Phytoplankton community dynamics during Alexandrium blooms in 2019 off the Qinhuangdao coast, Bohai Sea, China*
Yixuan XIE , Renye DING , Daojun ZHA , Yu LI , Guowang YAN , Yaya ZHANG ,Haiyan WU , Guanchao ZHENG , Zhijun TAN , , Tao JIANG ,
1 School of Marine Technology and Geomaties, Jiangsu Ocean University, Lianyungang 222005, China
2 School of Ocean, Yantai University, Yantai 264005, China
3 Key Laboratory of Testing and Evaluation for Aquatic Product Safety and Quality, Ministry of Agriculture and Rural Aff airs,Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao 266071, China
Abstract Alexandrium blooms in the northwest area of the Bohai Sea (Qinhuangdao coastal area),China, produce large amounts of toxins that could be enriched in shellf ish and consequently harm human bodies. To understand the succession of the phytoplankton community structure during Alexandrium bloom events in the northwest area of the Bohai Sea off Qinhuangdao from April 2 to May 7, 2019, microscopy observations and high-performance chromatography (HPLC)-pigment analysis were performed.Sixty species of phytoplankton were identif ied, mainly diatoms and dinof lagellates. The abundance of Alexandrium reached the maximum on April 16 (3.3×10 3 cells/L). HPLC-pigment CHEMTAX analysis showed that the phytoplankton community was composed mainly of diatoms, dinof lagellates, prasinophytes,and cryptophytes. Diatoms were the main contributor to the total Chl- a pool. There was a downward trend for the proportion of diatom biomass to the total Chl- a pool, followed by an upward trend. The proportion of dinof lagellate biomass showed the opposite trend, whereas that of the prasinophyte biomass presented an obvious increasing trend. Temperature, nutrients, and nutrient structures were the main factors on the distribution of diff erent phytoplankton groups in the study area as shown in the redundancy analysis. This work illustrates the succession of phytoplankton community structures during Alexandrium blooms and provided a theoretical basis for studies on the mechanism underlying the outbreak of harmful algal blooms in sea areas.
Keyword: Alexandrium bloom; phytoplankton population; environmental factor; high-performance chromatography (HPLC)-CHEMTAX; phytoplankton pigment; Qinhuangdao
1 INTRODUCTION
Phytoplankton is def ined as colonies and freef loating unicells that grow photoautotrophically in aquatic environments (Reynolds, 2006).Phytoplankton is an important primary producer given that it f ixes more than half of the photosynthetic carbon in the ocean (Pujari et al., 2019). However,under certain environmental conditions, the explosive proliferation or high aggregation of some phytoplankton species results in the ecological abnormality of local discoloration, namely, red tides or harmful algal blooms (HABs). HABs cause harm because the rapid and huge buildup of phytoplankton biomass leads to the depletion of oxygen as the blooms decay or to the destruction of f ish or shellf ish habitats by the shading of submerged vegetation(Anderson et al., 2002). HABs often cause serious damage to f isheries and mariculture in coastal areas.In addition, toxic algae, includingAlexandriumspp.,can produce biotoxins and seriously threaten the health of consumers throughout the food chain (Costa et al., 2021).
Alexandriumblooms have long attracted considerable attention due to their capability to produce highly toxic secondary metabolites, i.e.,paralytic shellf ish poisoning toxins (PSTs). Among marine biotoxins, PSTs are widely distributed and highly hazardous and have high incidence(Hallegraeff , 1993; Anderson et al., 1996). PSTs are responsible for 87% of global shellf ish poisoning incidents, which have a global mortality rate of 2%–14% (Azanza and Taylor, 2001; Raposo et al.,2020). In addition, Paralytic Shellf ish Poison (PSP)toxins may be associated with the deaths of birds and humpback whales (Nisbet, 1983; Geraci et al., 1989).Therefore, many countries have listed PSTs as routine detection objects in shellf ish farming areas (Nishitani and Chew, 1988; Shumway et al., 1988).Alexandriumspp. are important red tide species worldwide, and approximately 10 of these species produce toxins(Hallegraeff , 1993; Dai et al., 2020). Conditions featuring relatively lowAlexandriumcell densities(>103cells/L) without seawater discoloration are still considered asAlexandriumblooms because of their potential threat of PSTs (Anglès et al., 2012).Alexandriumbloom outbreaks have been reported in some coastal areas worldwide, including Chile(Jedlicki et al., 2012), Brazil (Persich et al., 2006),the United States (Townsend et al., 2001), Canada(McGillicuddy et al., 2014), the northwestern Mediterranean (Vila et al., 2001), and temperate Asian countries (Yu et al., 2021).
The blooms ofAlexandriumspp. are rarely monospecif ic in the natural marine environment. In HABs,Alexandriummay dominate the bulk or part of the whole phytoplankton population. For example,Alexandriumblooms usually co-occur with the largescale blooms ofProrocentrumspp. in the East China Sea (Zhou et al., 2008; Jiang et al., 2014). Jiang et al. (2014) showed thatAlexandriumtamarenseoccupy only a small proportion of the phytoplankton population and thatProrocentrumspp. accounts for a large proportion of the spring dinof lagellate blooms in the Nanji Islands. A similar result was also observed in the northwestern Mediterranean, whereProrocentrumspp. dominates the phytoplankton community duringAlexandriumblooms, and other species, such asSkeletonemacostatumandChaetocerosspp., are present in appreciable numbers (Delgado et al.,1990).Scrippsiellatrochoideawas the most abundant dinof lagellate species during theAlexandriumbloom in the Bay of Plenty, New Zealand, in 1993 (Chang et al., 1997). By contrast, inAlexandriumblooms in estuaries in France,Alexandriumminutumis the dominant species (60% of the total phytoplankton population) and is accompanied by a small portion ofNitzschialongissima(12%) andChaetoceros sp.(23%) (Maguer et al., 2004). In general, the occurrence ofAlexandriumblooms is a complex process that is aff ected by complicated environmental factors, under which the phytoplankton population structure is not static and shows variations with time and place.
