Fabricating a novel supramolecular light-activated platform based on internal-driven forces induced by the UV-light
Xiaoni Qi,Weichun Li,Bingbing Shi,Youming Zhang,Hong Yao,Qi Lin,Taibao Wei
Key Laboratory of Eco-Environmental Polymer Materials of Gansu Province,C ollege of Chemistry and Chemical Engineering,Northwest Normal University,Lanzhou 730070,China
Keywords:Light-activated platform Pillar[5]arene Phenazine derivatives Internal-driven forces UV-light
ABSTRACT Recently,exploiting a novel supramolecular fabrication pathway have drawn great attention.To this endeavor,we firstly designed and reported an original light-activated platform based on the internal-driven forces of macrocyclic host by hiring the pillar[5]arene as the host molecule(H)and phenazine derivatives acting as an energetic guest molecule(G).Surprisingly,after adding the H solution into G system,the intensive fluorescence emission of the G molecule rapidly decreased under the irradiation of the UV-light(254 nm)until absolutely quenching.Delightfully,different from the traditional supramolecular host-guest interaction,the fluorescent emission of G molecule could be recovered after irradiating under the nature light.In view of this interesting observations,the interaction mechanism was carefully investigated by a series of characterizations.Those results suggested that the G molecule was easily threaded into the macrocyclic cavity(H)under the internal-driven forces induced by the UV-light irradiation,forming a 1:1 host-guest complex.Moreover,taking advantage of this especial feature,the light-activated platform of host–guest complex was further applied for ink-free light-driven printing materials,exhibiting great potential in the real application.
With the rapid development of supramolecular self-assembly,it has drawn considerable interesting to fabricate supramolecular multifunctional systems[1–4].To control the self-assembly behavior based on host-guest interactionvianon-covalent driving forces is a major path to approach this goal[5–8].At present,a variety of advances have been derived from substantial efforts in the aspects of fabricating supramolecular multifunctional systems by regulating the behavior properties of host-guest complex[9–11].According to the related reports[12–15],with the aid of the macrocyclic host,the behavior properties(such as fluorescence performance)of some discrete guest molecule could be suitably adjusted by weak non-covalent interactions(including the hydrogen bond,π−πstacking,C–H···πinteraction,etc.),breaking thought the existing limitations[16–21].However,with comparison to the traditional host-guest complex,exploiting the external stimulus to force the formation of the self-assembly complexviasupramolecular interaction remains rarely explored[22,23].Therefore,attempting to develop the novel pathways that could controllably tune the behavior properties of guest molecule under different external stimulus(such as acid,base and heat)viathe weak non-covalent interactions is highly desirable.For instance,Liet al.[24]reported a supramolecular switch trigged by the acid/base based on a host–guest complex,exploiting a water-soluble pillar[5]arene as the macrocyclic host to dominate the fluorescence properties of tetraphenylethene(TPE)molecule.
This design strategy paved a way for controlling the fluorescent behavior of guest moleculeviahost-guest interaction.Although minor advances have been gained for achieving various host-guest complex,fabricating an effective platform to realize the formation of the host-guest complex by exploiting the itself internal-driven forces of macrocyclic host induced by the UV-light(254 nm)is rarely reported in the field of supramolecular self-assembly.
Herein,a novel strategy,that is,supramolecular light-activated platform based on internal-driven forces of macrocycle host induced by the UV-light was fabricated,by using the pillar[5]arene as macrocyclic host molecule(H)due to its good performance[25–29].Moreover,an original phenazine compounds was served as a vigorous player(G).After adding the H solution into the G system,the remarkable fluorescence emission of G molecule gradually decreased until absolutely quenching under the irradiation of the UVlight.According to the related characterization analysis,the macrocyclic host(H)easily produced the internal-driven forces under the UV-light irradiation and compelled the G molecule to encapsulate into/out the cavity of H molecule as shown in Scheme 1.Different from the previous efforts[30–34],it is the first example to achieve the host-guest complex by itself forces of macrocyclic host induced by UV-light.
