Two unique C21-diterpenoid alkaloids from Aconitum carmichaelii
Jingfang Zhang,Xiaoqiang Lei,Yazi Wei,Hui Liu,Qinglan Guo,Tiantai Zhang,Jiangong Shi
State Key Laboratory of Bioactive Substance and Function of Natural Medicines,Institute of Materia Medica,Chinese Academy of Medical Sciences and Peking Union Medical College,Beijing 100 050,China
Keywords:Ranunculaceae Aconitum carmichaelii C21-diterpenoid alkaloid Aconidenusulfonine Analgesic activity
ABSTRACT Two sulfonated diterpenoid alkaloids possessing different but related novel carbon skeletons,named aconidenusulfonine A(1)and 12,16-secoaconidenusulfonine A(2),respectively,were isolated as minor components from an aqueous extract of the lateral roots of Aconitum carmichaelii(“Fu Zi”).The structures of 1 and 2,representing the first two C21-diterpenoid alkaloids from nature,were determined by analysis of various spectroscopic data and chemical transformation,of which 1 was further proved by single-crystal X-ray diffraction.Especially,1 exhibited dose-depended analgesic activity consistent with the clinical function of Fu Zi.
Diterpenoid alkaloids,which are mainly divided into C18-,C19-and C20-subtypes,have been isolated as characteristic constituents ofAconitumgenus(Ranunculaceae)[1–3].Their impressive structural architecture and myriad activity have continuously sparked interest in chemistry and biology for many decades[1,2].The lateral root ofAconitum carmichaeliiDebx,namely“Fu Zi”(Chinese),is an irreplaceable traditional Chinese medicine with fatal toxicity and important clinic functions of pain remove,anti-inflammation,anti-arrhythmia,anti-angina,and anti-heart failure.To detoxify,Fu Zi is used after long-time decocting or appropriate processing[4–8].Considering the most common application method by decocting,a water extract of the raw drug materials of Fu Zi was investigated,to systematically search for unknown diverse constituents having pharmacological effects in agreement with the clinical functions of this drug[9–20].Previously,we reported 59 diterpenoid alkaloids including eight sulfonated C20-diterpenoid alkaloids,of which some exhibited analgesic effects consistent with the main function of Fu Zi[15–20].Herein,reported are isolation(Supporting information),structural elucidation,possible biogenetic route,and analgesic activity of two further unique C21-diterpenoid alkaloids,named aconidenusulfonine A(1)and 12,16-secoaconidenusulfonine A(2)(Fig.1)from the remaining fractions of the aqueous extract.
Compound 1 was isolated as colorless prisms(MeOH-MeCN,3:1;m.p.>300°C)with[α]20D+13.0(c0.09,H2O).Its IR spectrum indicated the existence of carbonyl(1684 cm−1)and hydroxyl(3334 cm−1)functionalities.The molecular formula of 1 was determined as C23H33NO7S by HR-ESIMS atm/z468.2059[M+H]+(calcd.for C23H34NO7S,468.2051)andm/z466.1895[M−H]−(calcd.for C23H32NO7S,466.1905),along with NMR spectroscopic data(Table 1).The1H NMR spectrum of 1 in D2O exhibited diagnostic resonances of a quaternary methyl[δH0.88(s,H3-18)]and oneN-ethyl unit[δH3.26(dq,J=14.0 and 7.0 Hz,H-22a),3.20(dq,J=14.0 and 7.0 Hz,H-22b),and 1.34(3H,t,J=7.0 Hz,H3-23)]for the diterpenoid alkaloids[12,16,18–20],and the partially overlapping resonances arising from methines and methylenes(betweenδH1.41−4.51).The13C NMR and DEPT spectra of 1 revealed the presence of the 23 carbons in the structure,containing two methyls,nine methylenes,six methines,and six quaternary carbons including an ester carbonyl carbon atδC175.7(C-17).When compared with the reported data of the chemical constituents previously isolated fromA.carmichaelii[16–19],the above mentioned spectroscopic data suggest that 1 must be a sulfonated diterpenoid alkaloid possessing 21 skeletal carbons,which is quite unusual and confirmed by 2D NMR experimental data analysis.
