Mn-mediated reductive C(sp3)–Si coupling of activated secondary alkyl bromides with chlorosilanes
Lingling Qi ,Xiobo Png ,Ki Yin,Qiu-Qun Pn,Xio-Xue Wei ,Xing-Zhong Shu,*
a State Key Laboratory of Applied Organic Chemistry(SKLAOC),College of Chemistry and Chemical Engineering,Lanzhou University,Lanzhou 730000,China
b School of Chemistry and Chemical Engineering,Southeast University,Jiangsu Optoelectronic Functional Materials a nd Engineering Laboratory,Nanjing 211189,China
c Zhejiang Nanjiao Chemistry Co.,Ltd.,Shangyu Economic and Technological Development Zone,Shangyu 312369,China
Keywords:Organosilanes Alkylsilanes Reductive coupling Chlorosilanes Organophosphorus Sulfones
ABSTRACT The construction of secondary alkylsilanes is a challenging subject in the synthetic community.The crosscoupling provides a practical solution to address this problem,but it typically relies on organometallic species.Herein,we report an Mn-mediated reductive C(sp3)–Si coupling to synthesize these compounds from alkyl and silyl electrophiles.This approach avoids the requirement for activation of Si–Cl by transition metals and thus allows for the coupling of various common chlorosilanes.The reaction proceeds under mild conditions and shows good functional group compatibility.The method offers access toαsilylated organophosphorus and sulfones with a scope that is complementary to those obtained from the established methods.
Alkylsilanes are key structural motifs in many functional molecules,and they have found broad applications in organic synthesis,material science,and medicinal chemistry[1–4].The streamlined synthetic approaches to these compounds involve the treatment of alkyl Grignard/lithium reagents with chlorosilanes[5]and catalytic hydrosilylation of alkenes[6–10].Despite formidable advances,several challenges remain,involving the construction of secondary alkylsilanes[11–16].Transitionmetal-catalyzed C–Si coupling offers an attractive solution to this problem[17–25].For example,the groups of Oestreich and Fu and others have recently reported several elegant works on the synthesis of alkylsilanes by coupling of secondary alkyl electrophiles with silylmetallic species (e.g.,Si–M,M=Li,B,Mg and Zn)(Scheme 1a,path a)[26–33].Meanwhile,Watson and coworkers have achieved the couplings of alkyl Grignard and Zinc reagents with silyl electrophiles(Scheme 1a,path b)[34,35].Alternatively,the reaction of carbon and silyl electrophiles may deliver the same compounds by using more readily available chemicals,but it remains largely unexplored(Scheme 1a,path c).
The cross-electrophile reaction has recently emerged as a promising tool for the construction of C-C bonds[36–44].This method has shown high step-economy,good functionality tolerance,and an orthogonal chemoselectivity to classic crosscouplings.Our group has an ongoing interest in the exploration of new coupling partners for this chemistry[45–50].Very recently,we reported the first reductive C–Si coupling reaction,which allows for the synthesis of aryl and vinylsilanes from carbon electrophiles and chlorosilanes[51].This method was later expanded to forming C(sp3)-Si bonds by Oestreich,Zhang,and our groups using nickel catalysis[52–54].However,due to the low reactivity of Si–Cl towards transition metals[55–57],the nickel-catalyzed route appears to be highly dependent on vinyl chlorosilanes.Moreover,the reaction of secondary alkyl electrophiles remains largely unexplored,and current studies focus on the synthesis ofα-cyano alkylsilanes(Scheme 1b)[52].
Herein,we report an Mn-mediated approach for reductive C(sp3)–Si bond formation.This protocol avoids the requirement for activation of Si–Cl by transition metals,and it thus allows for the synthesis of organosilanes using a wide range of chlorosilanes.The feasibility of this approach is illustrated by the coupling reaction ofα-functionalized alkyl bromides with chlorosilanes(Scheme 1c).