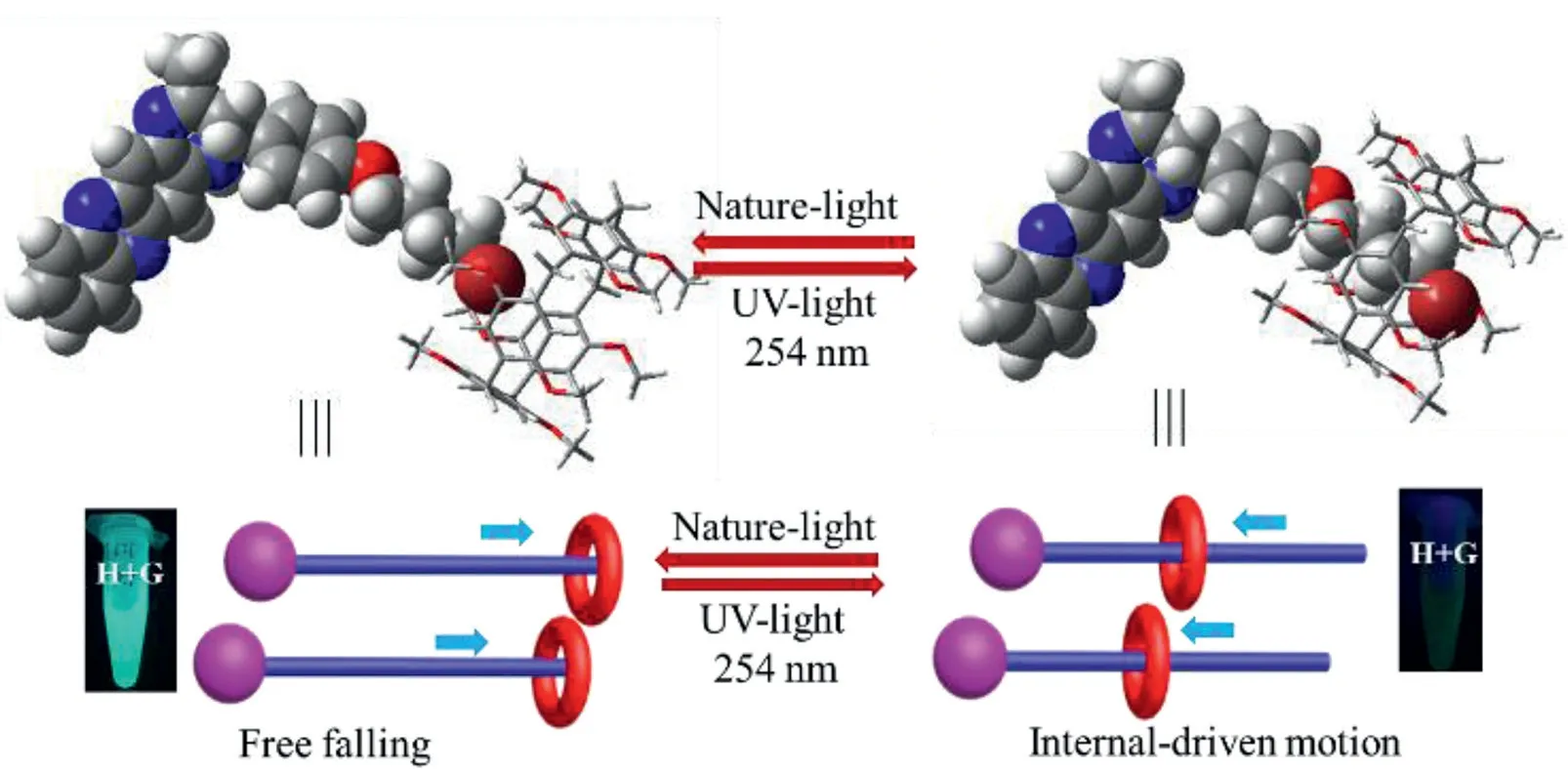
Scheme 1.Cartoon representations of internal-driven interaction induced by the UV-light(254 nm).
