Direct benzylation reactions from benzyl halides enabled by transition-metal-free photocatalysis
Pnjie Xing ,Ki Sun,*,Shung Wng ,Xioln Chen,*,Lingo Qu,Bing Yu,*
a Green Catalysis Center,College of Chemistry,Zhengzhou University,Zhengzhou 450001,China
b Zhengzhou Tobacco Research Institute of CNTC,Zhengzhou 450001,China
Keywords:Benzylation Photocatalysis Transition-metal-free Radical reaction N-Heterocyclics
ABSTRACT An SN2-based photochemical strategy using dithiocarbamate anion as catalyst was developed for the activation of benzyl halides,which are extremely challenging to be applied as radical precursors in visible light photocatalysis.With this transition-metal-free and oxidant-free protocol,the benzylation(or cyanomethylation)of various heterocycles including quinoxalin-2(1H)-ones,coumarin,2-phenyl-2H-indazole,1-methyl-5-phenylpyrazin-2(1H)-one,1-(fluoromethyl)cinnolin-4(1H)-one,and 2,4-dibenzyl-1,2,4-triazine-3,5(2H,4H)–dione could be realized(46 examples,up to 98%yield).Importantly,some biologically relevant 3-benzylquinoxalin-2(1H)-ones were also be synthesized under mild conditions.
N-Heterocyclics are becoming increasingly important due to their considerable bioactivities[1–5].Among them,quinoxalin-2(1H)-ones are of pivotal importance with significant pharmacological value[6–9],such as anti-inflammatory,antimicrobial,antithrombotic,and even antitumor activities[10–14].In addition,quinoxalin-2(1H)-one is also an effective framework for designing luminescent materials[15,16].Therefore,the preparation of different functionalized quinoxalin-2(1H)-ones has attracted widespread interest in the past few years,and increasing efforts have been devoted to developing efficient synthetic methods[17–23].In recent years,the direct C-H functionalization of quinoxalin-2(1H)-ones has been developed into a powerful platform for the preparation of structurally diverse quinoxalin-2(1H)-ones[24–26].The selective C-H bond alkylation is a sought-after native group transformation,which might result in significant changes in chemical and biological properties[27].In particular,the C-3 benzylation of quinoxalin-2(1H)-ones have attracted extensive interest due to an amazingly wide range of biological activities,such as calcium channel blocker[28],aldose reductase inhibitor[29],MAO-A inhibitor[30],and anti-thrombotic(Fig.1)[31].However,it is still a pressing task for the development of convenient and practical synthetic methods to fulfill the growing requirements related to the design of drugs using basic chemical materials.
Traditional methods to prepare 3-benzylquinoxalin-2(1H)-ones generally rely on condensation reactions[32].However,the low selectivities and yields were extremely unfavorable for practical applications.In 2018,the Yuan group reported a copper-catalyzed protocol for the C-3 benzylation of quinoxalin-2(1H)-ones,providing an elegant manner to access 3-benzylquinoxalin-2(1H)-ones in good yields[33].However,transition metal(CuCl)and excessive strong oxidant(tert–butyl peroxybenzoate,TBPB)were indispensable for this transformation.Visible light was considered as the clean,safe,renewable energy source[34–38].Over the last decade,photocatalysis has displayed great prospects in developing sustainable and eco-friendly organic conversions[39–42].Many meaningful and valuable photocatalytic reactions have been developed usually accompanied by a single electron transfer(SET)process[43–47].Very recently,the Xuan group developed a visiblelight-promoted protocol to access 3-benzylquinoxalin-2(1H)-ones efficiently by employing 4-benzyl-1,4-dihydropyridines(R-DHPs)as radical precursors and acetoxybenziodoxole(BI-OAc)as an oxidant[48].However,the synthesis of R-DHPs requires additional synthetic processes.Moreover,the employment of strong oxidants might be unfavorable for the tolerance of sensitive functional groups.Additionally,low step-/atom-economy are the inevitable shortcomings.Therefore,the exploration of inexpensive commercially available benzylation reagents,e.g.,benzyl halides,is highly desirable and promising.