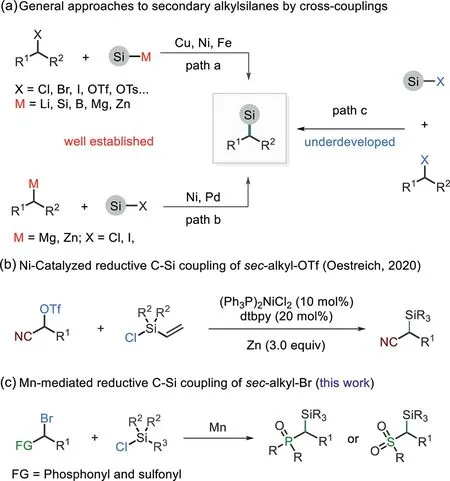
Scheme 1.General methods for the synthesis of sec-alkylsilanes.
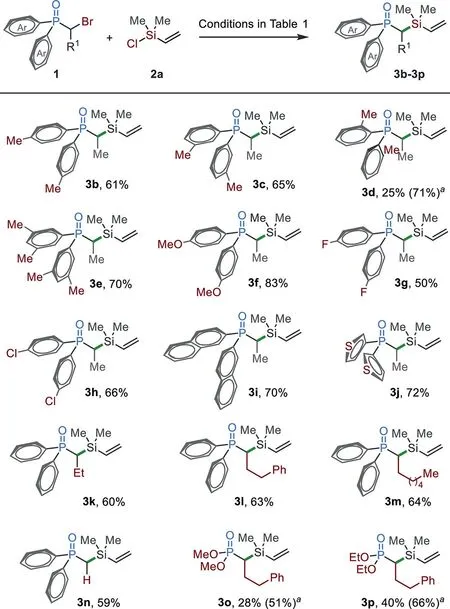
Scheme 2.The substrate scope ofα-bromo organophosphorus.The reaction conditions as shown in Table 1.Bromide 1(0.2 mmol)and chlorosilanes 2a(0.34 mmol)were used;isolated yields are given.a NiI2(5 mol%)was added.
α-Functionalized alkylsilanes are useful building blocks in organic synthesis[58–62].The importance of these structural motifs has triggered continuing efforts for their synthesis.The general approaches to these compounds involve the nucleophilic substitution reactions using strong basic conditions(e.g.,base=n-BuLi,LDA and NaHMDS)[63,64]and carbenes/silenes insertion reactions[65–68].The coupling method was realized by Oestreichet al.,which allows for the synthesis ofα-silyl nitriles/esters fromα-triflyloxy nitriles/esters and silyl organometallic species[29]and chlorosilanes[52].Considering the importance of phosphorus or sulfur in ligand design[69–71]and the broad application of silyl moieties in developing heterogeneous catalysts[72],we here report a new method for the synthesis ofα-silyl organophosphorus and sulfones by reductive C(sp3)–Si bond-forming reaction.
We started our investigation by studying the reaction ofαbromo phosphorus 1a with chlorosilane 2a(Table 1).We found the treatment of them with Mn(3.0 equiv.)in 1,4-dioxane/DMA(4:1,0.1 mol/L)gave the desired product 3a in 65%isolated yield(entry 1).The nitrogen solvent is essential to the success of this reaction,but the addition of 1,4-dioxane improved the yield from 53%to 69%(entries 1–3).Lowing the loading of Mn resulted in decreased yields of 3a;the use of Mn(4.0 equiv.)did not improve the reaction efficiency(entries 4–6).The use of other metal reductants also delivered the target product but gave relatively lower yields(entries 7–9).No reaction was observed in the presence of an organic reductant,TDAE,or in the absence of Mn(entries 10 and 11).