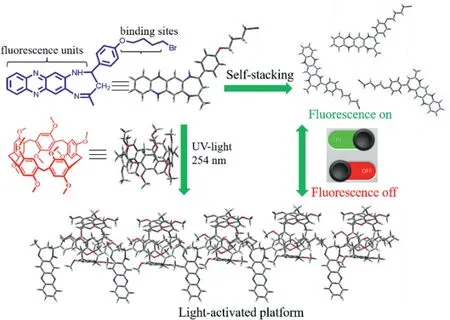
Scheme 2.The interaction mechanism of H+G light-activated platform.
In order to fabricate a light-activated platform based on host-guest interaction,a photo-response group with fluorescence switching characteristic and electron deficient alkyl chain serving as the axis of movement were desired.Herein,a phenazine derivatives(G)satisfying the above requirements was prepared.As shown in Scheme 2,fluorescence units of G molecule offered the switching characteristic and the butenyl chain acted as the mechanical motor upon irradiating by the UV-light.As described in Fig.1A,when added the H solution into the G solution and irradiated by the UV-light for few minutes,the corresponding fluorescence emission of G molecule changed from“turn on”to“turn off”.Nevertheless,the free G and H molecule have hardly distinct change on their fluorescence emission behavior under same irradiation conditions,which indicated that the G molecule could interact with H molecule after irradiation by the UV-light,forming the H+G complex and leading to fluorescence quenching of G molecule.
After carefully investigating,we observed that the fluorescence intensity of H+G system could be absolutely quenched in 4 min at most as shown in Fig.1B,but the fluorescence intensity of the free G and H emerged tiny change both irradiating in 0 min and 4 min,respectively.By comparing with the controlled arrays,fully illustrated that the UV-light was probably the main driven forces to prompt the formation of H+G complex according to the fluorescence response properties.Besides,in order to further verify the effect of irradiation time on the fluorescence performance of the H+G system,the different irradiation time were arranged to
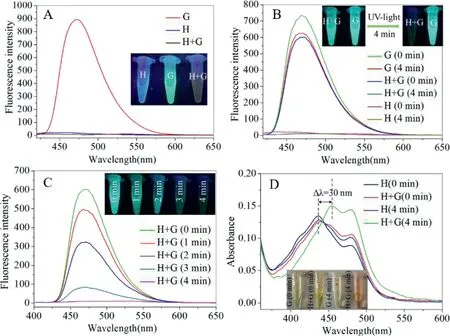
Fig.1.(A)The fluorescence spectrum of H(20μmol/L),G(20μmol/L)and H+G in the CHCl3 solution.(B)The fluorescence spectrum of H+G(1:1)and H after irradiating for 0 min and 4 min,respectively under UV-light.(C)The fluorescence spectrum of H+G(1:1)irradiating for different times.(D)The absorption spectrum of the G(irradiation 0 and 4 min)and H+G(irradiation 0 and 4 min)under UV-light.