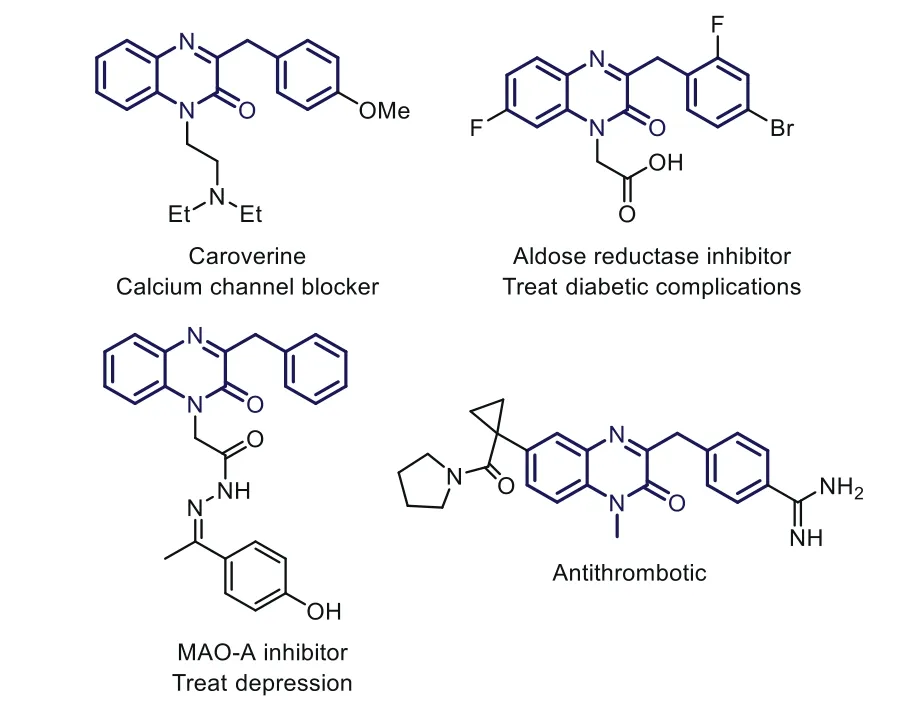
Fig.1.Representative 3-benzylquinoxalin-2(1H)-ones.
Benzyl halides,served as a kind of cheap and commercial raw materials,are generally difficult to be reduced(E1/2<−1.70 Vvs.SCE in MeCN)in visible light photocatalysisviaa SET process[49].Recently,the Melchiorre group developed elegant photocatalytic strategies using dithiocarbamates as nucleophilic organic catalysts for the alkylation and acylation of olefins[50–52].Encouraged by these exciting results,and with our continuous research interests in visible-light-promoted organic conversions[53–55],we herein reported a photochemical catalytic approach to access 3-benzylquinoxalin-2(1H)-ones by using a nucleophilic dithiocarbamate anion catalyst to activate benzyl halides(Scheme 1).To the best of our knowledge,this is the first example of the photocatalytic synthesis of 3-benzylquinoxalin-2(1H)-ones under transition-metal-free oxidant-free conditions.Importantly,this practical protocol is amenable for the synthesis of biologically relevant 3-benzylquinoxalin-2(1H)-ones in satisfactory yields.Additionally,the skeletons of several natural products and drugs could be successfully introduced into 3-benzylquinoxalin-2(1H)-ones,which probably promotes the discovery of pharmaceuticals and bioactive molecules.
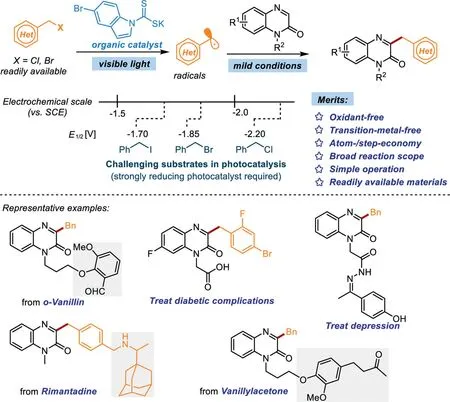
Scheme 1.Direct C-3 benzylation of quinoxalin-2(1H)-ones.