In most of the previous studies onAlexandriumblooms, phytoplankton identif ication was based on traditional microscopic observation. However, this method is time- and labor-consuming and cannot identify small or fragile algal cells (e.g., picoplankton).Such a constraint leads to biases in the assessment of the changes in phytoplankton population structure during algal blooms. In recent decades, HPLC-pigmentbased chemotaxinomic analysis has greatly improved the effi ciency of phytoplankton measurement and expanded the understanding of population structure.It has been widely used to reveal the temporal and spatial distributions of the phytoplankton assemblages in nearshore, coastal, and oceanic sea areas (Wright et al., 2010; Zhai et al., 2011; Das et al., 2017; Lu et al., 2018). Short-term changes in algal blooms and phytoplankton communities in some estuarydominated sea areas under the disturbance of storm events have also been studied by using the HPLCpigment method (e.g., Jiang et al., 2022). Notably,few studies have been conducted to characterize the succession of phytoplankton communities during the process ofAlexandriumblooms by using HPLCpigment analysis.
In the Bohai Sea, the largest inland sea in China,PSP toxins were f irst detected inCrassostreagigasandScapharcasubcrenatain 1996 (Lin et al.,1999). The detection of these toxins signif ied the possibility of the presence of the PSP-producing genusAlexandriumin the seawater. Zhang et al.(2018) reviewed the occurrence of PSP toxins during 1993 to 2016 and suggested that the detection rate and concentration of these toxins in shellf ish showed a signif icant upward trend starting in 2006. However,Alexandriumblooms rarely occurred in the Bohai Sea before 2016. For example, only oneAlexandriumbloom event was observed during 2000 and 2015 (Dou et al., 2020). Nevertheless, theAlexandriumgenus has been reported to be occasionally the dominant taxon in seawater (Cao et al., 2006; Xu et al., 2017).
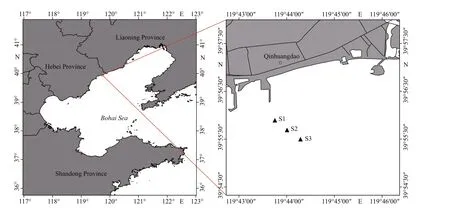
Fig.1 The sampling stations in the Qinghuangdao coastal waters
Qinhuangdao is located northwest of the Bohai Sea. In recent years, this sea area suff ered from a large amount of land-based pollution sources from industrial, agricultural, and aquaculture wastewaters due to vigorous economic development (Xu et al.,2017; Cui et al., 2018). Consequently, its ecological environment has been deteriorated, and eutrophication in this area was intensif ied, thus leading to the frequent occurrence of HABs (Peng, 2015; Liu et al., 2017). This area has experiencedAureococcusanophageff erensbrown tides on an annual basis since 2009 (Xu et al., 2017). These algal blooms have severely aff ected the development of the local marine economy and attracted widespread concern. Although brown tides have not occurred in this area since 2016,the Qinghuangdao coastal area has experienced high incidences ofAlexandriumblooms in recent years(Zhang et al., 2018).Alexandriumblooms have occurred every spring (in April to May) and resulted in excessive PSP content in shellf ish (Ding et al.,2017; Zhang, 2020). An outbreak of PSP poisoning was reported in the spring of 2016. Since then, the local government has issued bulletins every spring on the prevention of paralytic shellf ish poisoning because of the recurrence ofAlexandriumblooms.The annual outbreak ofAlexandriumblooms has exerted a massive eff ect on the local shellf ish farming industry.
Alexandriumblooms have become a recurring problem in the coastal areas of Qinhuangdao City in recent years. However, the detailed process ofAlexandriumblooms has yet to be reported. In this study, microscopy examination and HPLCCHEMTAX pigment analysis were used to track the process ofAlexandriumblooms in the spring of 2019.The aims of this study are to present the temporal dynamics of phytoplankton assemblages, especially those ofAlexandriumspp., and to investigate how environmental factors aff ect the short-term succession of phytoplankton communities in this area in spring.
2 MATERIAL AND METHOD
2.1 Site description and sampling method
Our previous investigations indicated that in the study area,Alexandriumblooms mainly occur in April to May (unpublished data). In this study, nine investigations were carried out at three stations in the Qinhuangdao sea area from April 2, 2019 to May 7, 2019 (Fig.1). After May 7, 2019, the cell density ofAlexandriumdecreased to <1 000 cells/L. The sampling stations were approximately 0.8–1.7 km away from the shoreline. Only surface seawater was sampled at shallow water depth (<7 m). Physical parameters, including salinity, temperature, and DO,were measured by using an YSI 556 multiparameter instrument (Yellow Springs Instruments, USA).Seawater samples with an accurate volume of 1.0 L were f iltered through GF/F f ilters (0.7 μm, Whatman).The f ilters were taken and immediately frozen in liquid nitrogen for pigment analysis. The f iltrate was stored at -40 °C in a freezer for the analysis of ammonium nitrogen (NH4+), NO3ˉ, NO2ˉ, DIP, and DSi concentrations. Phytoplankton samples (1.0 L)preserved with acidic Lugol solution were used for microscopy identif ication.