Analysis of the1H−1H COSY and HSQC spectroscopic data resulted in unequivocal assignment of the hydrogen and hydrogenbearing carbon resonances in the NMR spectra of 1(Table 1).In the1H−1H COSY spectrum of 1,the vicinal proton-proton coupling cross-peaks of H-1/H2-2/H2-3,H2-6/H-7,H-9/H2-11,H2-13/H2-14,H-15/H2-21 and H2-22/H3-23 revealed the presence of six struc-tural segments(Fig.2,bold lines).Based on the HMBC spectroscopic data of 1(Fig.2,red arrows),connections between the segments with the quaternary carbons,undefined methines,and heteroatoms were established.In the HMBC spectrum of 1,H3-18 correlatedviatwo-or three-bond to C-3,C-4,C-5,and C-19,revealing a linkage of the quaternary C-4 with C-3,C-5,C-18,and C-19.The connection of the quaternary C-8 by C-7,C-9,C-14,and C-15 was deduced from the HMBC correlations of H-7 to C-8 and C-9;H-9 to C-8,C-14,and C-15;and both H-15 and H2-21 to C-8,in combination with the chemical shifts of these proton and carbon resonances.A linkage of the quaternary C-10 with the oxygen-bearing C-1 as well as C-5,C-9,and C-20 was established by the HMBC correlations of H-1 to C-5,C-9,and C-20;H2-2 and H-9 to C-10,and H-20 to C-5,along with their chemical shifts.A bonding of the oxygen-bearing quaternary C-12 with C-11,C-13,and C-16 was elucidated by the HMBC cross-peaks from H2-11 to C-12,C-13,and C-16 and from H2-13 to C-12 and C-16.The further bonding of the quaternary C-16 with C-15 and the ester carbonyl C-17 as well as a lactone ring formation were unraveled by the HMBC