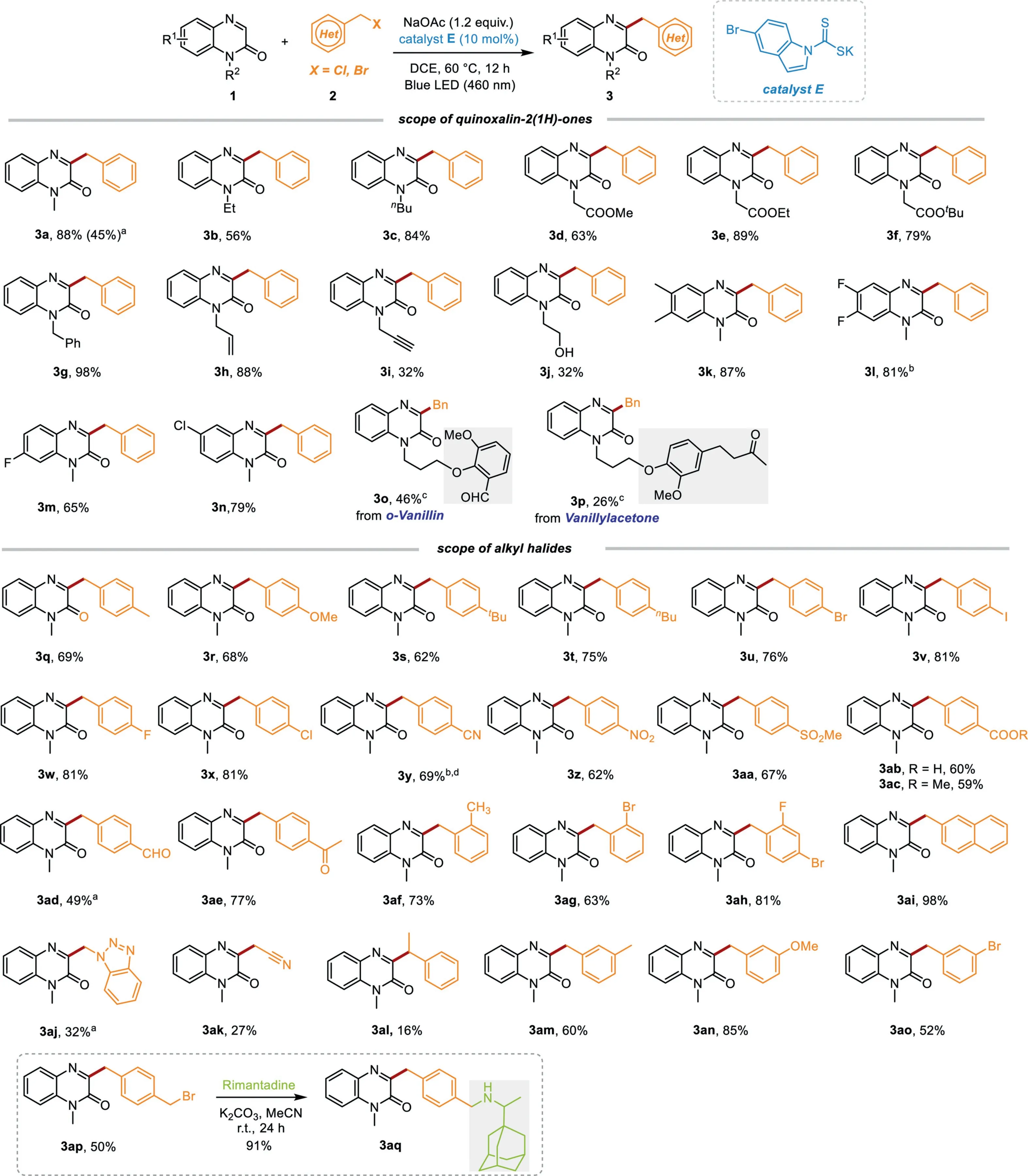
Scheme 2.Reaction scope.Reaction conditions:1(0.2 mmol),alkyl bromide(1.2 equiv.),catalyst E(10 mol%),and NaOAc(1.2 equiv.),DCE(4 mL)were irradiated with 10 W blue LED(460 nm)at 60°C under N2 atmosphere for 12 h;isolated yields were given based on 1.a Alkyl chloride was employed instead of alkyl bromide.b 24 h.c 72 h.d 40 W blue LED.
The preliminary study was commenced by exploring the model reaction betweenN-methylquinoxalin-2(1H)-one(1a)and benzyl bromide(2a)with various nucleophilic dithiocarbamate anion catalysts,bases,and solvents under visible light irradiation(Table 1).Initially,the desired product 3a could be obtained in 10%yield by using NaOAc as the base and potassium dimethylcarbamodithioate(A)as the catalyst at 60°C in DCE for 12 h(entry 1).After confirming the feasibility,a series of nucleophilic dithiocarbamate anions(B-E)were investigated to further improve the reaction efficiency(entries 2–5).To our delight,the yield was significantly improved to 90%when catalyst E was employed(entry 5).With this satisfactory result in hand,the effect of different solvents including CH3CN,CH3OH,DMSO,THF,1,4-dioxane,2-Me-THF was screened(entries 6–11).As a result,all of them showed poorer results compared to the solvent of DCE.In addition,other bases including Na2CO3,NaHCO3,NaOH,triethylenediamine(DABCO),N,N,N’,N’-tetraethylethylenediamine(TEEDA),and 2,6-lutidine were evaluated(entries 12–17).Unfortunately,no positive results were obtained,and NaOAc was still the best choice for this transformation.Further attempts at optimizing the reaction temperature failed to improve the yield(entries 18 and 19).However,when the reaction was performed by only using traditional heating without visible light irradiation,no desired products could be detected(entry 20).Finally,the control experiment without catalyst indicated the essential role of catalyst for this transformation(entry 21).Thus,the optimal reaction conditions were established as follows:1a(0.1 mmol),2a(1.2 equiv.),catalyst E(10 mol%),and NaOAc(1.2 equiv.)in DCE(2 mL)irradiated with 10 W blue LED(460 nm)at 60°C under N2atmosphere for 12 h(entry 5).