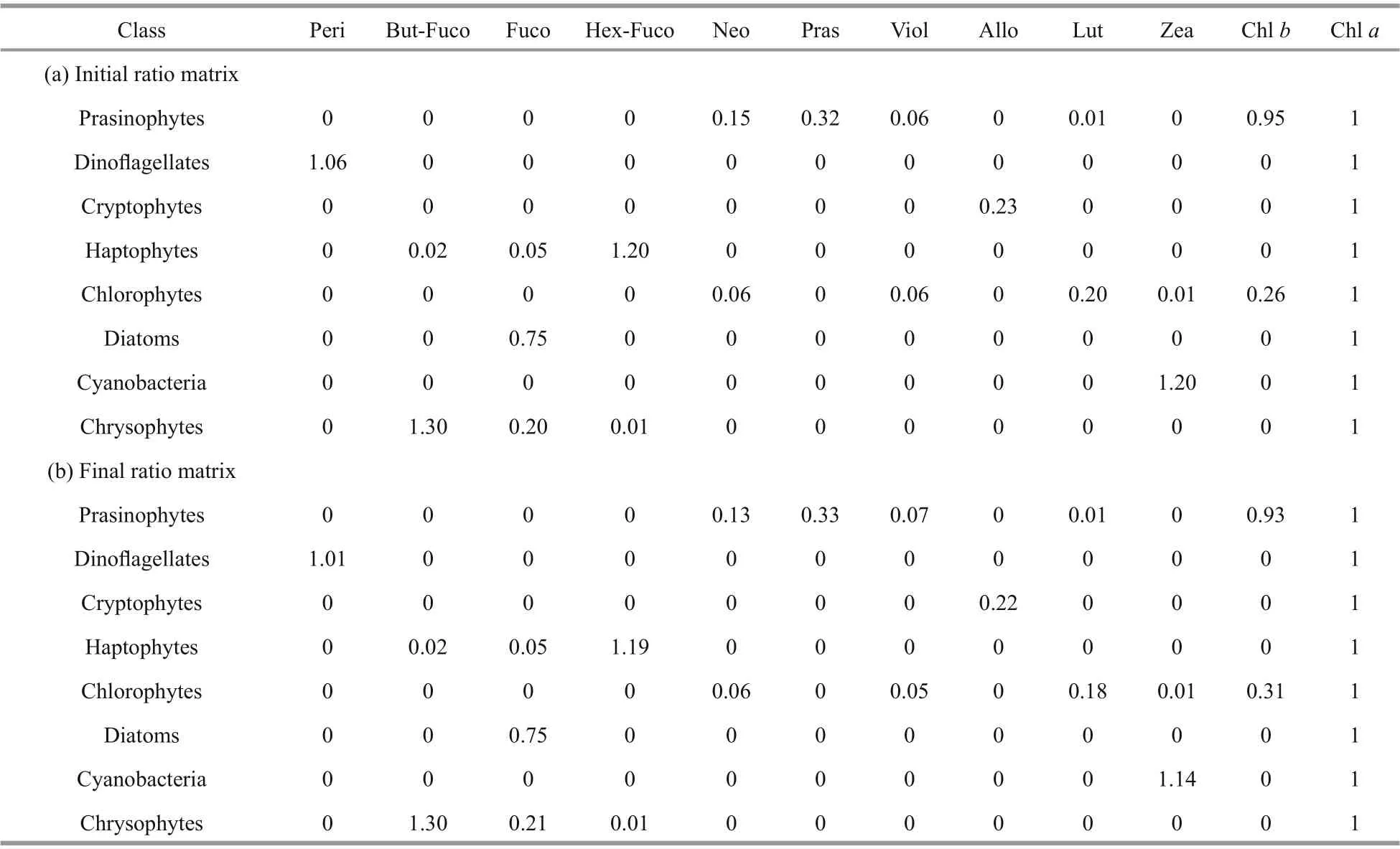
Table 1 The pigment vs. Chl a ratios used in CHEMTAX analysis of pigment data
2.2 Nutrient analysis
The concentrations of NH4+, NO3ˉ , NO2ˉ, and dissolved inorganic phosphate (PO4ˉ) and metasilicate(SiO3ˉ) were measured by using a Skalar San++continuous f low analyzer (Strickland and Parsons,1972). DIN refers to the sum of NO3ˉ, NO2ˉ, and NH4+contents.
2.3 HPLC pigment analysis
Photosynthetic pigments were extracted in darkness and low temperature as per Lu et al. (2018).HPLC analysis was performed as per Zapata et al. (2000) by using an Agilent series 1100 HPLC system equipped with a G1314A detector and Waters Symmetry C8 column (150×4.6 mm, 3.5-μm particle size, 100-Å pore size). The absorption spectrum at 440 nm and the peak time were used to identify the pigment peak, and the retention times were compared with the retention times of the authentic standards obtained from DIH Inc. (Høsholm, Denmark). The relative standard deviation for pigment analysis was controlled within ±5%. A total of 22 standards were used. They included chlorophyllc3, Mg-2,4-divinylpheopor-phyrin, chlorophyllc2, peridinin(Peri), pheophorbidea(Pheidea), 19-but-fucoxanthin(But-Fuco), fucoxanthin (Fuco), neoxanthin (Neo),prasinoxanthin (Pras), violaxanthin (Viol), 19ʹ-hexfucoxanthin (Hex-Fuco), diadinoxanthin, alloxanthin(Allo), diatoxanthin, zeaxanthin (Zea), lutein (Lut),canthaxanthin, gyroxanthin-diester, chlorophyllb(Chlb), chlorophylla(Chla), pheophythina(Phea),and β-carotene (β-Car).
2.4 CHEMTAX analysis
Version 1.95 of CHEMTAX software was used to calculate the contributions of diff erent phytoplankton groups to total Chla. The initial pigment ratio matrix was derived from a series of values given by Mackey et al. (1996). The initial and output matrixes are shown in the appendix as Table 1. Each cell of the initial matrix was multiplied with a random function to generate a series of 60 derivative pigment ratio matrixes. The macro was applied to calculate the best six output results, and the average was taken.
2.5 Phytoplankton identif ication and enumeration
The sample was concentrated to a volume of 10–20 mL after 48 h of precipitation. Identif ication and counting were performed under an inverted microscope in accordance with Utermöhl (1958). The references used for the identif ication of phytoplankton species included Illustrations of Common Planktonic Diatoms in Chinese Seas by Yang and Dong (2006),Dinof lagellates in China’s Seas Ⅲ ( Peridiniales)by Yang et al. (2019), and Illustrations of Plankton Responsible for the Blooms in Chinese Coastal Waters by Guo (2004).
2.6 Statistical analysis
The Pearson analysis was conducted by using SPSS Statistics (version 23) to test the correlation between the microscopy observation data and CHEMTAX results. Redundancy analysis (RDA) was performed with CANOCO 5 software to explore the relationships between environmental and biological(phytoplankton) variables.
Species diversity, ecological richness, species evenness, and dominance indexes were used to evaluate the diversity of the phytoplankton community structure. The main formulas are given below.
The Shannon-Wiener diversity index was used as the species diversity index (Hʹ) and is calculated as

The Makarev index was utilized as the ecological richness index (dMa) and has the formula:

The Pielou index was applied as the species evenness index (J) and is given as

The dominance index of phytoplankton (Y) is

whereNrepresents the total number of individuals;Pirepresents the ratio of the individual number of theithspecies in a sample to the individual number of the sample;Srepresents the total number of species in a sample;nirepresents the total number of individuals of theithspecies; and ƒirepresents the frequency of the occurrence of theithspecies in each sample. The dominant species are those whose dominance degrees exceeded 0.02.