correlations of both H-15 and H2-21 to C-16 as well as of H2-21 to C-17.Furthermore,the connection between C-5 and C-6,which was hardly distinguished by the1H−1H COSY spectroscopic data due to the partial overlap of H-3a and H-5 resonances,was determined by the HMBC correlations from H2-6 to both C-4 and C-5.Although the1H−1H COSY spectroscopic data did not display a cross-peak between H-7 and H-20 since the two vicinal protons possibly had a perpendicular dihedral angle,the connection between C-7 and C-20 was resolved by the three-bond HMBC correlations of H-20 to C-6 and C-8.The bonding of the nitrogen atom with C-19,C-20,and C-22 to form the unusual C21-diterpene skeletal architecture was finalized by the HMBC cross-peaks from H2-19 to C-20 and C-22,together with their chemical shifts.Two hydroxyl and one sulfonic acid functional groups were positioned at C-1,C-12,and C-16,respectively,to satisfy requirements of substitution and chemical shifts of these carbons as well as of the molecular formula.Considering the presence of relative strong acidic and alkali functional groups in the same molecule,an inner salt was proposed to be formed in 1.Accordingly,the planar structure of 1 was elucidated as shown in Fig.2.
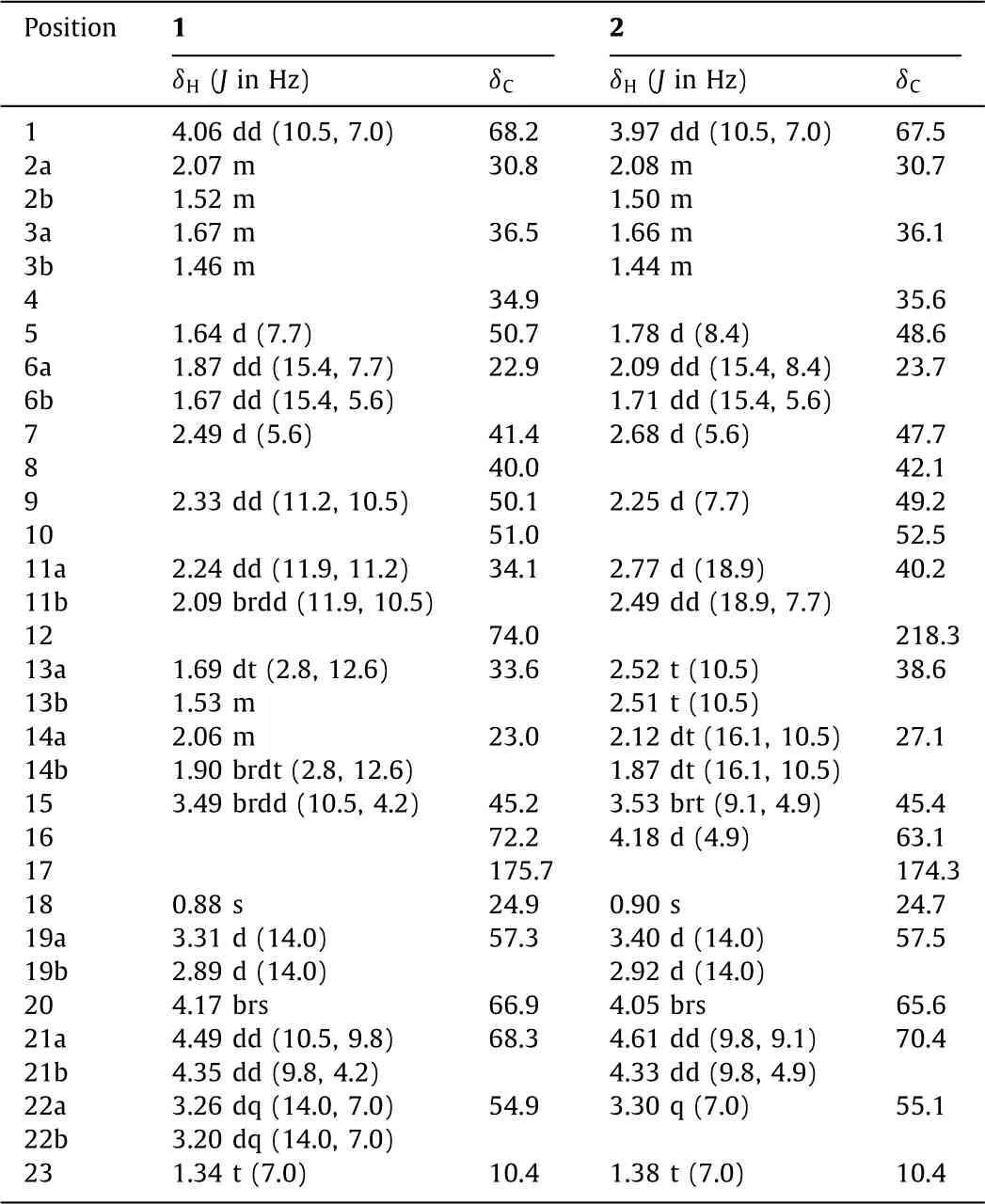
Table 1 NMR spectroscopic data for 1 and 2 in D2O.a
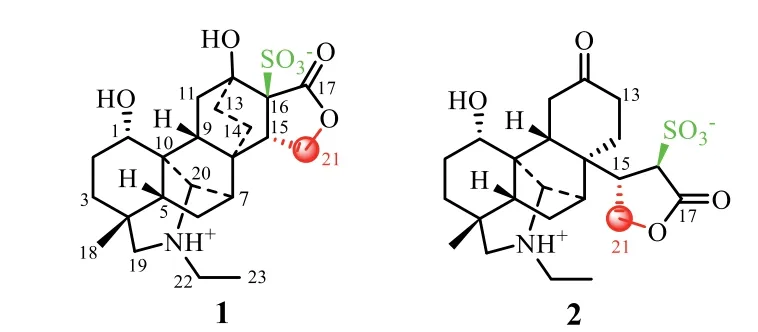
Fig.1.Structures of 1 and 2.
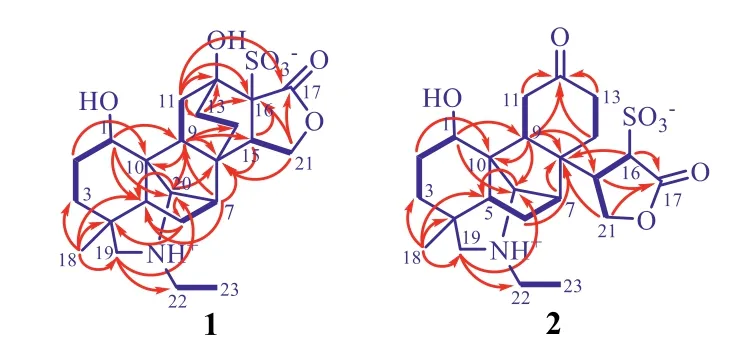
Fig.2.Main 1H−1H COSY(blue bold lines)and HMBC correlations(red arrows,from 1 H to 13C)of 1 and 2.