measure the fluorescence response properties.As shown in Fig.1C,with irradiation time increasing from 0 to 4 min,the fluorescence intensity gradually reduced and almost fully quenched in 4 min.However,different from an obvious change of H+G system,the fluorescence emission of the free G has no evident change under the same irradiation conditions as shown in supporting information(Video S1 in Supporting information).The corresponding fluorescence photographs were displayed in the Fig.1C.The above results richly demonstrated that the optical behavior of single G and H molecule was completely different from the H+G complex system under UV-light irradiation,which further verified that it is probably a new constructing pathway to form host-guest complex based on the UV-light driven forces.Interestingly,the fluorescence emission of H+G complex could recover after being exposed on the nature light for 24 h as shown in Fig.S11(Supporting information),illustrating a reversible process to disassembly the H+G complex.Meanwhile,the arrays of reversible cycle of H+G complex were performed as shown in Fig.S12(Supporting information).According to the UV-spectrometry characterizations,the maximum absorption of the H+G complex suppressed with irradiation appeared an obvious red-shift as shown in Fig.1D,but no distinct variation was observed at the controlled array with no irradiation.This result also can be discovered in the1H NMR without irradiation as shown in Fig.S14(Supporting information),which adequately explained that the UV-light irradiation might be a primary driven factors to form the host-guest complex.Hence,we pictured the interaction mechanism of H and G as shown in Scheme 2,the H molecule could produce the internal-driven forces irradiated by the UV-light,compelling the G molecule to encapsulate into/out the cavity,changing the packing modes of G molecule and regulating the fluorescence emission of G at“turn on”or“turn off”state.
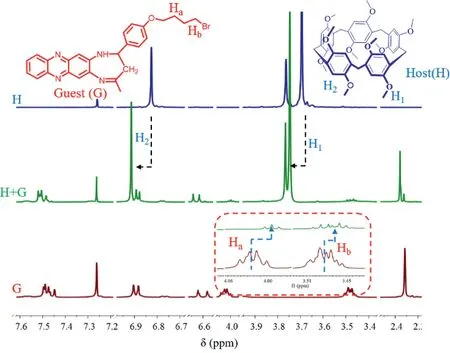
Fig.2.Partial 1H NMR spectra of H(5 mg),G(5 mg)and H+G(5 mg)irradiation for 4 min in CDCl3.
Afterwards,to further characterize the formation of H+G complex,we carried out a series of characterizations to get the evidence of forming H+G complex,including the1H NMR spectrometry,2D-NOSEY NMR,HR-MS,SEM analysis,theoretical calculation(DFT)and controlled arrays.As displayed in Fig.2,the chemical shift of H1and H2centering on the H molecule remarkably shifted downfield and the Haand Hblocating at butenyl chain(G molecule)shifted up-field,which revealed that the alkyl group have been encapsulated into the macrocyclic cavity of H molecule,occurring the weak-interaction.Meanwhile,the HR-MS analysis have also given valid evidence to support the formation of H+G complex and indicated a 1:1 host-guest binding ratio as shown in Fig.S13(Supporting information).We collected the corresponding data under the positive mode and suggested that the theoretical molecular weight of H+G should be[M+H]+=1239.46940(formula:C71H76N4O10Br)and found the actual data was to be[M+H]+=1239.46558.Besides,the 2D-NOSEY NMR characterizations illustrated that the certain correlation of H2and Haas shown in Fig.S15(Supporting information).In order to further explore the self-assembly behavior of H+G complex,the scanning electron microscope(SEM)was applied to inspect the morphology characterizations.As shown in Fig.S17(Supporting information),the H+G complex exhibited an obvious coral-like layered structure,completely different from the morphology features of the single G and H molecule bearing with a ball-bubble structure and prismatic structure,respectively.This observation also suggested that the host-guest assembly process of H+G complex certainly occurred in microscopic behavior.