3 RESULT
3.1 Physical and chemical parameters
The sampling time for this survey was set at 9꞉00–11꞉00 am each time and did not take tidal eff ects into account. During the survey period, the sea surface temperature continued to increase from 6.8 °C to 14.2 °C (Fig.2a). Salinity remained very stable and ranged from 32.0 to 32.2. Although the DO concentration showed a downward trend,hypoxia (<3.0 mg/L) did not occur. The average concentrations of DIN, DIP, and DSi were 2.9, 0.2,and 2.6 μmol/L, respectively. During the investigation period, the concentrations of DIN and DIP showed a downward trend, whereas the concentration of DSi exhibited an upward trend (Fig.2b). The potential Si limit appeared during April 2–16 when the DIN/DSi ratio ranged from 2.08 to 2.89 and the DSi/DIP ratio was less than 11.58 (Fig.2d). However, after April 19,DIP concentration decreased to less than 0.1 μmol/L,and the ratios of DIN/DIP and DSi/DIP increased to 30, thus indicating P limitation (Fig.2b & c) (Dortch and Whitledge, 1992). The concentration of NO2ˉ was relatively stable and ranged from 0.18 μmol/L to 0.31 μmol/L (Fig.2c). NO3ˉ concentration decreased from 1.7 μmol/L to 0.05 μmol/L, and NH4+concentration decreased from 3.36 μmol/L to 1.28 μmol/L, with both indexes generally showing a downward trend (Fig.2c).
3.2 Phytoplankton pigment concentrations
A total of 17 pigments were identif ied. They included Peri, Pheidea, But-Fuco, Fuco, Neo, Pras,Viola, Hex-Fuco, Diad, Allo, Diat, Zea, Lut, Chlb,Chla, Phea, and β-Car. Fuco, followed by Chla,and Peri, was the most abundant. The concentration of Chlaremained relative high (>0.62 μg/L) before April 16, decreased sharply to 0.24 μg/L on April 19,and then presented an increasing trend (Fig.3a). The average concentration of Fuco reached 0.69 μg/L and showed a change trend that was similar to that exhibited by the concentration of Chla. By contrast,Peri concentration increased before April 19 and then decreased with an average value of 0.20 μg/L (Fig.3a).The average concentrations of Chlb, Pras, and Zea were 0.07, 0.11, and 0.005 μg/L, respectively. Pras concentration showed an upward trend during the investigation period (Fig.3b).
3.3 Phytoplankton community structure based on CHEMTAX
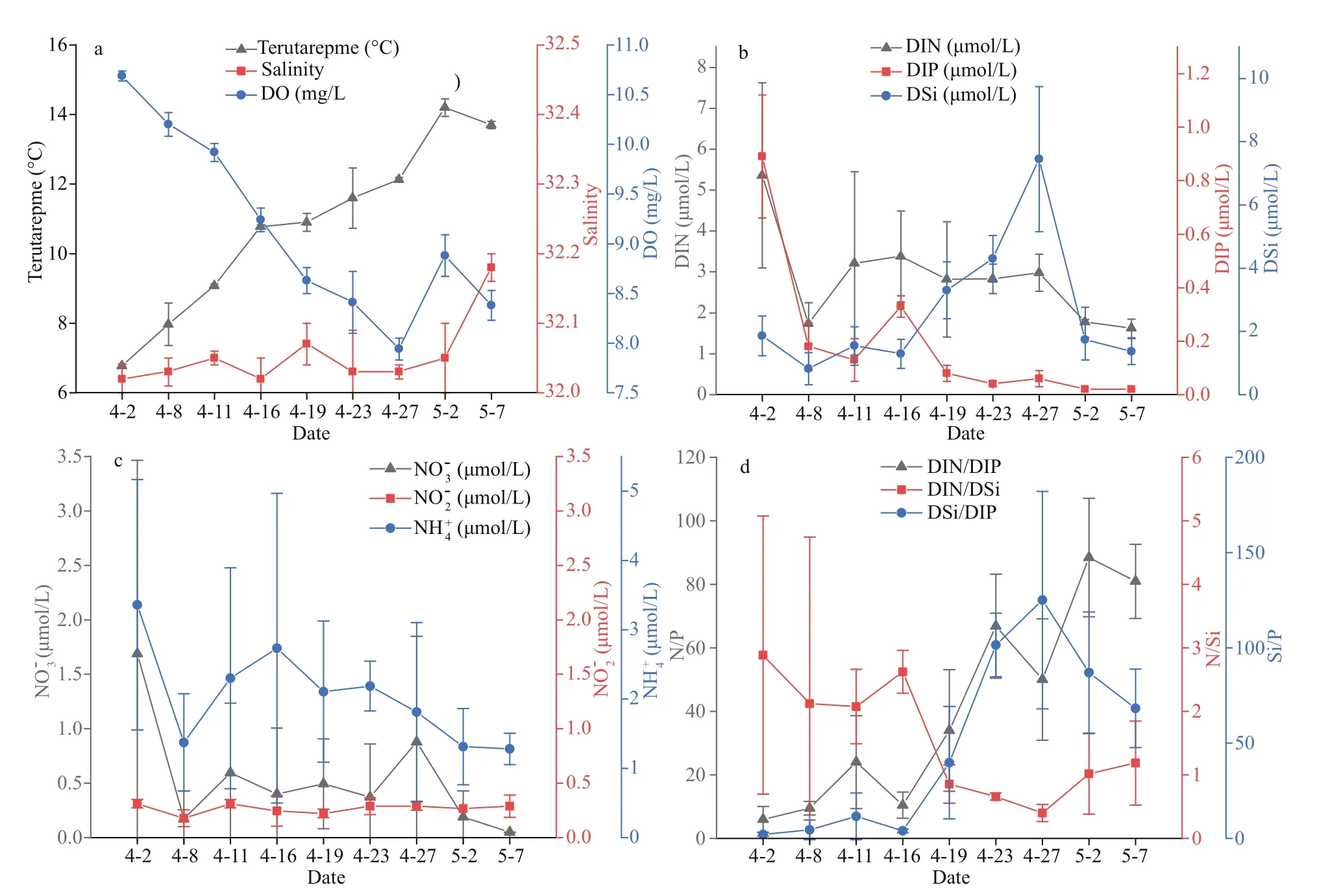
Fig.2 Values of the environmental factors of the Qinghuangdao coastal waters in spring
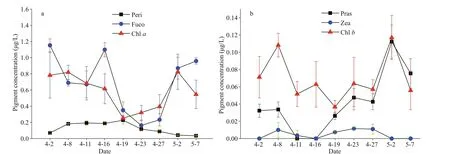
Fig.3 Temporal distribution of the main pigments in the Qinghuangdao coastal waters in spring
CHEMTAX analysis revealed the variation in the composition of the phytoplankton community during the study period. Diatoms, dinof lagellates,prasinophytes, and cryptophytes were the main phytoplankton groups in the study area, whereas chlorophytes, haptophytes, cyanobacteria, and chrysophytes accounted for a low proportion of the total phytoplankton biomass (Fig.4). The CHEMTAXderived diatom biomass accounted for the highest percentage of the total biomass (43.1% of Chlaon average). The proportion of diatom biomass showed an upward trend after decreasing, whereas that of the dinof lagellate biomass presented the opposite trend(Fig.4). Prasinophyte biomass was low (<0.11-μg/L Chla) before April 19 and gradually increased to the maximum value of 0.30 μg/L Chlain the late stage of the algal bloom (Fig.4a). On average, chlorophytes,haptophytes, cyanobacteria, and chrysophytes accounted for only 13.8%, 0.7%, 1.1%, and 2.1% of the biomass, respectively (Fig.4b).