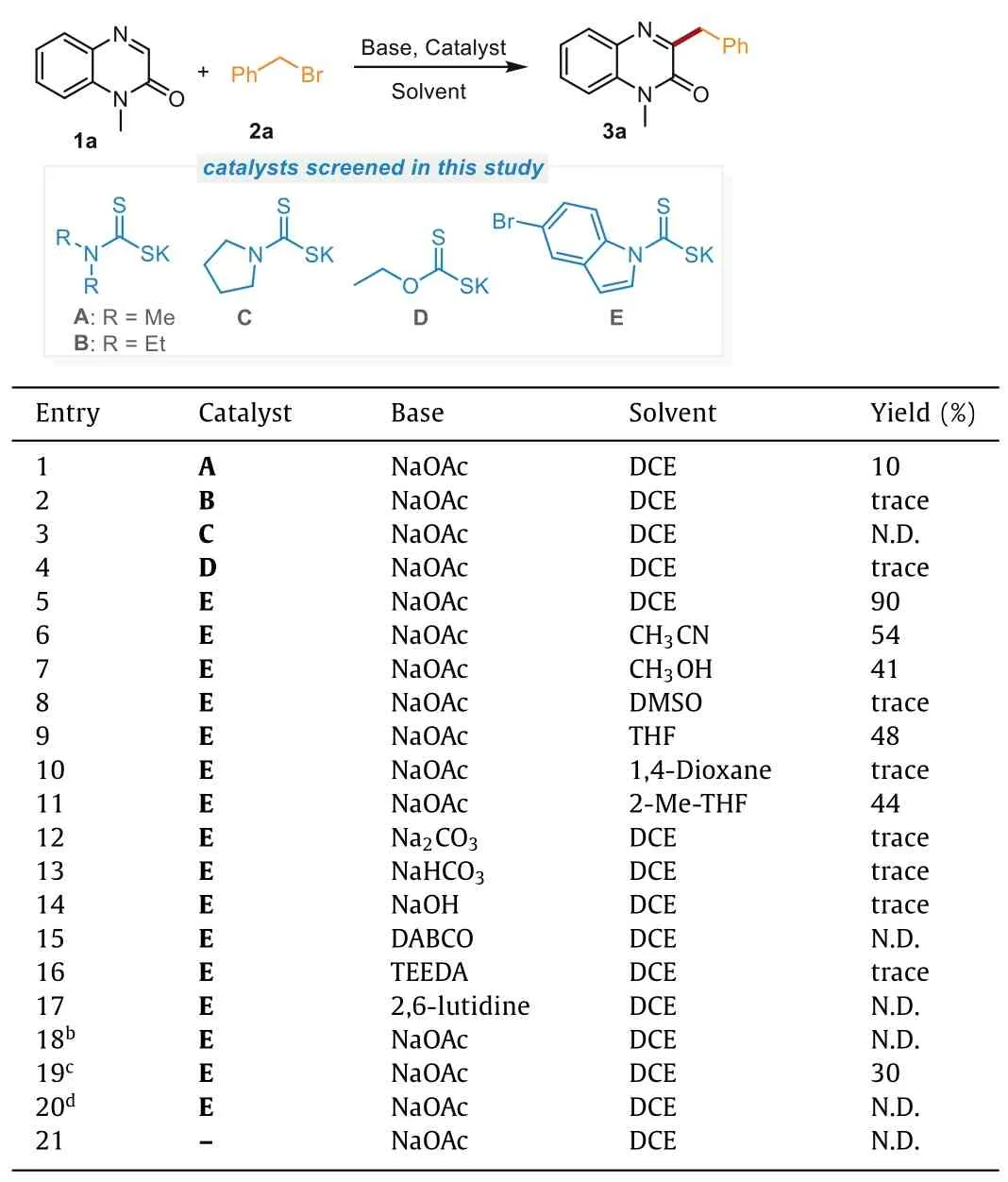
Table 1 Optimization of reaction conditions.a
With the optimized reaction conditions in hand,the scope of this efficient benzylation process was then investigated.As can be seen in Scheme 2,a wide range of quinoxalin-2(1H)-ones were proven to be suitable substrates to afford the desired 3-benzylquinoxalin-2(1H)-ones 3a-3n in satisfactory yields.Notably,some sensitive functional groups,such as hydroxy,alkynyl,and alkenyl are all well tolerated.Within the realm of late-stage functionalization,the possibility for employing valuable scaffolds containing drug-like molecules and natural isolates as reaction substrates were assessed.Satisfactorily,this approach displayed a high level of tolerance foro-vanillin,vanillylacetone to access products 3o-3p,suggesting highly useful applications in drug discovery research.The substrate scope of benzyl halides was then evaluated.To our delight,both electron-donating groups(–Me,–OMe,–tBu,–nBu)and electron-withdrawing groups(–F,–Cl,–Br,–I,–NO2,–SO2Me,–COOH,–COOMe,–CHO,–COCH3)on thepara-positions of phenyl ring of benzyl bromides moiety were all compatible for this transformation,affording the desired products 3q-3ae in average good yields.When 4-(bromomethyl)benzonitrile was employed as the substrate,the desired product 3y could be obtained in 69%yield albeit the high-intensity blue light(40 W)and slight long reaction time(24 h)are needed.Moreover,when more challenging benzyl chloride was employed as a substrate to instead of benzyl bromide,the reaction still reacted smoothly to access desired product 3a.However,when benzyl iodide