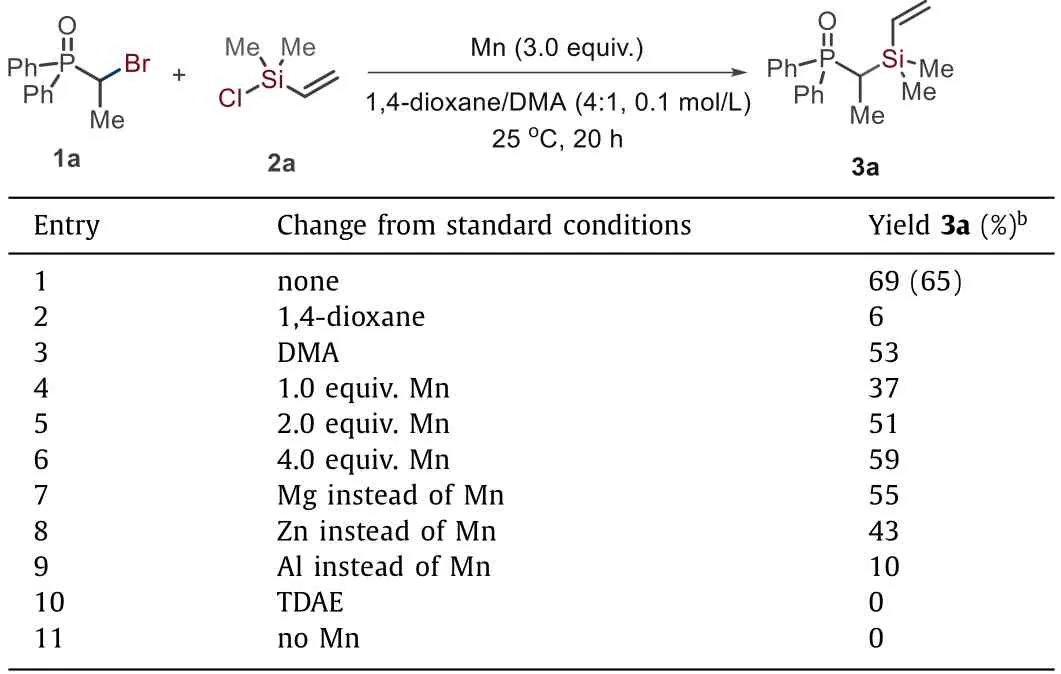
Table 1 Optimization of reaction conditions.a
With the optimized reaction conditions in hand,we then studied the reactions of variousα-bromo organophosphorus 1 with chlorosilane 2a(Scheme 2).Vinyl chlorosilane 2a was used as a coupling partner since this moiety has found promising applications in organic synthesis and material science[73–75].Diarylphosphine oxides,either bearing an electron-rich(3b–3f)or electron-poor(3g and 3h)substituent,were coupled with 2a efficiently.Substituents at thepara-andmeta-positions were tolerated(3b and 3c).However,when anortho-substituted substrate was used,the reaction gave a low yield of 3d.This challenge