3.4 Phytoplankton community structure based on microscopy observation
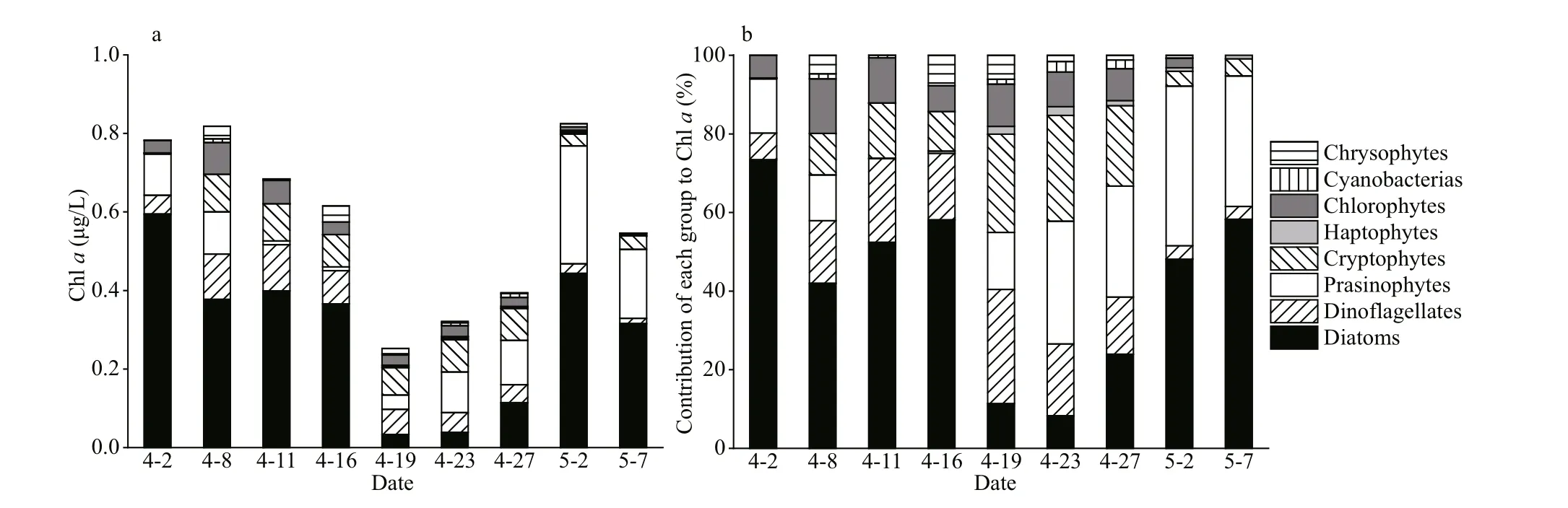
Fig.4 Contributions of various phytoplankton functional groups to Chl a and various phytoplankton biomasses in the Qinghuangdao coastal waters in spring
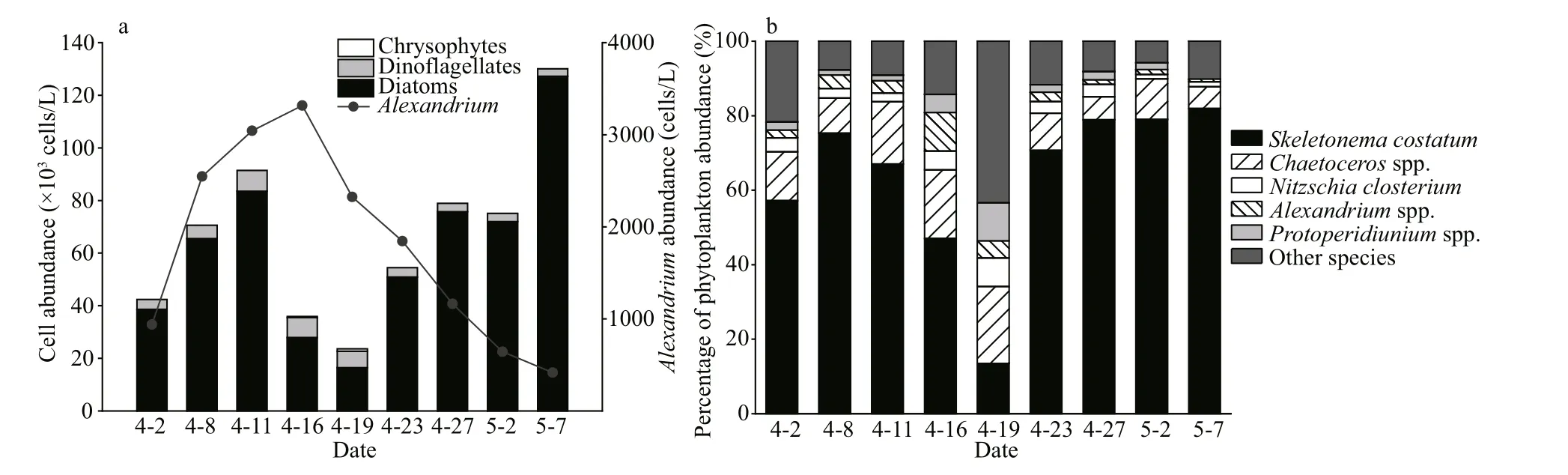
Fig.5 The cell abundances of phytoplankton in the Qinghuangdao coastal waters in spring based on microscopy observations
A total of 60 algal species were identif ied via microscopy observation. These species belonged to three phytoplankton groups: diatoms, dinof lagellates,and chrysophytes. The microscopy results showed that the total phytoplankton abundance ranged from 23.0×103cells/L to 130.1×103cells/L (Fig.5a). The phytoplankton community was mainly composed of diatoms (71.7%–97.8%) and dinof lagellates (2.2%–27.0%). The abundance of diatoms increased at f irst before April 11 and then decreased until April 19. Finally, it increased gradually. By contrast, the abundance of dinof lagellates f irst increased and then decreased. Chrysophytes were detected with low cell abundances (≤3.8% of the total phytoplankton abundance) on April 16 and 19 (Fig.5a).