was used to react with 1a under the optimized reaction conditions,3a could only be obtained in 11%yield,which might be caused by the instability of alkyl iodine to light[56].Furthermore,the scope of this protocol was extended to 2-substituted benzyl bromides to access corresponding products 3af-3ah with high efficiency.There was no major electronic effect and steric effects were observed in these cases.The reactivities of somemeta-substituted benzyl bromides were also investigated with quinoxalin-2(1H)-ones(1a)under the optimized reaction conditions.To our delight,the corresponding products 3am-3ao could be obtained in moderate yields.Moreover,2-(bromomethyl)naphthalene,1-(chloromethyl)-1H-benzo[d][1,2,3]triazole, 2-bromoacetonitrile,and(1-bromoethyl)benzene could also react smoothly withNmethylquinoxalin-2(1H)-one(1a)to afford the desired products 3ai-3al.Unfortunately,when long-chain halogenated hydrocarbons(e.g.,1-chlorohexane,1-bromohexane,4-chlorobutyronitrile)were employed as reactants to react with 1a,no desired products were detected.Notably,when 1,4-bis(bromomethyl)benzene was employed as the reaction substrate,a benzyl bromide group in product 3ap could be maintained for further functionalization.Subsequently,compound 3aq containing biologically important rimantadine could be synthesizedviasimple operation.Finally,the model reaction betweenN-methylquinoxalin-2(1H)-one(1a)and benzyl bromide(2a)was carried out on a 1 mmol scale.To our delight,the target product 3a could be obtained in 86%yield.
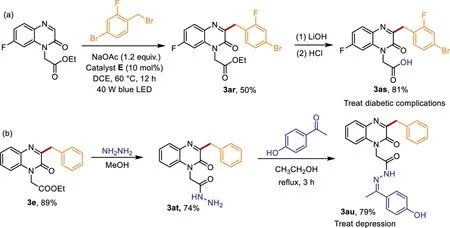
Scheme 3.The synthesis of the biologically significant molecules.
