Visible-light-induced novel cyclization of 2-(2-(arylethynyl)benzylidene)-malononitrile derivatives with 2,6-di(tert-butyl)-4-methylphenol to bridged spirocyclic compounds
Xiofei Xie ,Lei Wng ,c,*,Qun Zhou,Yongmin M ,Zhi-Ming Wng,Pinhu Li
a Advanced Research Institute and Department of Chemistry,Taizhou University,Taizhou 318000,China
b Department of Chemistry,Anhui Polytechnic University,Wuhu 241000,China
c State Key Laboratory of Organometallic Chemistry,Shanghai Institute of Organic Chemistry,Shanghai 200032,China
Keywords:2-(2-(Arylethynyl)benzylidene)malononitriles 2,6-Di(tert-butyl)-4-methylphenol Bridged spirocyclic compounds Visible-light-induced organosynthesis
ABSTRACT A green and highly efficient strategy for the preparation of bridged spirocyclic compounds via visiblelight-induced cyclization of 2-(2-(arylethynyl)benzylidene)malononitrile derivatives with 2,6-di(tertbutyl)-4-methylphenol(BHT)at room temperature was developed.The photoinduced radical reactions generated the corresponding products in good yields under simple and mild reaction conditions.
Spirocycles are a class of privileged structural motifs,which occur in biologically active molecules,natural products,and chiral ligands,and have attracted a great deal of attention[1–3],especially for the bridged spirocyclic compounds,such as(−)-cylindricine C,FR901483 and TAN1251C as novel tricyclic azaspirane cores,spiroaspertrione A,andiconin B and grayanotoxin VII as shown in Fig.1[4–6].Therefore,the high value of these compounds and the growing interest in chemistry call for efficient synthetic methods[7–9].Generally,the synthesis of spirocyclics mainly depends onipso-annulation through dearomatization,including transition-metal-mediated dearomatization,nucleophilic or electrophilic dearomatization,and oxidative dearomatization[10–12],as well as cascadeipso-cyclization reactions of alkynesviaradical process[13–15].
Quinones,quinonemethides,and their phenolic precursors are widely distributed in natural products.A variety of phenolic and quinonoid compounds as biologically active secondary metabolites[16],are present in plants[17]and insects[18,19].The quinone derivatives have aroused great interest for chemists due to their unique structures and high reactivity,and they widely used in the synthesis of organic compounds,electron chemistry[20]and materials chemistry[21–23].In order to realize the quinones,Kita
*Corresponding authors.
E-mail addresses:leiwang@chnu.edu.cn(L.Wang),pphuali@126.com(P.Li).[24–26]and Ciufolini[27,28]reported the pioneering works to construct spirocyclic compounds by the oxidiation of phenol and subsequently transformations(Schemes 1a and b).Very recently,Zhang and Yang reported the electrooxidative dearomatization of biaryls for the synthesis of tri-and difluoromethylated(CF3and CHF2)spiro[5.5]trienones(Scheme 1c)through a cascade radicalipso-cyclization reactions of alkynes,respectively[29,30].
As we all known,photoredox catalysis emerging as a powerful tool in synthetic chemistry have met to the demands of reaction economy,operational simplicity and environmental friendliness,and it received great attention and a number of important achievements have been made[31–37].On the basis of our exploration in photocatalysis and related works[38–46],we herein reported a highly efficient strategy for the preparation of bridged spirocyclic compoundsviaa visible-light-induced cyclization of 2-(2-(arylethynyl)benzylidene)malononitrile derivatives with 2,6-di(tert-butyl)-4-methylphenol(BHT)at room temperature.The photoinduced radical reactions generated the corresponding products in good yields under simple and mild reaction conditions(Scheme 1d).