has been addressed by the addition of NiI2(5 mol%),which significantly improved the yield from 25%to 71%.The reactions of naphthalenyl and heteroaryl substrates afforded 3i and 3j in good yields.The alkyl substituents could be expanded from methyl(3b–3j)and ethyl(3k)groups to long alkyl chains(3l and 3m).The reaction of primary alkyl bromide afforded silylated product 3n in 59%yield.In addition to phosphine oxides,the reactions of phosphonates also afforded the target products 3o and 3p in good yields in the presence of additional nickel.
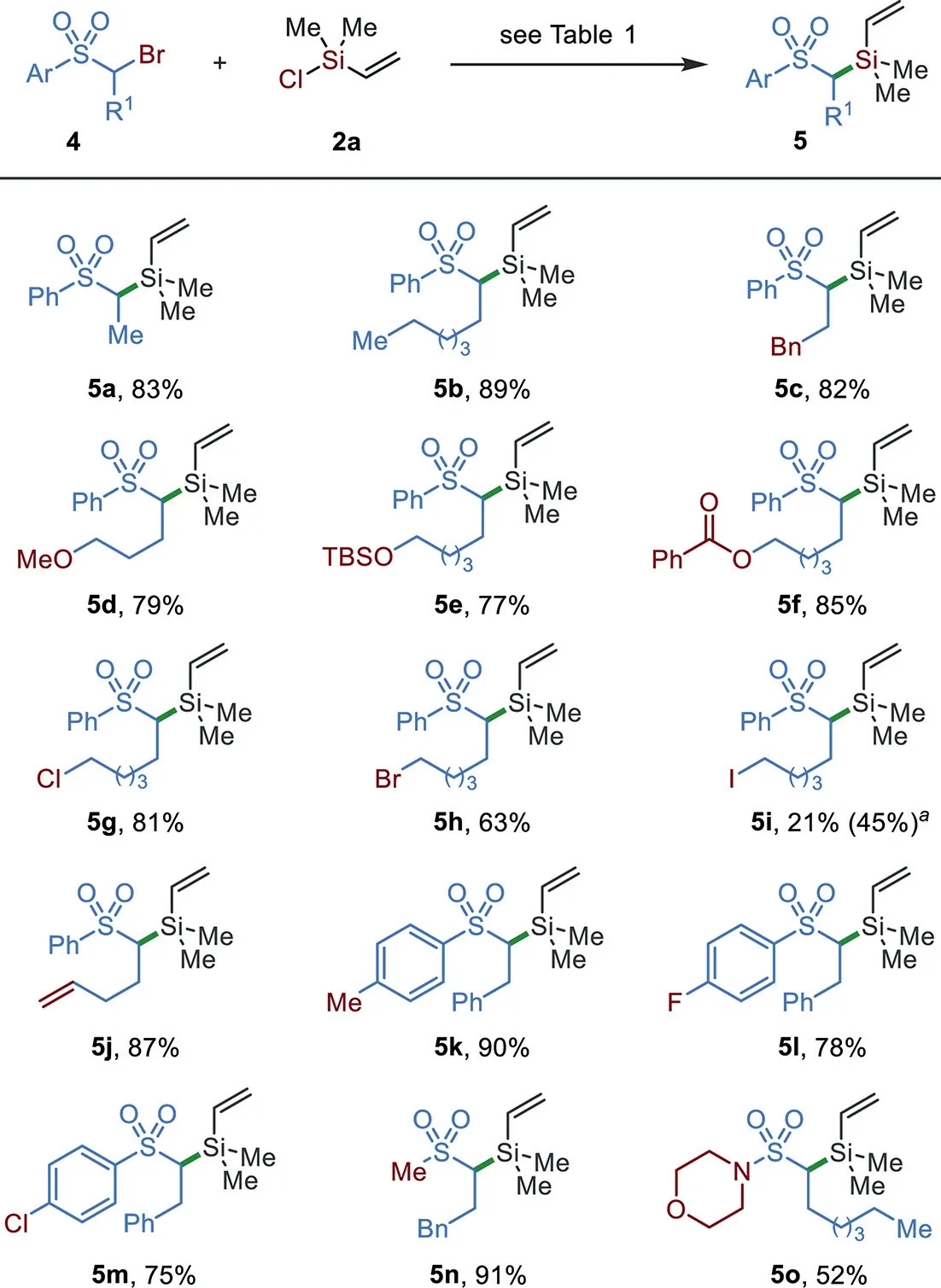