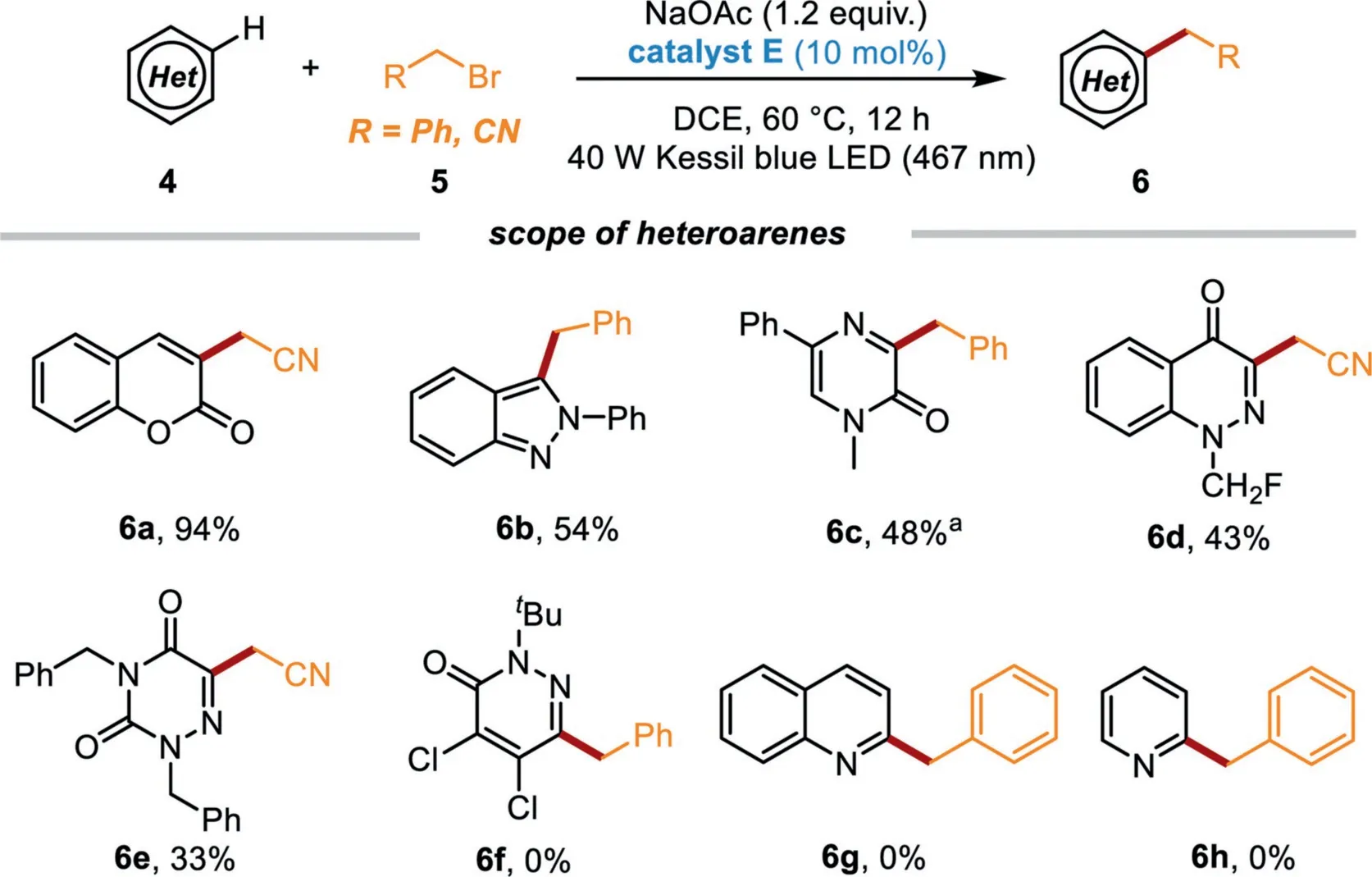
Scheme 4.Reaction scope of heteroarenes.Reaction conditions:5(0.2 mmol),heteroarene(5 equiv.),catalyst E(10 mol%),and NaOAc(1.2 equiv.),DCE(2 mL)were irradiated with 40 W blue LED at 60°C under N2 atmosphere for 12 h;isolated yields were given based on 5.a Heteroarene(0.2 mmol),benzyl bromide(2 equiv.),catalyst E(10 mol%),and NaOAc(2 equiv.),CH3 CN(2 mL)were irradiated with 40 W blue LED at 60°C under N2 atmosphere for 36 h;isolated yields were given based on 4.
In order to further demonstrate the practicality of this benzylation reaction,two biologically significant molecules were synthesized based on the benzylation products(Scheme 3).To our delight,the benzylation product 3ar and 3e were conveniently converted into the corresponding valuable compounds 3as and 3au through simple treatment.As a result,the desired molecules 3as and 3au were obtained in good yields(81%and 79%),which could be used to treat diabetic complications and depression[29,30].
Furthermore,the reactivities of some other heterocycles,including coumarin,2-phenyl-2H-indazole,1-methyl-5-phenylpyrazin-2(1H)-one,1-(fluoromethyl)cinnolin-4(1H)-one,and 2,4-dibenzyl-1,2,4-triazine-3,5(2H,4H)–dione were also explored(Scheme 4).Satisfactorily,these substrates were also well compatible in this powerful protocol to afford the desired benzylated (or cyanomethylated)products 6a-6e in 33%−94%yields under the irradiation of 40 W blue LED.Unfortunately,2-(tert–butyl)-4,5-dichloropyridazin-3(2H)-one,quinoline or pyridine were not a suitable substrate to products 6f-6h.
