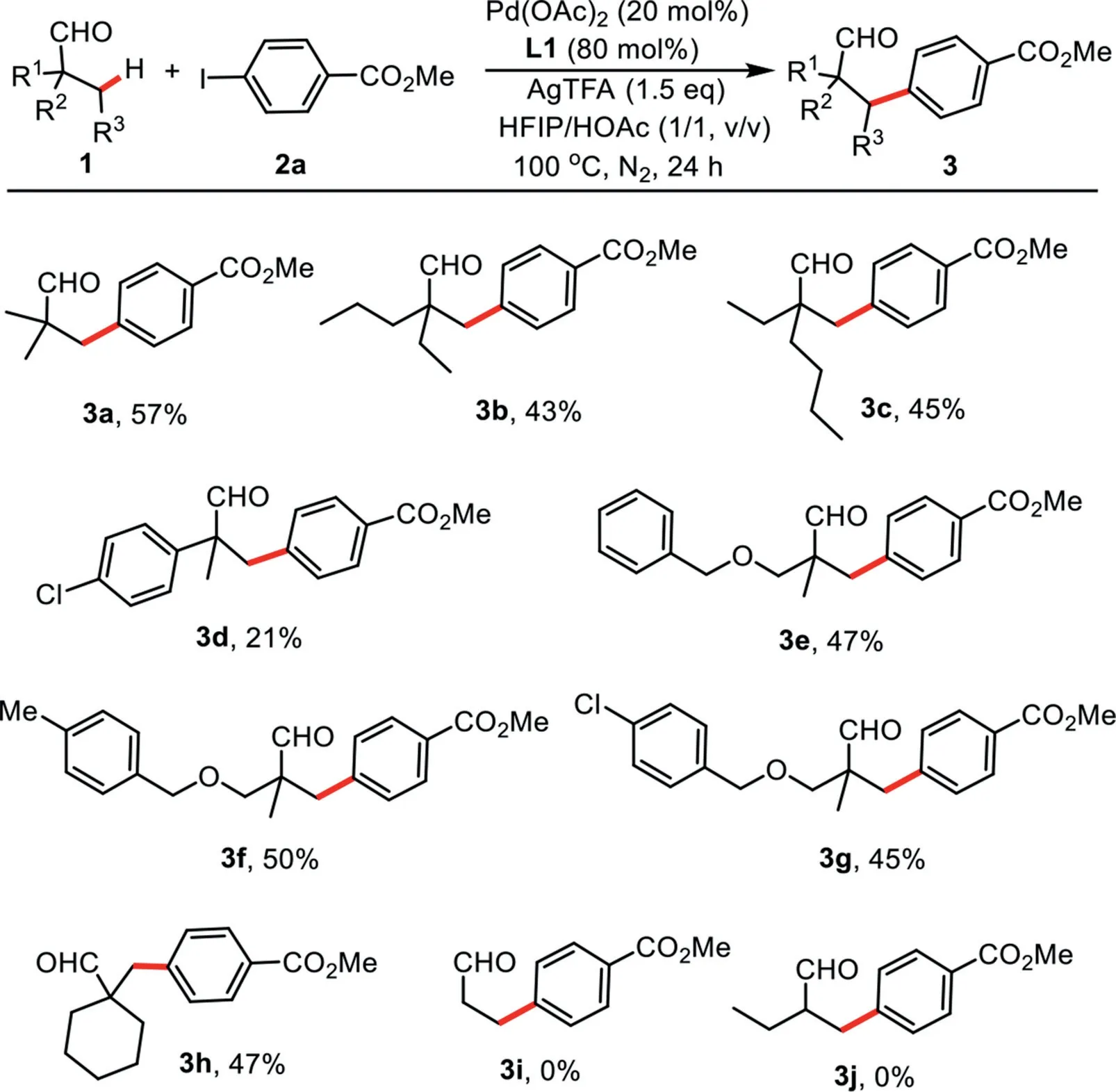
Scheme 1.Scope of aliphatic aldehydes.Reaction conditions:1(0.2 mmol),2a(0.4 mmol),Pd(OAc)2(0.04 mmol),AgTFA(0.3 mmol),L1(0.16 mmol),HFIP(1.0 mL),HOAc(1.0 mL),N2,100◦C,24 h.Isolated yields.