Fig.3.Main 2D NOESY correlations(between the pink double arrowed hydrogens)of 1 and 2.
The relative configuration of 1 was deduced from analysis of the NOESY(Fig.3)and NOE difference(Figs.S18−S20 in Supporting information)spectroscopic data.The cofacial orientation of H-1,H-5,H-9,H-15,H3-18 and H-21a on the same side of the ring system of 1 was assigned by the NOESY cross peaks of H-1/H-9;H-5/H-9 and H3-18;H-6a/H-9 and H-15;and H-9/H-15(Fig.3).This was supported by NOE enhancements of H-5 and H-9viairradiation of H-1 as well as the enhancements of H-5 and H-15viairradiation of H-9.Meanwhile,the NOESY cross peak between H-14a and H-20,together with the NOE enhancement of H-14aviairradiation of H-20,indicated that these protons oriented on another side of the fused ring system.The orientation of the ionizing sulfonic acid group was temperately assigned to be the same as H-15 to avoid a possible strong steric interaction with the bridging C-13 and C-14 unit in the highly fused ring system of 1.
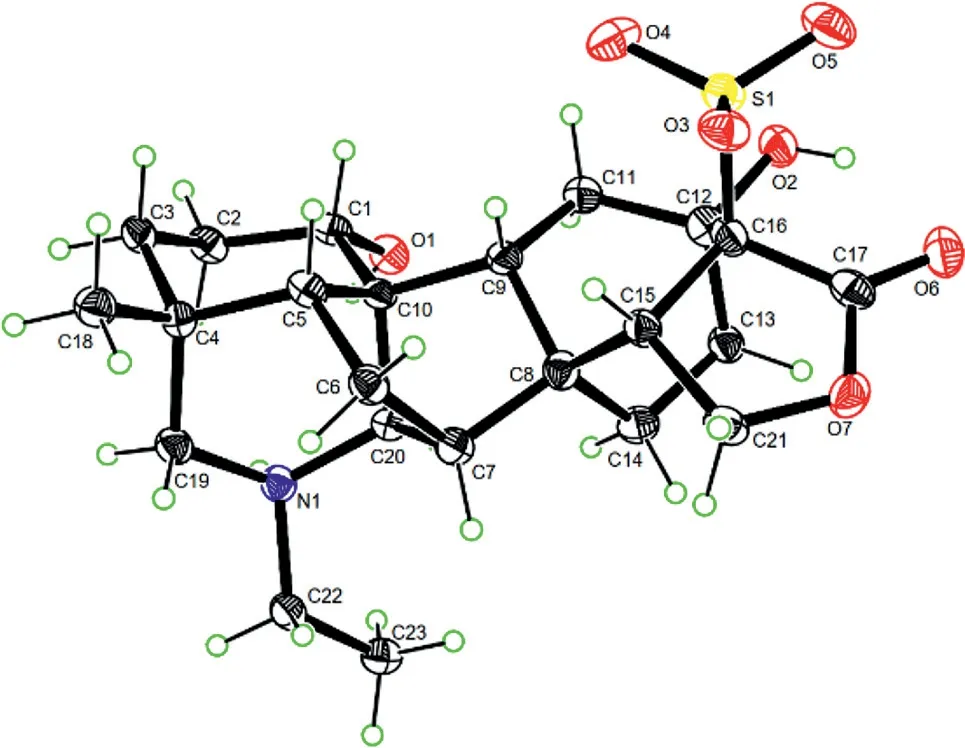
Fig.4.ORTEP diagram of 1.