Subsequently,to gain the deeper insights into the assembly mechanism of H and G,we exploited the quantum chemical calculations to investigate the host-guest interactions of H+G complex.The structure optimization of the H,G and H+G complex are performed by density functional theory(DFT)calculations by using the B3LYP/6-311G*(d,p)to investigate the change of energy gap and weak interaction.Firstly,the energy gap of the H+G(0.10220 eV)presented a pronounced decrease by comparing with the energy gap of the single G(0.11296 eV)and H(0.39507 eV)as shown in Fig.S16(Supporting information),which powerfully indicated that assembly system is much more advantageous to motivate the electronic from the excited state to ground state[35],which was consistence with the UV-spectrum analysis.Secondly,according to the electrostatic surface potential(ESP)analysis of the H,G and H+G,the ESP of H exhibited a deep red,manifesting that H endows exclusive electron-rich structure.The ESP of the G emerged a light green,which suggested that the alkyl group of the G molecule endowed with an electron-deficient features.Nevertheless,the ESP of the H+G displayed a light green after forming the host-guest complex,explaining that the part of alkyl units of G molecule was easily embedded in the cavity of H molecule though the weak interactions and leading to the transformation in charge distribution.Moreover,in order to verify the weak interactions between G and H,we analyzed the reduced density gradient(RDG).The visual results of weak interactions of H+G were displayed in Figs.3A and B,a solid weak interaction manner was clearly exhibited in the interlayer spaces(light green parts).Meanwhile,in order to further discuss the weak interactions,the interaction iso-surfaces between H and G were described by the independent gradient model(IGM)(Fig.3C).As we expected,with the low-density,low-gradient spike lying at negative values,suggested a stable weak interaction between G and H.Those observation indicated that C–H···πinteractions and the van der Waals force surely existed between the G and H according to the iso-surfaces analysis.Certainly,the chemical shift of Hc,Hdand Helocating at the G molecule shifted downfield as shown in Fig.S18(Supporting information)and further supported the above proposed mechanism occurring in the H+G complex.
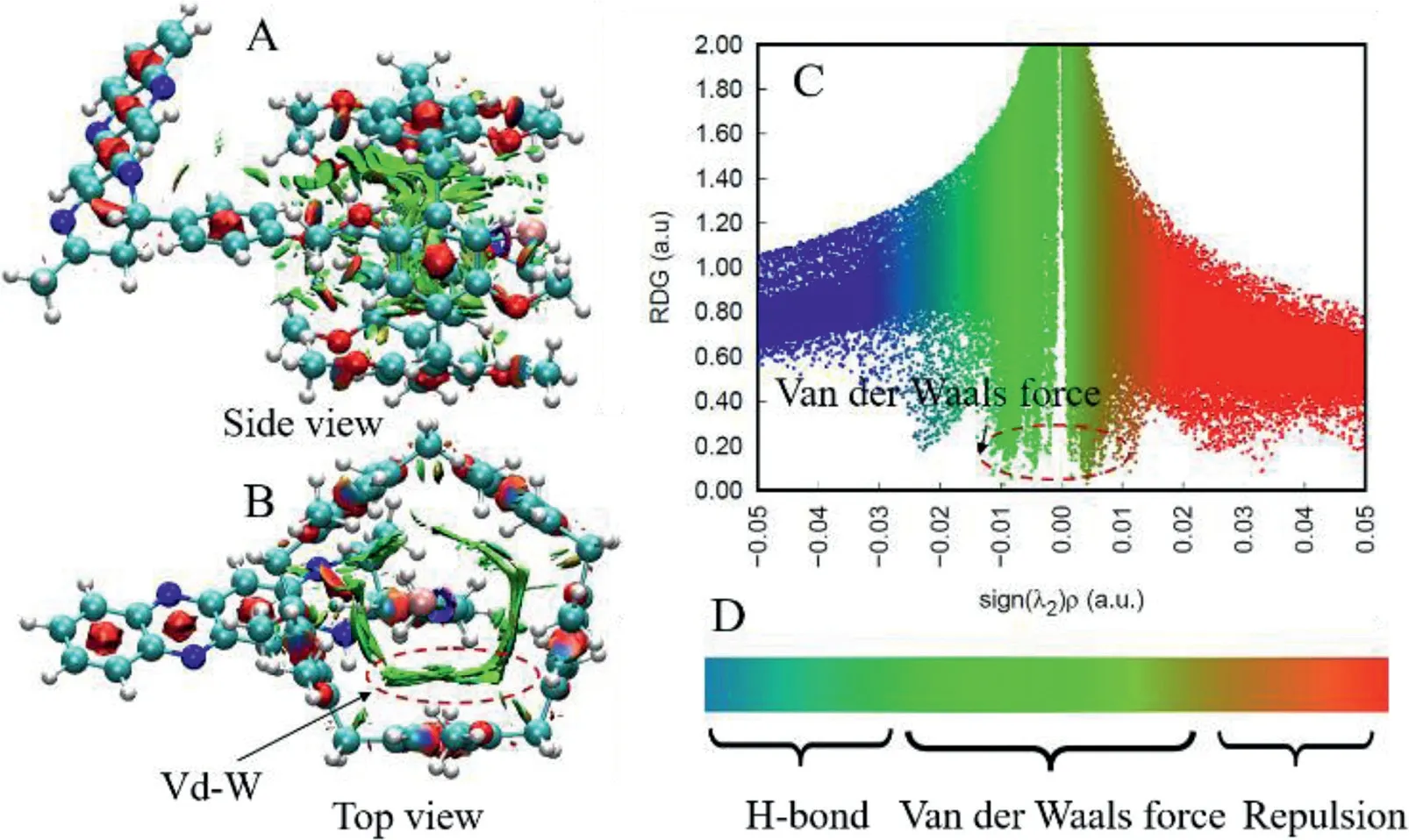