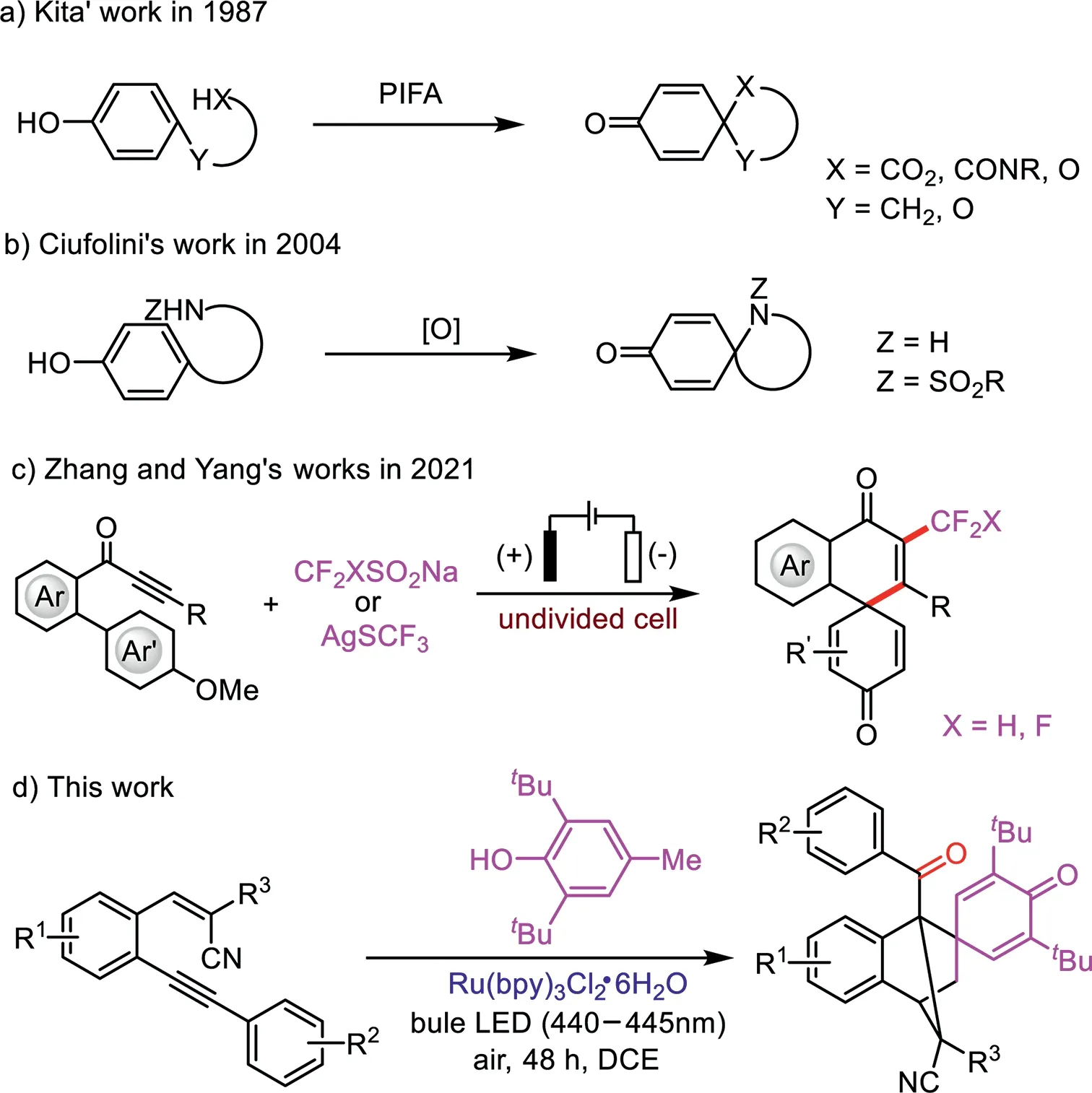
Scheme 1.Representative synthesis of spirocyclic quinones.
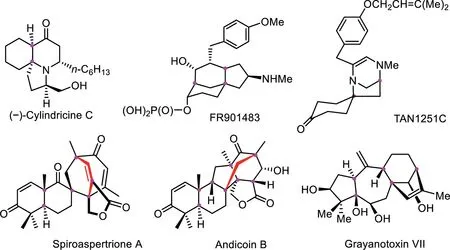
Fig.1.Bridged spirocyclic compounds with biological activity.