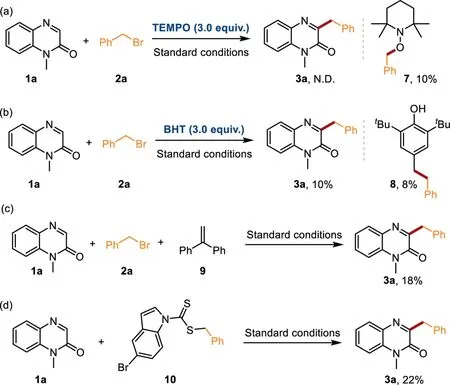
Scheme 5.Control experiments.
Some control experiments were conducted to gain insights into the reaction mechanism.The treatment of model reaction with 3 equiv.of radical scavengers such as 2,2,6,6-tetramethylpiperidin-1-yl)oxidanyl(TEMPO)or 2,6-di–tert–butyl–4-methylphenol(BHT)failed to access the desired product efficiently,indicating the possible involvement of radical pathway in this transformation(Schemes 5a and b).Furthermore,compounds 7 and 8 were successfully detected by high-resolution mass spectrometry(HRMS),implying the existence of benzyl radical intermediates.Likewise,the addition of 1,1-diphenylethylene(9)to the model reaction strongly suppressed the reaction giving the corresponding product 3a only in an 18%yield,which further confirms a radical process(Scheme 5c).When compound 10 is directly used in this photocatalysis reaction,product 3a could be obtained in a 22%yield.The reason for the low reactivity might be caused by the different concentrations of free radicals in the reaction solution(Scheme 5d,details see Supporting information).This result confirmed that compound 10 might be a key intermediate in this transformation.
According to the above experiments and previous reports[57],a plausible mechanism is proposed as illustrated in Scheme 6.Firstly,benzyl bromide 2 undergoes an SN2 reaction with nucleophilic catalyst E to obtain the intermediate 10,in which the weak C–S bond is cleaved under visible light to produce benzyl radical 11 and dithiocarbonyl radical 15.Then benzyl radical 11 adds to the C=N bond of quinoxalin-2(1H)-ones 1 to afford the intermediate 12,which afterward undergoes 1,2-H migration to generate the carbon-centered radical intermediate 13.Subsequently,a single electron transfer(SET)process between the intermediate 13 and radical 15 occurs to produce the cation 14 along with the regeneration of nucleophilic catalyst E for the next cycle.Finally,radical cation 14 undergoes deprotonation in the presence of a base to yield the desired product 3.
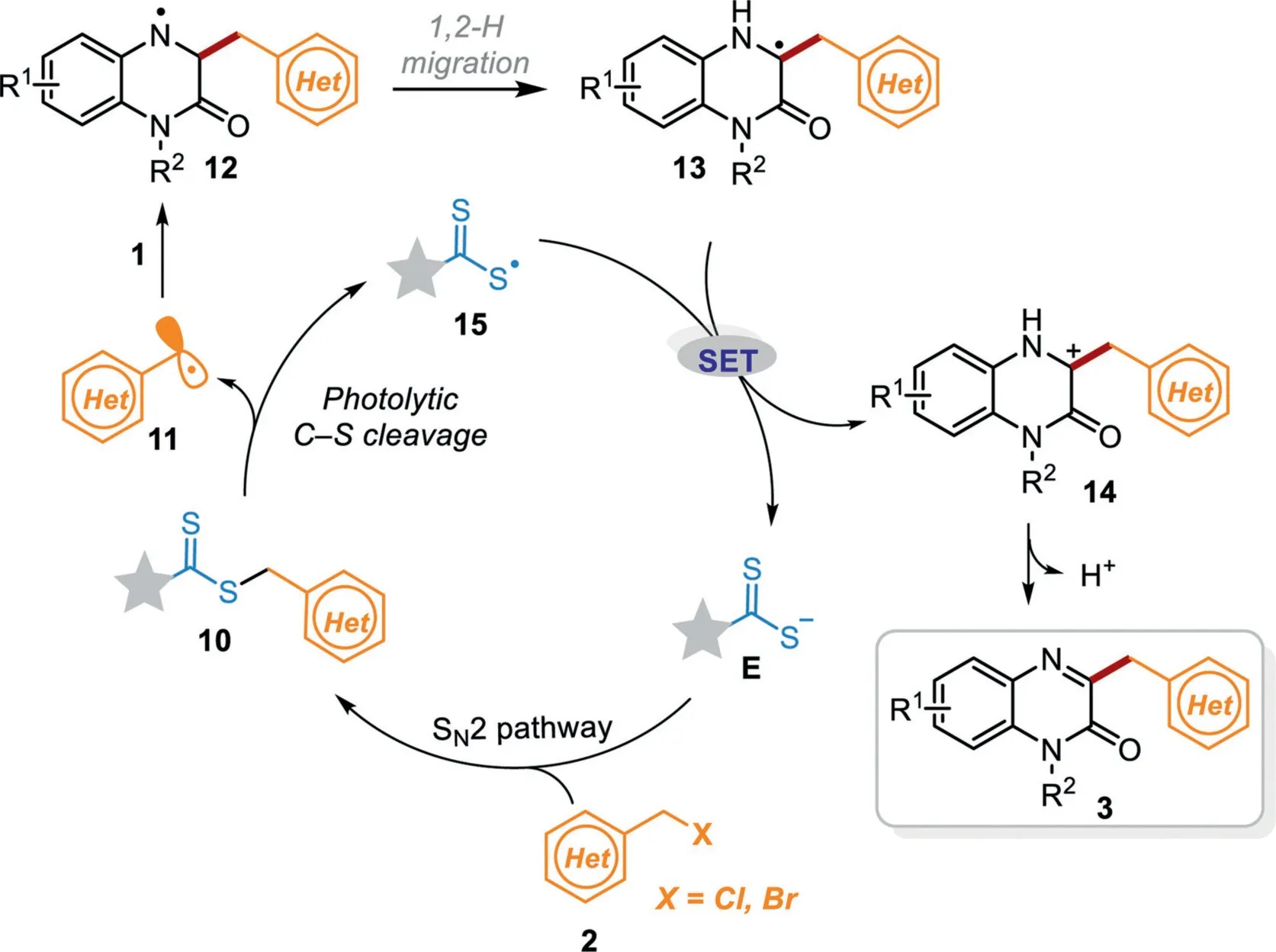
Scheme 6.Proposed reaction mechanism.