The dominant species wereSkeletonemacostatum,Chaetocerosspp.,Nitzschiaclosterium,Alexandriumspp., andProtoperidiuniumspp.S.costatumwas the f irst dominant species. It accounted for 69.6%of the total phytoplankton cells on average (Fig.5b).Chaetocerosspp. was the second dominant genus(11.5% on average). Its abundance f irst increased and then decreased (Fig.5b).Alexandriumspp. (2.6% on average) andProtoperidiuniumspp. (2.1% on average)were the two dominant dinof lagellate genera (Table 2). The abundance ofAlexandriumspp. continued to increase during the pre-bloom period. It reached the maximum value of 3.3×103cells/L on April 16 and then decreased to 0.4×103cells/L on May 7 (Fig.5a).
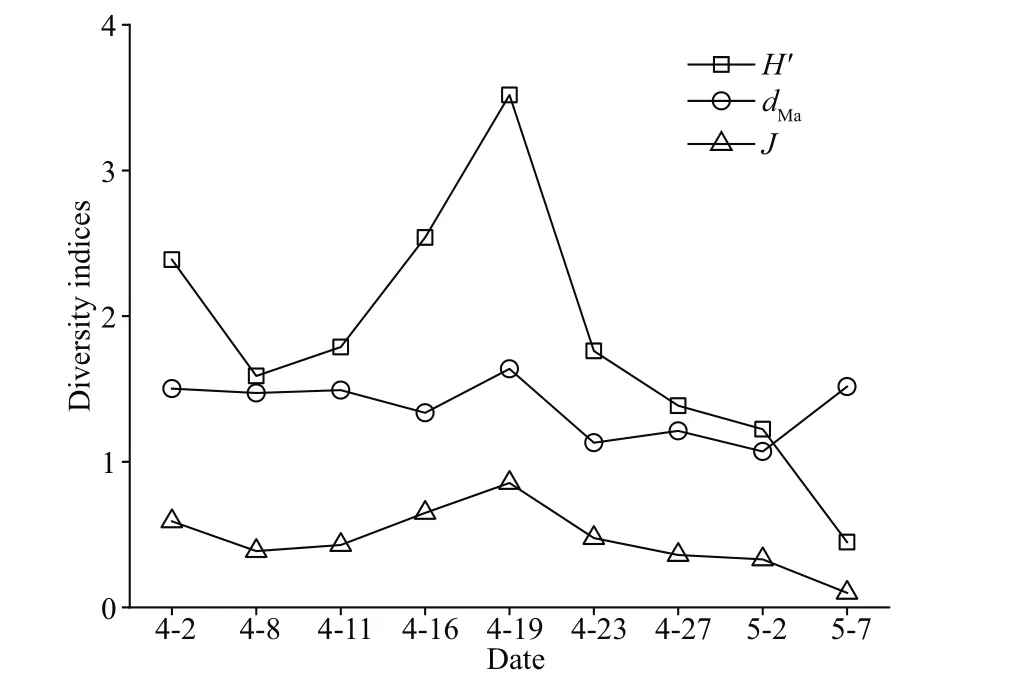
Fig.6 Diversity indexes of the phytoplankton community in the Qinghuangdao coastal waters in spring
Hʹ(0.44–3.52),dMa(1.07–1.64), andJ(0.10–0.85)reached their maximum values on April 19 (Fig.6).HʹandJincreased before April 19 and then decreased to their lowest values on May 7 (Fig.6).
3.5 Comparison of cell counts and CHEMTAX estimates
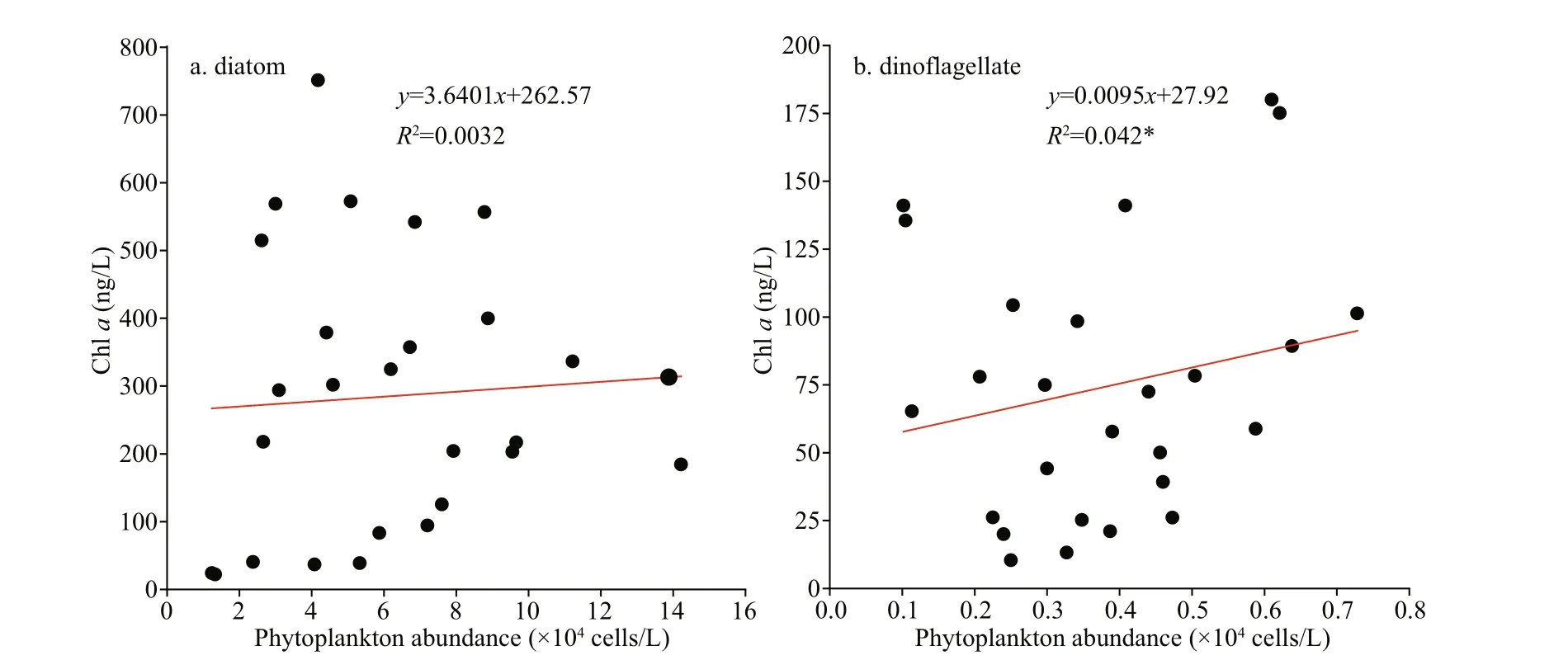
Fig.7 Linear relationship of the phytoplankton abundances obtained through microscopy cell counting with the CHEMTAXderived biomass (* P<0.05)
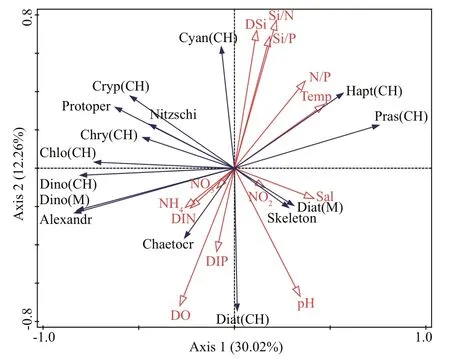
Fig.8 RDA ordination plots showing the relationships of phytoplankton species with environmental variables in the Qinghuangdao coastal waters
The CHEMTAX-derived Chlaestimates and phytoplankton cell abundances were compared.Statistical analysis showed that the algal cell abundance and CHEMTAX estimates for diatoms were not signif icantly correlated (P>0.05; Fig.7).The microscopy counting results and CHEMTAX estimates for dinof lagellates were signif icantly correlated (P<0.05).