Structural similarity between 1 with denudatine-and napellinetype C20-diterpenoid alkaloids[16–20]previously reported from theAconitumspecies suggests that these different types of C20-diterpenoid alkaloids share similar biosynthetic paths,so that the absolute configuration of their skeletal chiral carbons(e.g.,C-4,C-5,C-7−C-10,and C-20)are retained when ignored substitution influence.Therefore,the absolute configuration of these chiral carbons in 1 was proposed to be the same as those of napellineand denudatine-type C20-diterpenoid alkaloids,which was proved by X-ray crystallography of the different analogues with various substitution patterns[12,16,17,19,21-28].This proposition was supported by comparison of experimental CD and calculated ECD spectra of 1(Fig.S2 in Supporting information).Happily,a single crystal suitable for X-ray diffraction analysis was obtained in a MeOH−MeCN(3:1)solution of 1,follow-up crystallography using anomalous Cu Kαradiation scattering proved the above proposition and the structure of 1,with a Flack parameter of 0.04(4),an ORTEP diagram shown in Fig.4.Hence,the structure of compound 1 was determined and named aconidenusulfonine A.
Compound 2 was isolated as a white amorphous powder with[α]20D+40.0(c0.12,MeOH).The spectroscopic data indicated that 2 is an isomer of 1.Comparison of NMR spectroscopic data of the two compounds(Table 1)indicated that the hydroxyl-and ionizing sulfonic acid-bearing quaternary carbons in 1 were replaced by a ketone carbonyl carbon[δC218.3(C-12)]and a methine[δH4.18(d,J=4.9,H-16),δC63.1(C-16)]in 2,respectively.The key difference suggested that 2 must be a 12,16-seco derivative of 1 to match the requirement of chemical shift changes and to retain the molecular composition.The deduction was proved by 2D NMR spectroscopic data analysis of 2,especially by the1H−1H COSY cross-peaks of H-16/H-15/H2-21 and the HMBC correlations of the carbonyl carbon C-12 with H-9,H2-11,H2-13,and H2-14 and of the lactone carbonyl carbon C-17 with H-15,H-16,and H2-21(Fig.2).The NOESY(Fig.3)and NOE difference(Figs.S34−S36 in Supporting information)spectroscopic data of 2 indicated that the relative configuration at the chiral carbons was identical to that of 1.Particularly,the NOESY cross-peaks of H-9/H-15,H-16/H-14b,and H-14a/H-20 revealed that H-15 and H-16 weretrans-oriented,while the NOESY cross-peak between H-11b and H-16 indicated that the lactone ring back faced to the top of the cyclohexanone ring in 2 with a free rotation restriction of the C-8−C-15 bond due to the lactone ring bulkiness.The relative configuration assignment was supported by transformation between 1 and 2.According to the abovementioned reasons for 1,the absolute configuration of 2 was assigned as depicted in Fig.3.The assignment was supported also by comparing the experimental CD and calculated ECD spectra(Fig.S4 in Supporting information).Additionally,the CD spectrum of 2 displayed a weak Cotton effect at 284(Δε+0.02)nm,arising from n−π*transition of the ketone chromophore.Application of the octant rule for the cyclohexanones further supported the assignment(Fig.S22 in Supporting information).Thus,the structure of compound 2 was assigned as 12,16-secoaconidenusulfonine A.
Compounds 1 and 2 are the first two C21-diterpenoid alkaloids possessing different but related carbon skeletons.Their natural occurrence was supported by UPLC−HR-ESIMS analysis of the crude aqueous extract of raw Fu Zi(Figs.S37 and S38 in Supporting information).The similar structural features among 1,2,and those deriving from the napelline-and denudatine-type C20-diterpenoid alkaloids isolated from the same extract[16,18-20]suggest that these natural products share a basic biosynthetic path breaching out to produce diverse skeletons.Based on our previous propositions on several sulfonated C20-diterpenoid alkaloids[16–19],1 and 2 are proposed to be biosynthesized through an extension of the pathways of aconicarmisulfonines A−C[16,18],sharing the same precursor songorine(3)and enzyme-assisted initial steps of the double bond oxidation,epoxide ring opening,and semipinacol rearrangement(Scheme 1 and Scheme S1 in Supporting information).Scheme 1 illustrates the extension of the pathwayviathe C-15−C-16 bond migration of ii to produce 1 and 2 through the same intermediate iii as that of aconicarmisulfonine A[16].The intermediate iii undergoes either intramolecular dehydration,followed by hydrogenationviaiv,or directly dehydroxylation to give v.Aldol reaction of v with formaldehyde produces vi.Subsequent retro-Claisen rearrangement of vi through vii′,followed by intramolecular Aldol additionviavii,generates viii.Oxidative hydrolysis of vii and viii give 2 and 1,respectively.For production of 1 and 2 from the extension of the pathwayviathe C-13−C-16 bond migration of ii to form another intermediate(same as that of aconicarmisulfonines B and C[18])is provided in Supporting information(Scheme S1).