According to the strategy outlined in Scheme 1d,the model substrates 2-(2-(phenylethynyl)benzylidene)malononitrile (1a)and 2,6-di(tert-butyl)-4-methylphenol(2)were chosen to explore the optimal reaction conditions through the variations of photocatalyst,solvent,light source,molar ratio of 1a/2 and reaction time.To our delight,the reaction underwent smoothly under the irradiation of light emitting diode(LED,380−385 nm)in air with Ir(ppy)3(2 mol%)as a photocatalyst and 1,2-dichloroethane(DCE)as a solvent for 12 h,the desired product 3a was obtained in 22%yield(Table 1,entry 1).As shown in Table 1,an investigation of photocatalysts(PCs),including Ir(ppy)3,Mes-Acr+ClO4−,4CzIPN,TPPT (2,4,6-triphenylpyrylium tetrafluoroborate),Ru(bpy)3(PF6)2,Ru(bpy)3Cl2,rose bengal,eosin Y,Ir[dF(CF3)ppy]2(dtbpy)PF6,and the selected LED source matching them in their maximum absorption ranges,respectively.For example,blue LEDs used for Ru(bpy)3(PF6)2,Ru(phen)3Cl2,TPPT,Mes-Acr-Me+ClO4−and Ru(bpy)3Cl2;while green LED used for eosin Y and UV LED used for Ir(ppy)3[12],demonstrated that Ru(bpy)3Cl2and blue LED irradiation was the most effective ones(entries 1−10).Meanwhile,blue LED(440−445 nm)was selected for the irradiation source for its highest yield of the desired product among the selected blue LEDs(450−455 nm,435−440 nm,and 440−445 nm)(entry 7vs.11vs.12).Thereafter,the different solvents such as acetone,MeCN,hexane,EtOAc and dichloromethane(DCM)were examined,indicating that DCE as a solvent is the best of choice(entries 13−17).Moreover,increasing the molar ratio of 2/1a could improve the yield of cyclization product 3a(entry 18vs.12).Further investigation of the reaction time indicated that moderate to good yields of the product 3a were obtained as the reactions were prolonged to 24−48 h,respectively(entries 19−21).In the absenceof light irradiation or photocatalyst,no any product was observed(entries 22 and 23).It is worth noting that only trace amount of the desired product 3a was detected observed when the reaction was performed in nitrogen atmosphere,(entry 24).Finally,the optimal reaction conditions are consist of 1.0 equiv.of 1a,3.0 equivalents of 2,and 5 mol%of Ru(bpy)3Cl2dissolved in DCE and irradiated with a blue LED(450−455 nm)for 48 h.