Fig.3.The weak interaction of H+G system(A)top view;(B)side view;(C)plots of the independent gradient model(IGM)of the H+G;(D)the scale label of interaction forces.
Moreover,to verify the availability of this novel approach for constructing host-guest complex based on the UV-light driven forces,we prepared the other relevant controlled host compounds(H1,H2,H3)and guest molecule(G1,G2,G3)as displayed in Figs.S19–S29(Supporting information)and corresponding Video(Videos S2–S7 in Supporting information).According to corresponding observations,suggested that the H+G1,H1+G and H2+G system could produce light-driven behavior being similar with the H+G system.Furthermore,we also carried out the other comparatives experiments in different conditions,including the darkness,daylight,in candescent light,single wavelength visible light,temperature and concentration as shown in Table S1(Supporting information).Those complementary results suggested that the UVlight(254 nm)might be the main factor to induce the H molecule to produce the internal-driven forces.In addition,taking into consideration of this excellent properties,this novel host-guest(H+G)system was applied for light-driven printing technology as shown in Fig.4[36,37].
This observation indicated that this novel light-activated platform could be used for exploring the light printing materials and presented great application potential in near future.
In conclusion,we firstly constructed and reported a novel supramolecular light-activated platform by exploiting the internaldriven force of the H induced by the UV-light.Serving as the macrocyclic host,controllably regulate the luminescent behavior of G molecule based on supramolecular force.Those results suggested that the G molecule was easily threaded into the macrocyclic host(H)cavity,flexibly dominating the fluorescence performance on the“on”or“off”stateviaan effective shuttle movement upon irradiating by the UV-light.Meanwhile,other controlled arrays have also further verified this fabrication strategy was available to design and realize other similar host-guest complex by UV-light driven forces.Moreover,in view of this features of the new host-guest complex,the lightactivated platform of host–guest complex was further applied for ink-free printing materials,not only exhibiting great potential in the practical application,but also providing a referable guidance for exploring and constructing new supramolecular synthesis path.