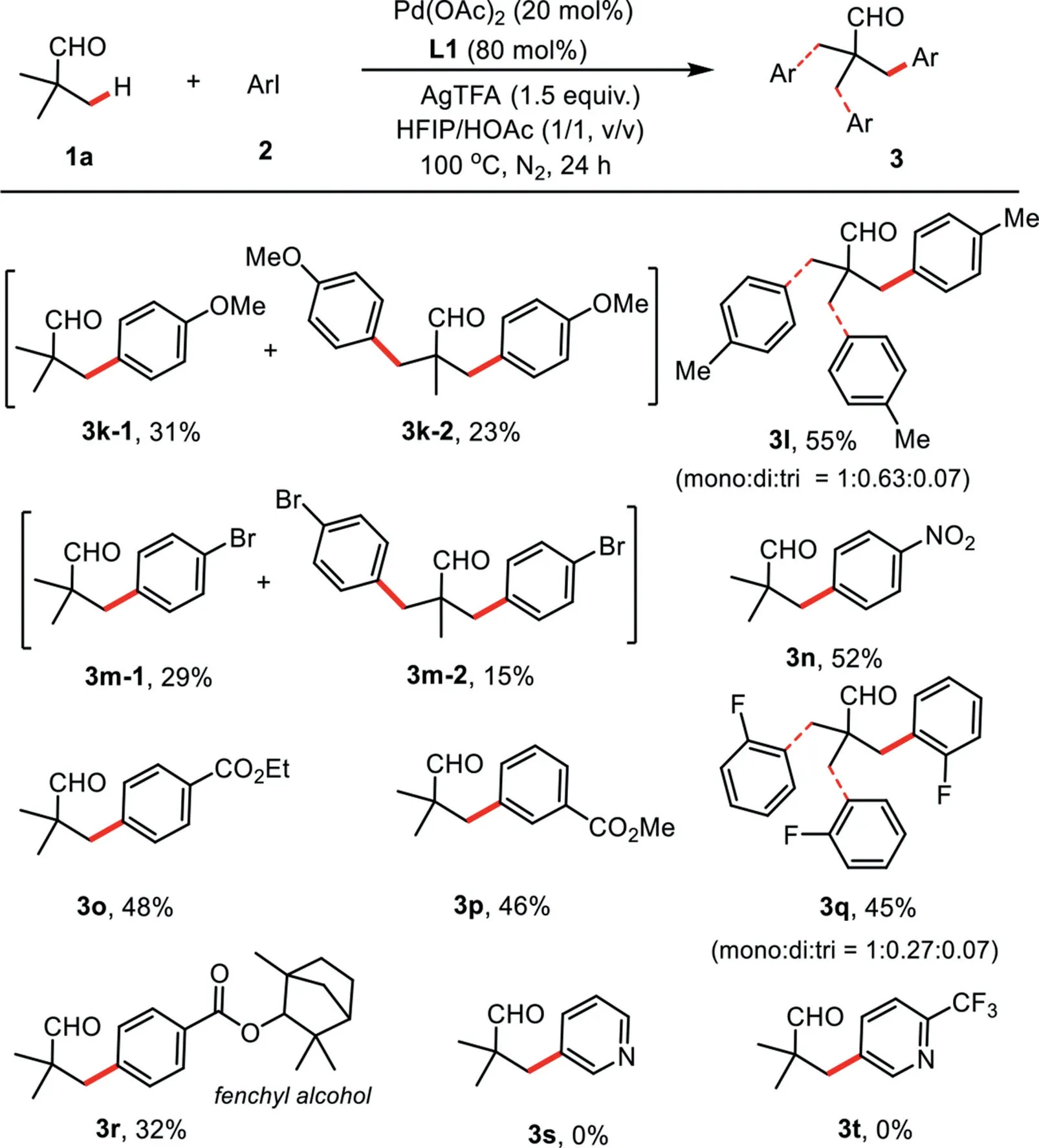
Scheme 2.Scope of(hetero)aryl iodides.Reaction conditions:1a(0.2 mmol),2(0.4 mmol),Pd(OAc)2(0.04 mmol),AgTFA(0.3 mmol),L1(0.16 mmol),HFIP(1.0 mL),HOAc(1.0 mL),N2,100◦C,24 h.Isolated yields.
Next,the substrate scope study of aryl iodides was then examined in Scheme 2.Both electron-donating(MeO and Me)and electron-withdrawing groups(F,Br,ester and NO2)at thepara-,meta-orortho-position on the aromatic ring were compatible under the current catalytic system,providing the desired products 3k–3q in moderate yields.It should be noted that these important functional groups were well-tolerated in this reaction,enabling the further transformation of the initial products.Furthermore,the aryl iodide derived from fenchyl alcohol could also be utilized in this process to produce the desired product 3r in 32%isolated yield.Normally,a mixture of mono-,di-and tri-arylated products were obtained by using the substrates containing two or three equivalent methyl groups at theβ-position.Finally,neither 3-iodopyridine nor 5-iodo-2-(trifluoromethyl)pyridine failed to afford the desired products 3s and 3t.