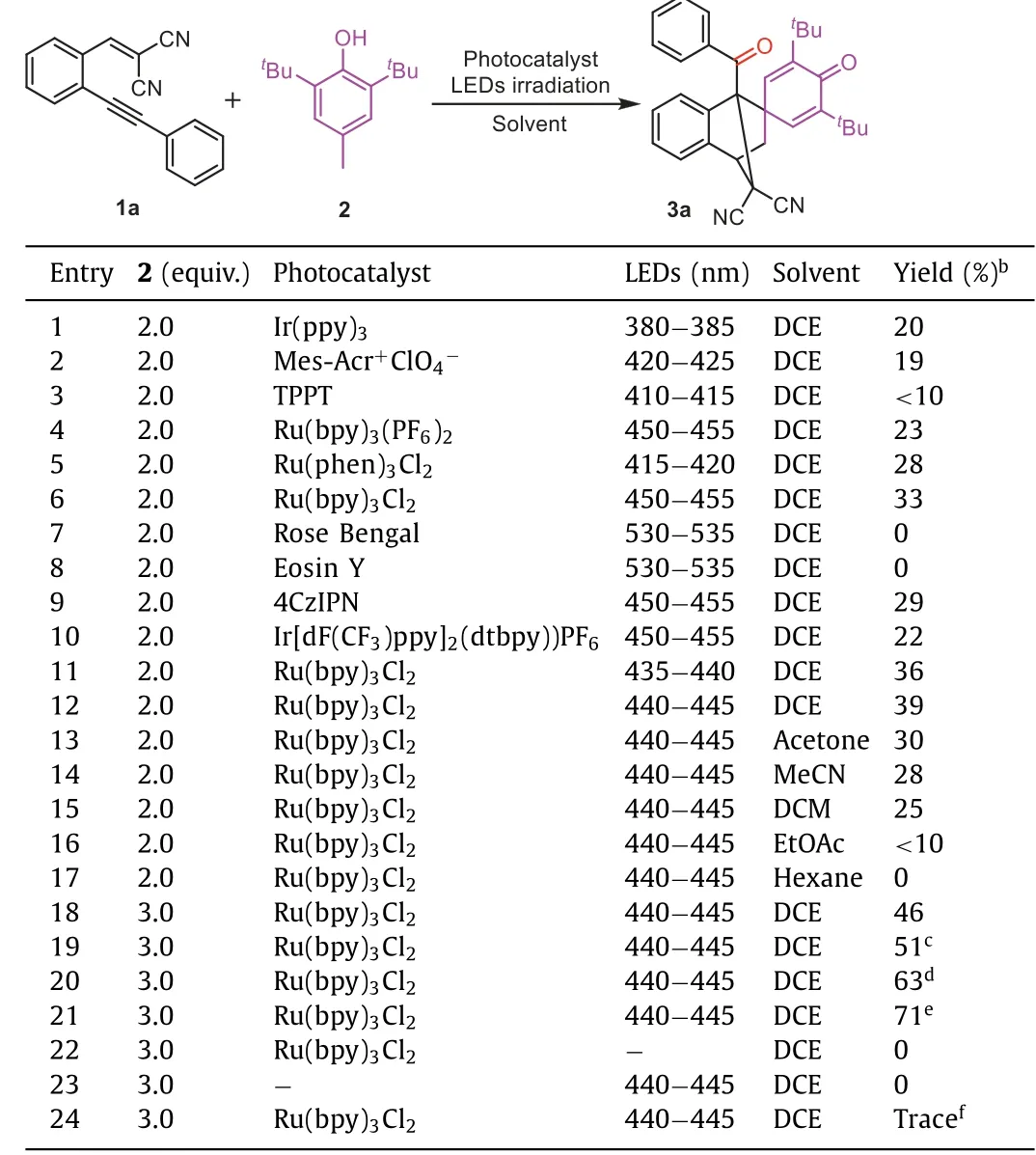
Table 1 Optimization of the reaction conditions.a
After learning the optimal conditions of the reaction,we investigated the universality of this transformation.First,a variety of 2-(2-(arylethynyl)benzylidene)malononitriles(1),derived fromortho-arylethynyl arylaldehydes and malononitrile,reacted with BHT(2)smoothly to generate the corresponding products 3a−3r in moderate to good yields,as shown in Scheme 2.2-(2-(Arylethynyl)benzylidene)malononitriles(1)with an electronwithdrawing group(R2=F,Cl,Br)or an electron-donating group(R2=Me,Et,tBu)at thepara-position of the benzene rings provided the desired products(3b–3g)in 50%–65%yields.It should be noted that 2-(2-(phenylethynyl)benzylidene)malononitrile(1a)and 2-(2-((p-phenyl)phenylethynyl)benzylidene)malononitrile(1h)produced the corresponding products 3a and 3h in 71%and 55%yields,respectively.When a substituent(R2=F,or MeO)was placed at themeta-position of the phenyl rings,the reaction produced 60%yield of 3i,and 50%yield of 3j.Subsequently,the scope of the substituted group(R1)on substrates 1 was also examined(Scheme 2).The electron-rich group(R1=Me)or electron-poor group(R1=F,Cl,F3C)on the aromatic rings delivered the corresponding products(3k–3o)in 45%−61%yields,neglecting the steric hindrance effect(3o).In addition,substrates containing naphthyl(1p)or thiophenyl group(1q)reacted with 2 to produce the anticipated products 3p and 3q in 72%,and 63%yields,respectively.Remarkably,substrate derived fromortho-(cyclopropyl)ethynylbenzaldehyde(1r)was also well tolerated in this transformation,and the desired product(3r)was obtained in 44%yield.Furthermore,this visible-light-induced intermolecular cyclization of 1a with 2 was scaled up to 2.0 mmol for practical applications,generating 3a in 62%yield.

Scheme 2.The scope of substrates 1.Reaction conditions:1(0.20 mmol),2(0.60 mmol),Ru(bpy)3 Cl2(5.0 mol%),DCE(2.0 mL),room temperature,air atmosphere,under blue LED(440–445 nm)irradiation for 48 h;isolated yield of the product;a isolated yield of 3a in 2.0 mmol scale.