3.6 Eff ects of environmental factors on phytoplankton community structure
RDA was conducted to investigate the correlation between phytoplankton and environmental factors(Fig.8). The cumulative contribution of the f irst two axes to the relationship between species and the environment was 72.14%. RDA showed thatAlexandriumwas negatively correlated with temperature. Diatom cell density was positively correlated with salinity and pH. Dinof lagellate cell density was positively correlated with NH4+, DIN, and DIP and negatively correlated with temperature. The Chl-avalues of diatoms were positively correlated with DIP and negatively correlated with DSi, N/P,Si/N, and Si/P. The Chl-avalues of dinof lagellates,chlorophytes, and chrysophyta were positively correlated with DIN and negatively correlated with DSi, N/P, Si/N, and Si/P. Temperature and salinity were the main environmental factors aff ecting cryptophytes and haptophytes (Fig.8).
4 DISCUSSION
4.1 Dynamics of Alexandrium spp. in the Qinghuangdao sea area
Two conditions are needed for the formation of HABs: an algal density exceeding 105or 106cells/L and water discoloration (Anderson, 2014). However,events wherein the density of toxic algae (e.g.,Alexandriumspp.) is low are considered as algal blooms in consideration of environmental safety,especially food safety. In the Gulf of Maine, shellf ish harvest is banned whenAlexandriumconcentrations reach 100 cells/L to prevent paralytic toxin poisoning(Wells et al., 2020). In this study,Alexandriumspp.dinof lagellates in seawater samples were observed under microscopy but could not be accuratelyidentif ied. Previous investigations have suggested thatA.catenellaandA.pacif icumare the most likely causative species of poisoning outbreaks in the Qinhuangdao coastal area (Gao et al., 2015; Yu et al., 2021). A parallel experiment conducted by Zhang(2020) revealed that the toxin content in mussels is highly consistent with the abundance ofAlexandriumcells in this area. For example, the highest density ofAlexandriumwas 3.3×103cells/L (on April 16)(Fig.5a), and the PSP content in mussels reached the highest value (929 μg STXeq/kg meat, exceeding the regulation limit of 800 μg STXeq/kg meat)during the whole investigation period (Zhang, 2020).Consequently, in the present study, the minor intrusion of an undesirable species (i.e., genusAlexandrium)into the common phytoplankton community was considered as anAlexandriumbloom event (Wells et al., 2020).
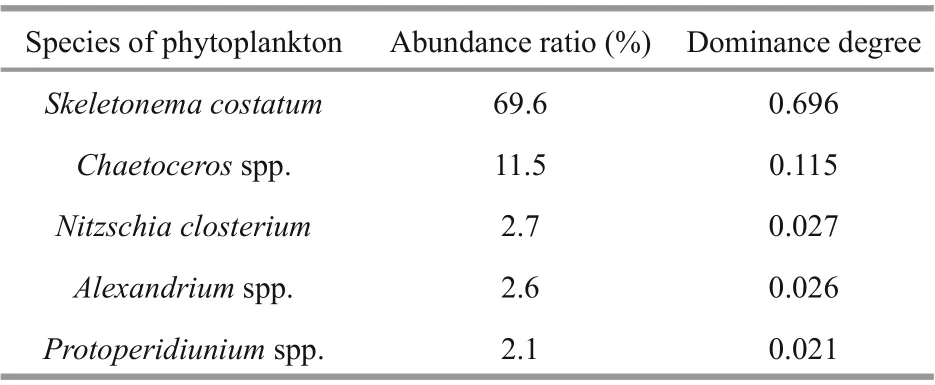
Table 2 Dominant species of phytoplankton in the Qinghuangdao coastal waters in spring
In this study,Alexandriumspp. showed good growth potential at 8.0–11.0 °C. Regression analysis revealed that the highest cell abundance ofAlexandriumspp. appeared at approximately 10 °C (Fig.9). Similarly, the highest cell densities were observed at 8–9 °C in Oppa Bay (Ichimi et al.,2001). In addition, the temperature associated with the highest cell density was higher in some sea areas than in Qinhuangdao. For example, the maximum cell density ofAlexandriumin Northport-Huntington Bay and the southern coast of Korea was observed at approximately 15 °C (Hattenrath et al., 2010; Kim et al., 2020). The highest cell densities ofAlexandriumminutumwere observed in the Penze Estuary (France)at 16.3–18.9 °C (Maguer et al., 2004). The small variations in salinity (32.00–32.20) suggested that in the study area, temporal variation was less inf luenced by salinity than by other factors (Figs.2a & 8).