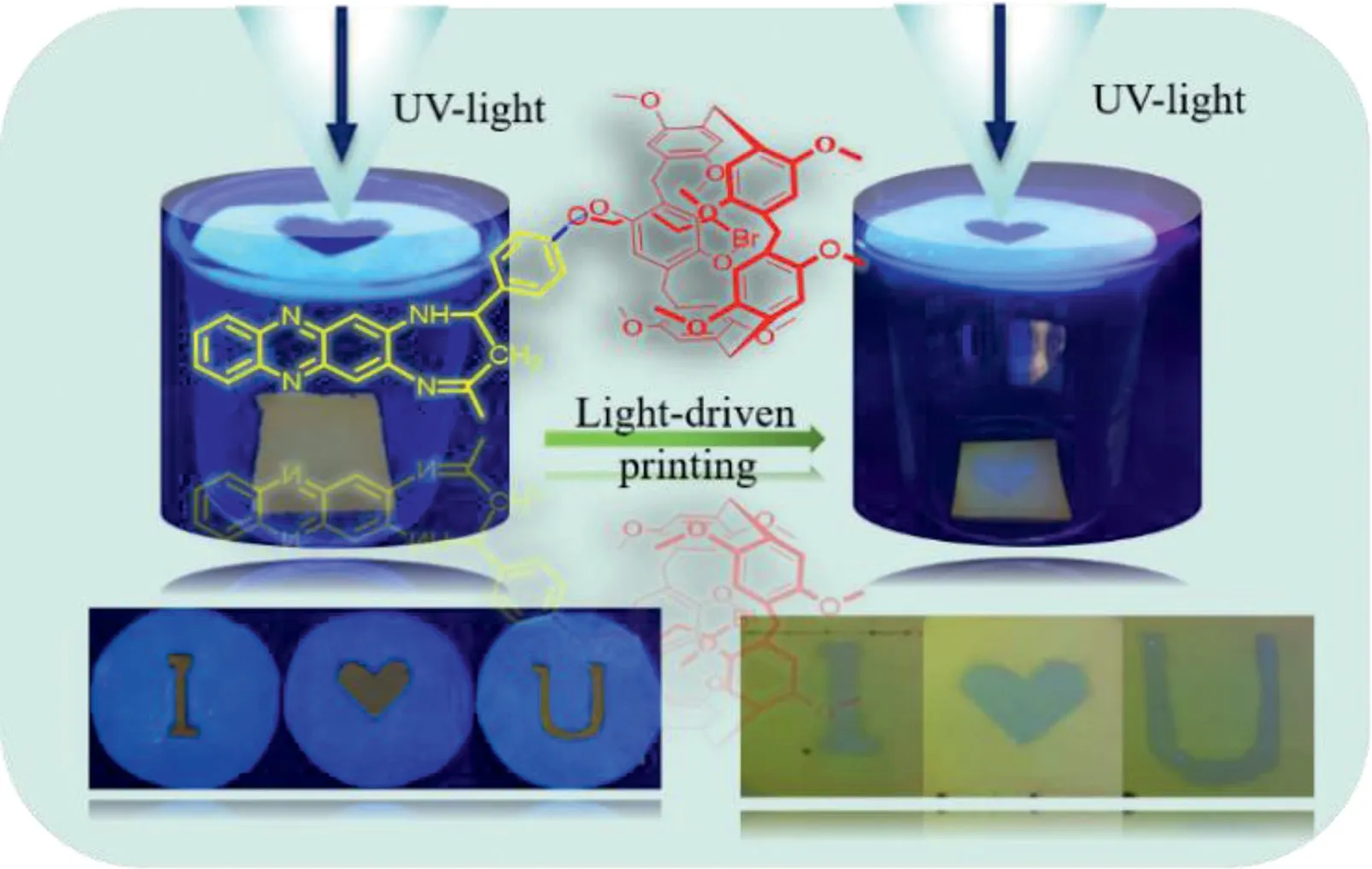
Fig.4.The application of light-driven printing based on the H+G complex.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China(NSFC,Nos.22165027,22061039,22001214),Gansu Province Innovation Star(No.2021CXZX-183).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.03.081.
 Chinese Chemical Letters2022年12期
Chinese Chemical Letters2022年12期
- Chinese Chemical Letters的其它文章
- Diverse strategic approaches en route to Taxol total synthesis
- Recent advances in gold-complex and chiral organocatalyst cooperative catalysis for asymmetric alkyne functionalization
- Unmodified methodologies in target discovery for small molecule drugs:A rising star
- Recent advances in single-crystalline two-dimensional polymers:Synthesis,characterization and challenges
- Environmental applications of graphene oxide composite membranes
- Recent advances in the application of metal organic frameworks using in advanced oxidation progresses for pollutants degradation