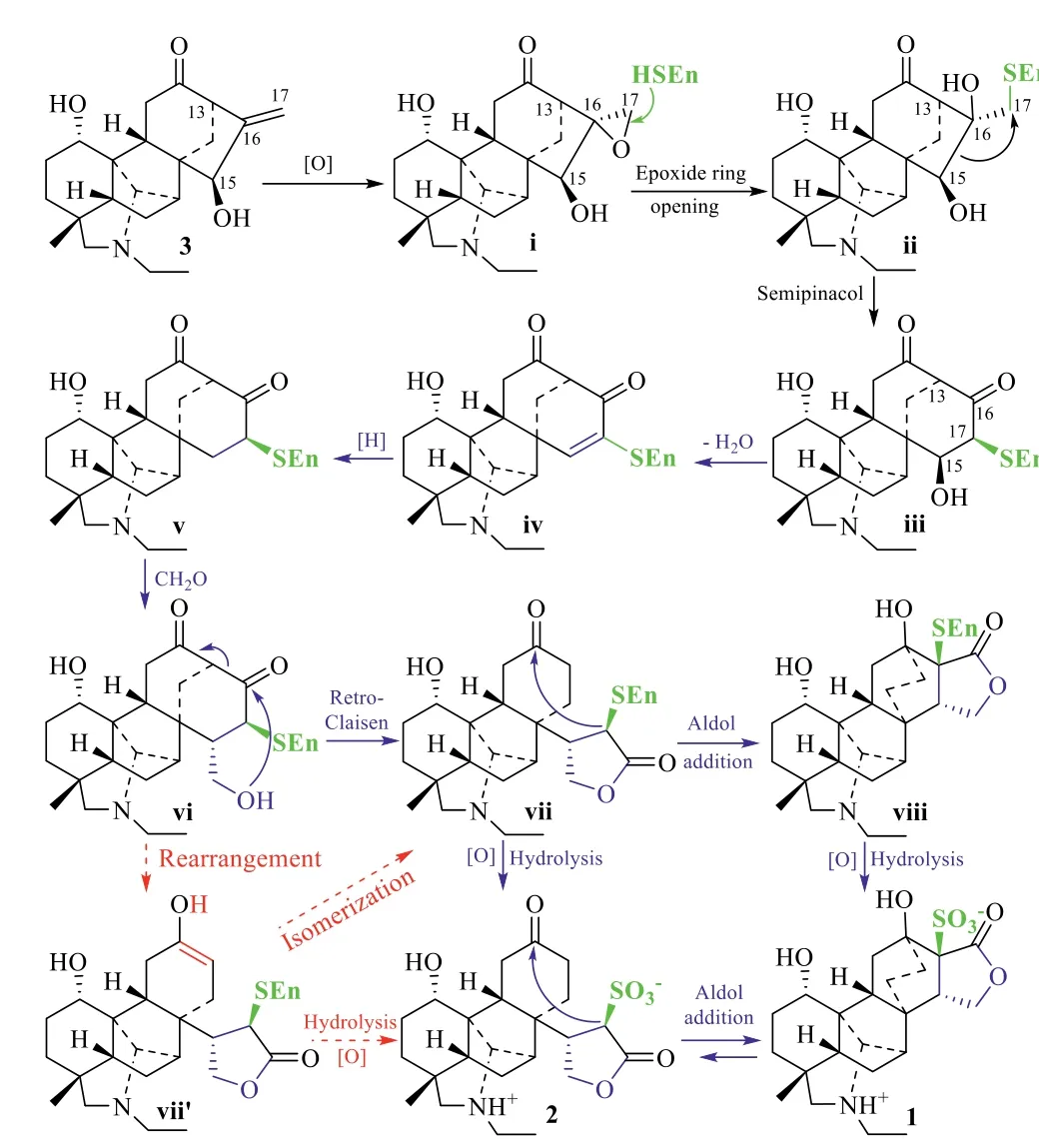
Scheme 1.Proposed biogenetic pathways of 1 and 2.