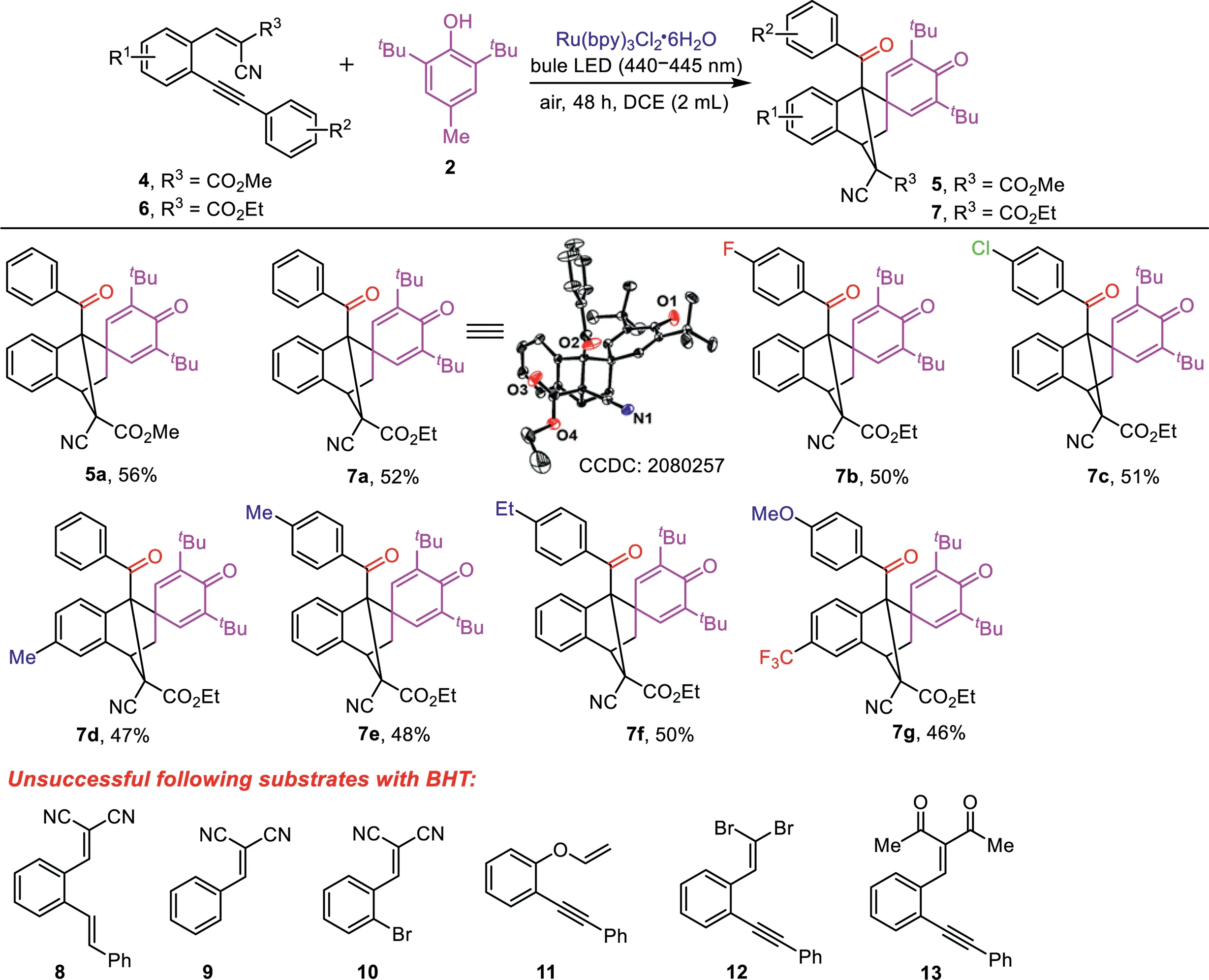
Scheme 3.The scope of substrates 4 and 6.Reaction conditions:4 or 6(0.20 mmol),2(0.60 mmol),Ru(bpy)3 Cl2(5.0 mol%),DCE(2.0 mL),room temperature,air atmosphere,under blue LED(440–445 nm)irradiation for 48 h;isolated yield of the product.
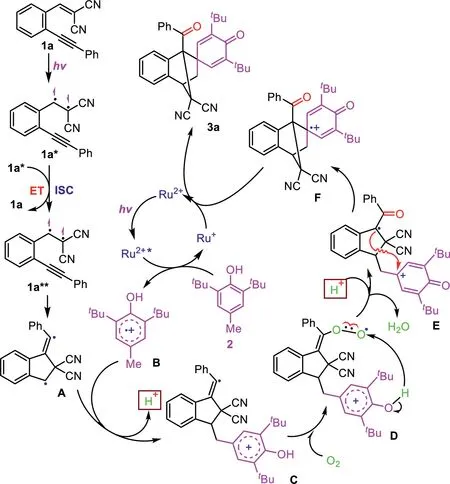
Scheme 4.The proposed mechanism.