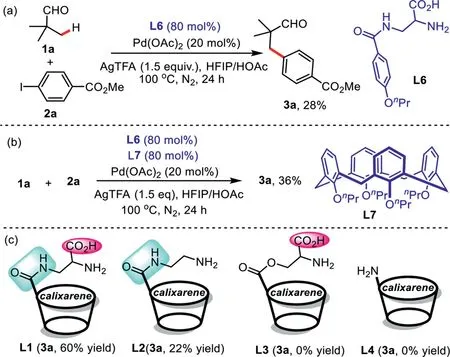
Scheme 3.Mechanistic studies.
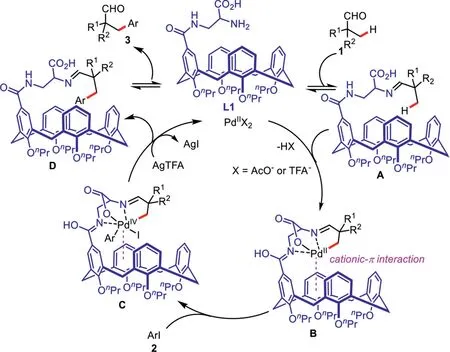
Scheme 4.The plausible reaction mechanism.
To gain some insights into the reaction mechanism,several control experiments have been performed(Scheme 3).The comparative experiment demonstrated that a lower yield of desired product 3a was obtained in the presence of ligand L6(Scheme 3a).We speculated that cationic-πsupramolecular interaction between calixarene cavity and cationic palladium may be existed.Meanwhile,we also examined the reaction of pivalaldehyde 1a and methyl 4-iodobenzoate 2a with ligand L6 and L7 under the current conditions,the desired product 3a was observed in 36%yield,which indicating that the synergistic effect between calixarene cavity and catalytic center promotes this process(Scheme 3b).In addition,our previous results of the ligands indicate that both amide-linked and carboxyl group are crucial for this catalytic reaction(Scheme 3c).
Based on the above results and the previous literature reports[23–31,54–56],a plausible reaction pathway of this process is proposed as shown in Scheme 4.Condensation of aliphatic aldehyde 1 with the ligand L1 provides the imine intermediate A.Next,the coordination of imine intermediate A to a palladium species followed by a subsequent cyclometallation process produces the corresponding bicyclic palladium intermediate B,which is promoted by the cationic-πsupramolecular interaction between calixarene cavity and palladium catalyst center.Then,the oxidative addition of the intermediate B with an aryl iodide generates the palladium species C.Finally,the reductive elimination of the palladium complex C followed by the ligand dissociation and iodide abstraction process affords the imine intermediate D,which can be further transformed into the desired product 3.
In summary,a site-selective arylation reaction of a tertiary aliphatic aldehyde with an aryl iodide has been developedviaa Pd(II)-catalyzedβ-C(sp3)−H functionalization by using an upperrim functionalized calix[4]arene ligand L1 as a novel transient directing ligand.Moreover,a good functional group compatibility has been observed in the reaction,and both electron-deficient and electron-rich aryl groups were smoothly incorporated into the tertiary aliphatic aldehydes.Mechanism studies indicate that cationic-πsupramolecular interaction and synergistic effect between calixarene cavity and palladium catalyst may be involved in this catalytic cycle.In view of the important role of aliphatic aldehydes in organic and pharmaceutical research,this strategy would have a board application prospects in organic chemistry and medicinal chemistry.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We gratefully acknowledge the financial support from the National Natural Science Foundation of China(Nos.21572026,21702019),Postgraduate Research&Practice Innovation Program of Jiangsu Province(No.KYCX20_2525),Advanced Catalysis and Green Manufacturing Collaborative Innovation Center,Changzhou University.We also acknowledge the analytical testing support from Analysis and Testing Center,NERC Biomass of Changzhou University.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.03.125.
 Chinese Chemical Letters2022年12期
Chinese Chemical Letters2022年12期
- Chinese Chemical Letters的其它文章
- Diverse strategic approaches en route to Taxol total synthesis
- Recent advances in gold-complex and chiral organocatalyst cooperative catalysis for asymmetric alkyne functionalization
- Unmodified methodologies in target discovery for small molecule drugs:A rising star
- Recent advances in single-crystalline two-dimensional polymers:Synthesis,characterization and challenges
- Environmental applications of graphene oxide composite membranes
- Recent advances in the application of metal organic frameworks using in advanced oxidation progresses for pollutants degradation