In summary,we have developed a transition-metal-free and oxidant-free protocol for the direct C-3 benzylation of quinoxalin-2(1H)-ones under the irradiation of visible light by using an experimental simplicity and low-cost nucleophilic dithiocarbamate anion catalyst to activate benzyl halides.A series of quinoxalin-2(1H)-one and benzyl bromides/chlorides were compatible with this mild protocol to produce the desired products in satisfactory yields,showing wide reaction scope and excellent functional group tolerance.In addition,this practical protocol was amenable for the synthesis of biologically relevant 3-benzylquinoxalin-2(1H)-ones,making it a promising procedure in the design and discovery of novel pharmaceuticals and bioactive molecules.Some other meaningful heterocyclics were also suitable substrates.To the best of our knowledge,this is the first example for photosynthesis of 3-benzylquinoxalin-2(1H)-ones under transition-metal-free and oxidant-free conditions.Further synthetic applications of this protocol are currently ongoing in our laboratory.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work,there is no professional or other personal interest of any nature or kind in any product,service and/or company that could be construed as influencing the position presented in,or the review of,the manuscript entitled“Direct benzylation reactions from benzyl halides enabled by transition-metal-free photocatalysis”.
Acknowledgments
We acknowledge the financial support from the National Natural Science Foundation of China(Nos.21971224,22071222,22171249),111 Project(No.D20003),the Key Research Projects of Universities in Henan Province(No.21A150053),the Natural Science Foundation of Henan Province(No.202300410375),China Postdoctoral Science Foundation(No.2021M692906)and Henan Postdoctoral Foundation(No.202003014).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.03.096.
 Chinese Chemical Letters2022年12期
Chinese Chemical Letters2022年12期
- Chinese Chemical Letters的其它文章
- Diverse strategic approaches en route to Taxol total synthesis
- Recent advances in gold-complex and chiral organocatalyst cooperative catalysis for asymmetric alkyne functionalization
- Unmodified methodologies in target discovery for small molecule drugs:A rising star
- Recent advances in single-crystalline two-dimensional polymers:Synthesis,characterization and challenges
- Environmental applications of graphene oxide composite membranes
- Recent advances in the application of metal organic frameworks using in advanced oxidation progresses for pollutants degradation