Eventually,the scope of substrates(4 and 6),which derived fromortho-(arylethynyl)arylaldehydes with methyl 2-cyanoacetate and ethyl 2-cyanoacetate was investigated,the results are illustrated in Scheme 3.Substrate 4a and a variety of substrates 6,including 6a−6g reacted with BHT(2)to provide the corresponding products 5a and 7a−7g in 46%−56%yields.It is important to note that the structure of obtained product 7a was confirmed by X-ray crystal diffraction analysis(see Supporting information for detail).The influence of the substituents(R1=Me,F3C)from the arylaldehydes,and the substituents(R2=F,Cl,Me,Et,MeO)in the aromatic alkyne units,on the reaction indicated that they are well tolerated.
However, the following substrates (E)-2-(2-styrylbenzylidene)malononitrile (8), 2-benzylidenemalononitrile(9),2-(2-bromobenzylidene)malononitrile(10),1-(phenylethynyl)-2-(vinyloxy)benzene(11),1-(2,2-dibromovinyl)-2-(phenylethynyl)benzene(12)and 3-(2-(phenylethynyl)benzylidene)pentane-2,4-dione(13)reacted with 2(Scheme 3),and the substrates including phenol,p-cresol,p-benzenediol, 2,6-dimethyl-p-benzenediol,2,4-dimethylphenol, 2,4,6-trimethylphenol, 2,6-di-tert-butyl-4-ethylphenol,6-tert-butyl-2,4-xylenol and naphthalen-1-ol reacted with 1a under the standard conditions,but failed.
On the basis of the above results and related reports[47,48],a plausible mechanism is proposed in Scheme 4.First,1a is excited by blue LED irradiation to generate its excited singlet species 1a*,which decays to its triplet exciplex 1a**through an intersystem crossing(ISC)process with formed another 1a*along with the formation of 1aviaan energy transfer(ET).The formed 1a**undergoes an intramolecular radical addition to afford a five-membered ring diradical intermediate A,which is trapped by BHT and confirmed by HRMS analysis(see Supporting information for detail).On the other hand,[Ru(bpy)3]2+is excited by the blue LED irradiation to its excited state[Ru(bpy)3]2+*,which then undergoes a single-electron transfer(SET)process with BHT(2)to afford a radical cation intermediate B and a[Ru(bpy)3]+.Then,a reaction of intermediates A and B generates intermediate C,which is oxidized by oxygen in the air to give intermediate Eviaan intermediate D.Finally,the obtained E undergoes an intermolecular cyclization to afford intermediate F,which is followed by an SET reduction with[Ru(bpy)3]+to provide the desired product 3a along with the formation of[Ru(bpy)3]2+for the catalytic cycle.To support the reaction mechanism,the ultraviolet-visible spectra of 1a indicated that it has stronger absorption around 430 nm and acts as a photosensitizer(Fig.S1 in Supporting information).In addition,the key intermediate A was captured BHT and its corresponding adduct was detected by high-resolution mass spectrum(HRMS)analysis(Fig.S2 in Supporting information).
In conclusion,we have developed a novel radical cyclization strategy to access bridged spirocyclic compounds from 2-(2-(arylethynyl)benzylidene)malononitriles and their derivatives with 2,6-di(tert-butyl)-4-methylphenol(BHT)under the irradiation of visible light(blue LED with 440–445 nm).The reactions generated the corresponding products in good yields under mild conditions.Further investigations on the detailed mechanism and the applications of this transformation are currently underway in our laboratory.
Declaration of competing interest
The authors declare that they have no financial and personal relationships with other people or organizations that can inappropriately influence their work.There is no professional or other personal interest of any nature or kind in any product,service and/or company that could be construed as influencing the position presented in,or the review of,the manuscript entitled.
Acknowledgments
We gratefully acknowledge the National Natural Science Foundation of China(No.22071171)and the Natural Science Foundation of Zhejiang Province(No.LZ22B020003)for financial support of this work.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.03.084.
 Chinese Chemical Letters2022年12期
Chinese Chemical Letters2022年12期
- Chinese Chemical Letters的其它文章
- Diverse strategic approaches en route to Taxol total synthesis
- Recent advances in gold-complex and chiral organocatalyst cooperative catalysis for asymmetric alkyne functionalization
- Unmodified methodologies in target discovery for small molecule drugs:A rising star
- Recent advances in single-crystalline two-dimensional polymers:Synthesis,characterization and challenges
- Environmental applications of graphene oxide composite membranes
- Recent advances in the application of metal organic frameworks using in advanced oxidation progresses for pollutants degradation