Scheme 3.The substrate scope ofα-bromo sulfones.The reaction conditions as shown in Table 1;bromide 4(0.2 mmol)and chlorosilane 2a(0.28 mmol)were used;reaction at 15°C for 20 h;isolated yields are given.a NiI2(5 mol%)was added.
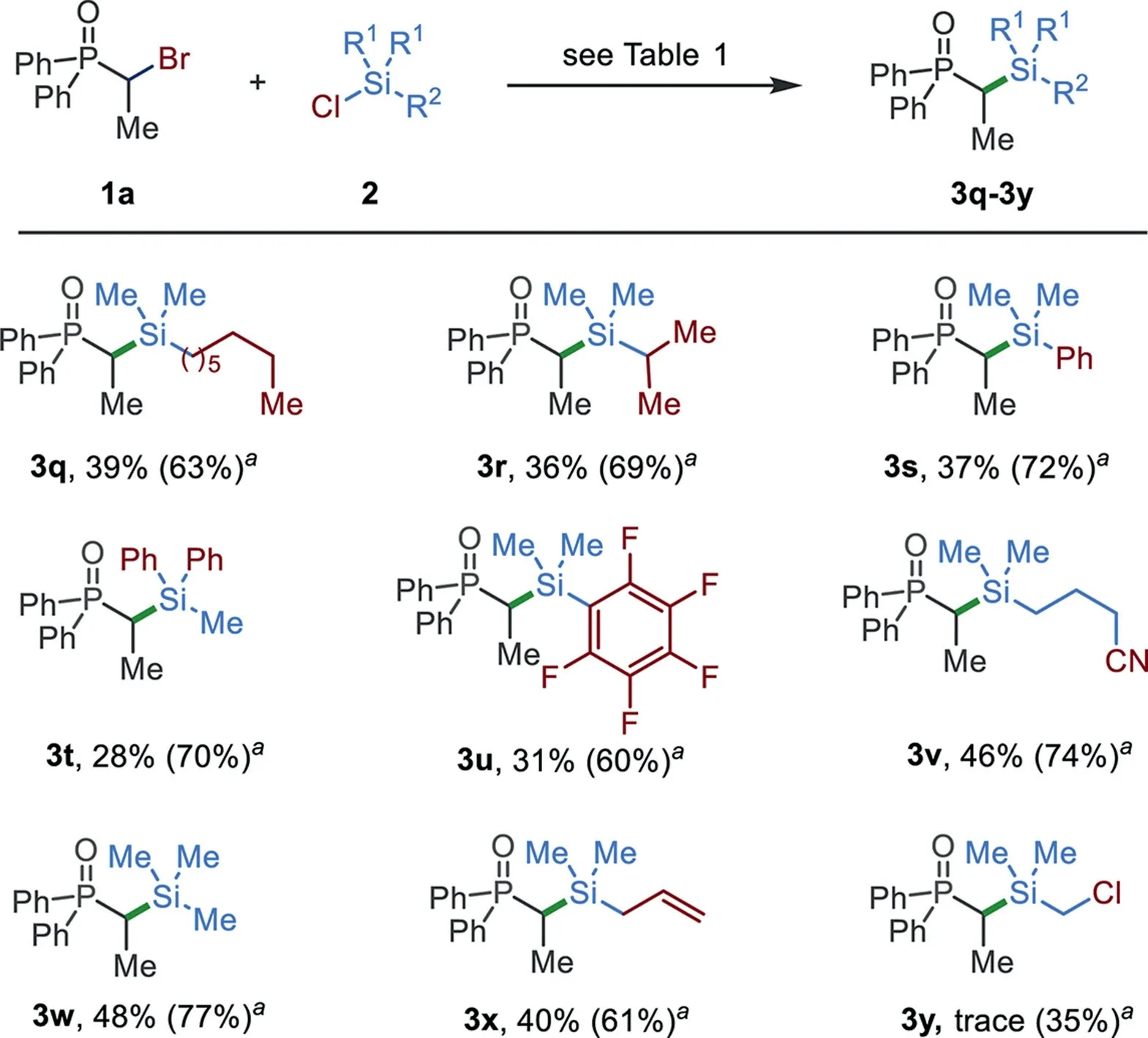
Scheme 4.The substrate scope of chlorosilanes.The reaction conditions as shown in Table 1;bromide 1a(0.2 mmol)and chlorosilanes 2(0.34 mmol)were used;isolated yields are given.a NiI2(5 mol%)was added.
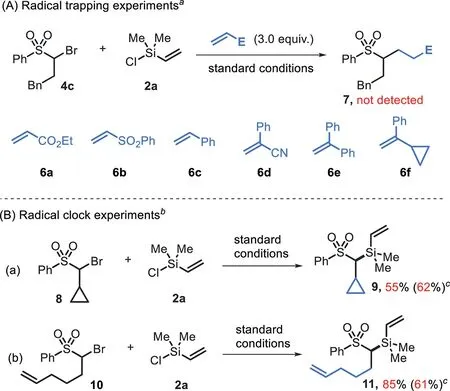
Scheme 5.Mechanistic studies.a Reaction conditions as shown in Scheme 3;bromide 4(0.2 mmol),chlorosilane 2a(0.28 mmol),and Michael acceptor 6a-6f(0.6 mmol)were used.b Bromides 8/10(0.2 mmol)and 2a(0.34 mmol)were used,isolated yields were given.c NiI2(5 mol%)was added.