It is worth noting that,at any step of the proposed pathways,oxidative hydrolysis of the enzyme-adducts might take place to produce the corresponding diterpenoid alkaloids while the rearrangement may undergo with the sulfonates.This is partially supported by previous isolation of aconicarmisulfonines A−C[16,18]as well as by interconversion between 1 and 2.The two compounds were stable after stored under ambient conditions for 7 days and after heated at 50°C for 12 h(Figs.S39−S43 in Supporting information)as indicated by UPLC-HR-ESIMS and NMR spectroscopic data analysis.However,when their aqueous solutions were separately heated at 110°C(liquid alloy bath temperature)for 12 h,1 and 2 were interconverted each other to reach an equilibration with 1:2 in an approximate ratio of 1.2:1.0 according to integration of the NMR signals(Figs.S44−S46 in Supporting information).This indicates that 1 is relatively more stable than 2.
According to the abundant occurrence of the possible precursor songorine(3)in this plant and the proposed biosynthetic pathways,1 and 2 should originate from the napelline-type C20-diterpenoid alkaloids.However,structural features of 1 and 2 are closer to denudatine-type C20-diterpenoid alkaloids[20].This finding suggests that at least part of denudatine-type C20-diterpenoid alkaloids might be biosynthetically transformed from napelinetype analogues.Additionally,although the positions of C-16 and C-17 are exchanged in the pathways,the skeletal carbons of 1 and 2 in Fig.1 are numbered to keep the consistency with that of the known diterpenoid alkaloids.
Because“Fu Zi”is clinically applied as an analgesic agent[1–3],along with the positive results of our previous work[15–20],1 and 2 were pharmacologically evaluated using thein vivomodel of acetic acid-induced mice writhing for the analgesic effect[29].For 1,writhes were reduced by 24.63%,26.35%,and 74.94%at dosages of 1.0,2.0,and 4.0 mg/kg(i.p.),respectively,while the positive control 3-acetylaconitine gave a 98.6%writhing reduction at 1.0 mg/kg(i.p.).However,at 2.0 mg/kg(i.p.),2 showed only 15.84%writhing inhibition,and was not further evaluated at a higher dose due to lack of enough sample.The lower effect of 2 indicated that the C-12−C-16 bond cleavage of the ring system in 1 decreased the analgesic effect.
In summary,two unique sulfonated C21-diterpenoid alkaloids were discovered as the minor chemical constituents of the aqueous extract of“Fu Zi”.Compound 1 exhibited the analgesic effect consistent with the clinical function of Fu Zi.This discovery adds a new subtype of diterpenoid alkaloids,of which the existence in nature has never been realized in the past studies.As predicted by the proposed biosynthetic routes,the C21-diterpenoid alkaloids are from a post modification of the napelline-type C20-diterpenoid alkaloids through a relative longer pathway.This suggests that more members of the C21-diterpenoid alkaloids possibly occur in the plants as minor metabolites like 1 and 2,which have been ignored in the past studies mainly due to difficulties of isolation and structural characterization.Thus,investigations of the minor constituents are essential for the medicinal plants to unravel their active substances consistent with the clinical functions.
Declaration of competing interest
The authors declare no competing financial interest.
Acknowledgments
Financial support from the National Natural Sciences Foundation of China(Nos.81630094 and 21732008),and CAMS Innovation Fund for Medical Science(No.2021-I2M-1-028)is acknowledged.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.cclet.2022.03.104.
 Chinese Chemical Letters2022年12期
Chinese Chemical Letters2022年12期
- Chinese Chemical Letters的其它文章
- Diverse strategic approaches en route to Taxol total synthesis
- Recent advances in gold-complex and chiral organocatalyst cooperative catalysis for asymmetric alkyne functionalization
- Unmodified methodologies in target discovery for small molecule drugs:A rising star
- Recent advances in single-crystalline two-dimensional polymers:Synthesis,characterization and challenges
- Environmental applications of graphene oxide composite membranes
- Recent advances in the application of metal organic frameworks using in advanced oxidation progresses for pollutants degradation