Slightly adjustment of reaction conditions,including the concentration(0.2 mol/L)and temperature(at 15°C),enables the silylation ofα-bromo sulfones to be established(Scheme 3).The presence of different alkyl groups was tolerated(5a–5j).Functionalities,including ether(5d),silyl ether(5e),ester(5f),alkyl halides(5g–5i),and terminal alkene(5j),were tolerated.The scope of aryl sulfones was extended from electron-neutral(5a–5j)to electron-rich(5k)and–poor(5l and 5m)derivatives without the loss of reaction efficiency.The reactions of dialkyl sulfone and sulfamide also worked well and afforded silylation products 5n and 5o in 91%and 52%yields,respectively.
The substrate scope of the reaction with respect to chlorosilanes is shown in Scheme 4.The reactions of non-vinyl substituted chlorosilanes generally gave useful yields of target products.The use of 5 mol%of NiI2significantly improved the reaction efficiency.The presence of a long alkyl chain and sterically hindered substituent were tolerated(3q and 3r).Aryl chlorosilanes,including PhMe2SiCl,Ph2MeSiCl,and(C6F5)Me2SiCl,also worked well;the reactions afforded target products 3s–3u in good yields.Functionalized dimethyl chlorosilanes,such as those bearing nitrile(3v),allyl(3x),and chloride(3y)groups,were also tolerated.
To insight into the mechanism of this process,several control experiments were performed.(1)In the presence of Michael acceptors 6a-6f(3.0 equiv.),the reactions proceeded well to afford the desired product in 58%−76%yields(see Scheme S1 in Supporting information for details),and they did not result in any radical trapping product 7(Scheme 5A).(2)The reaction ofα-cyclopropyl substrate 8 afforded the directly coupling product 9 in 55%yield with no ring-opening derivative was observed(Scheme 5B-a).(3)The reaction of bromo(pent-4-en-1-yl)sulfone gave silylated product 11 in 85%yield and did not result in any cyclized side-product(Scheme 5B-b).Similar results were also obtained in the presence of nickel(Scheme 5B).These results suggest that a free radical is not generated in this process.
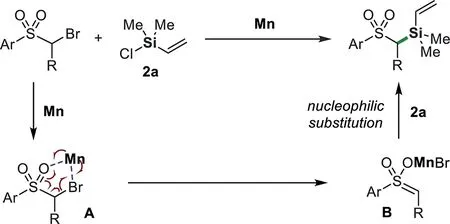
Scheme 6.Proposed mechanism of Mn-mediated reaction.
Based on the above results and literature reports,we tentatively proposed a mechanism for Mn-mediated C(sp3)–Si bond-forming reaction(Scheme 6).The coordination ofα-bromo substrate to Mn gives complex A,which may undergo a concerted oxidative addition reaction to afford complex B.The nucleophilic substitution of this complex with chlorosilanes affords the desired product[63,64,76–78].The formation of enol intermediate B is consistent with the deuterium experiments;in the presence of D2O(1.0 equiv.),the reaction of alkyl-Br 4c and 2a gave alkyl-D in 75%yield and a trace of alkyl-Si 5c(Scheme S2 in Supporting information).At present,the effect of nickel is unclear.We suppose it might be reduced to low-valent species like nanoparticle,which promote the reaction similar to the process of Mn.
In conclusion,we have reported a reductive C(sp3)–Si bondforming reaction of secondary alkyl bromides with chlorosilanes and thus established a new method for the synthesis ofα-silylated organophosphorus and sulfones.This ligand-free reaction proceeds under mild conditions and tolerates various functional groups.Further expansion of the scope of reductive C–Si coupling reactions are ongoing in our laboratory.
Declaration of competing interest
All authors declare no competing financial interests
Acknowledgment
We thank the National Natural Science Foundation of China for its financial support(No.22071084).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.03.070.
 Chinese Chemical Letters2022年12期
Chinese Chemical Letters2022年12期
- Chinese Chemical Letters的其它文章
- Diverse strategic approaches en route to Taxol total synthesis
- Recent advances in gold-complex and chiral organocatalyst cooperative catalysis for asymmetric alkyne functionalization
- Unmodified methodologies in target discovery for small molecule drugs:A rising star
- Recent advances in single-crystalline two-dimensional polymers:Synthesis,characterization and challenges
- Environmental applications of graphene oxide composite membranes
- Recent advances in the application of metal organic frameworks using in advanced oxidation progresses for pollutants degradation