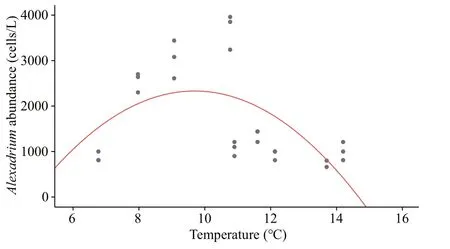
Fig.9 Curve f itting of Alexandrium cell abundance with the temperature in the Qinhuangdao sea area
Nutrient concentration and structure are also important factors that inf luence the dynamics ofAlexandriumin nearshore and coastal areas(Hattenrath et al., 2010; Jiang et al., 2014). An investigation in Northport by Hattenrath et al.(2010) showed that N input from sewage effl uent plays an important role in the development and toxicity ofAlexandriumfundyenseblooms. Among diff erent N components, NH4+may be the most eff ective in increasing the density ofA.fundyense(Hattenrath et al., 2010). Our results showed that NH4+concentration was higher than NO3ˉ and NO2ˉ concentrations (Fig.2c) with the former seeming to have a greater eff ect onAlexandriumthan the latter as shown by RDA (Fig.8). However, the abundance ofAlexandriumspp. decreased gradually when DIP limitation (<0.1 μmol/L) occurred, and the ratios of DIN/DIP and DSi/DIP increased to 30 starting on April 19 (Figs.2 & 5). Previous studies suggested thatAlexandriumspp. are poorer phosphorus competitors than other algal species (Jiang et al., 2014). RDA further corroborated the above conclusions by demonstrating thatAlexandriumspp. had a positive correlation with dissolved inorganic nitrogen and phosphate (Fig.8). Similar results were observed in the sea areas of the Nanji Islands, whereA.tamarenseblooms are terminated by low DIP concentrations(<0.1 μmol/L) (Jiang et al., 2014). In summary, we suggest that NH4+has a great eff ect onAlexandriumblooms, whereas DIP limitation may be responsible for the collapse ofAlexandriumblooms. Moreover,dissolved organic nitrogen (DON) may play an important role in supportingAlexandriumblooms(Hattenrath et al., 2010). This role, however, was not quantif ied in this study. Future studies should pay further attention to DON concentrations because they may stimulate the blooms ofA.anophageff erensin the Qinhuangdao coastal areas (Ou et al., 2018).
4.2 Temporal variability in phytoplankton assemblages and related inf luencing factors in spring
Previous results have suggested that the abundance of dinof lagellates along the coastal areas of Qinhuangdao often peak in spring (Chen et al., 2016;Xu et al., 2017). In this study, microscopy observation revealed that in the study period, diatoms and dinof lagellates coexisted; however, the former was more dominant than the latter (Fig.5; Table 2). One reasonable explanation for this phenomenon is that dinof lagellates generally exhibit slower growth rates than diatoms, and the latter can apparently outcompete dinof lagellates even when silicate decreases to nearlimiting concentrations in late spring (Grenney et al.,1973; Parsons et al., 1978). The dominant diatom taxa, i.e.,Skeletonemaspp. (warm-water species) andChaetocerosspp. (oceanic species), have rapid growth rates and can quickly exploit available resources and dominate the phytoplankton community (Banse, 1982;Yang et al., 1996). Thus, diatoms can apparently still outgrow and outcompete dinof lagellates even when silicate decreases to near-limiting concentrations in the late spring and early summer (Grenney et al.,1973; Parsons et al., 1978).
The comparison of algal cell abundance and CHEMTAX biomass revealed a signif icant positive correlation only for dinof lagellates but not for diatoms (Fig.7). Only two dinof lagellate taxa,i.e.,Alexandriumspp. andProtoperidiuniumspp.,coexisted in the seawater (Fig.5b) and showed similar cell sizes (approximately 18–31 μm). By contrast, the species number of diatoms was considerably larger than that of dinof lagellates (Fig.5a), and the cell sizes of diff erent diatom taxa were quite diff erent (Pan et al., 2020). The latter may be the key reason for the nonsignif icant linear correlation between the cell abundance and CHEMTAX biomasses of diatoms.The omission of small diatoms by microscopy was another inf luencing factor (Agirbas et al., 2015).
Small nondiatoms can be regarded as the background component of the planktonic community that is responsible for the recycling of organic matter within the euphotic layer (Seoane et al., 2011). However, some algal species, such as cryptophytes, cyanobacteria,and prasinophytes, are often neglected in microscopy observation due to the limitations of the method. This study showed that HPLC-pigment CHEMTAX is a suitable tool for assessing the composition and biomass of phytoplankton groups in the Qinhuangdao sea area.To the best of our knowledge, the CHEMTAX analysis of the phytoplankton community in this area has yet to be reported. Our results conf irmed that prasinophytes,chlorophytes, and cryptophytes substantially contributed to the total Chl-apool; these results were not observed via microscopy (Figs.4–5). The combination of microscopy and HPLC-pigment CHEMTAX highlighted the complementary advantages of the two methods in studies on algal blooms.
The present study showed through microscopy and HPLC analyses that the phytoplankton community changed obviously in just 36 days (Figs.3–5). Several physicochemical variables, such as temperature,salinity, and nutrient availability and structure,inf luence the temporal variations in phytoplankton communities (Álvarez-Góngora and Herrera-Silveira,2006; Hunt et al., 2010). These changes conf irmed that the phytoplankton community structure in the Qinhuangdao coast was not invariable in spring.This variability might be a ref lection of the complex interplay between diff erent phytoplankton taxa and other abiotic and biotic factors (Kremp et al.,2009). Considering this phenomenon, representing the seasonal variations in phytoplankton community structure through a single sampling event in spring may result in deviations from the results of previous studies (e.g., Lu et al., 2018; Wang et al., 2018;Miranda-Alvarez et al., 2020).
The rapid increment in seawater temperature in the spring (Fig.2a) may be an important environmental parameter that aff ects marine biological processes (Cui et al., 2018; Lu et al., 2018; Pan et al., 2020). Previous studies have suggested that temperature could induce changes with a regular and predictable pattern in phytoplankton community structure (Mendes et al.,2015; Pan et al., 2020). In general, diatoms usually f lourish at low temperatures in nearshore areas(<18 ℃) (Wasmund et al., 2011; Pan et al., 2020).In this study, the seawater temperature was low(<14.2 °C), and diatoms and temperature were not closely correlated (Fig.8). The signif icant increase in the proportion of prasinophytes and haptophytes in the total phytoplankton biomass with temperature was a prominent phenomenon (Figs.4 & 8). Similarly, Lu et al. (2018) reported that in the central Bohai Sea,phytoplankton assemblages transition from diatomdominated in spring to f lagellate-dominated (mainly haptophytes and prasinophytes) in early summer. Thus,temperature rise might be the key factor promoting the growth of prasinophytes and haptophytes in the sea area under study, as well as in the central Bohai Sea(Pan et al., 2020; Yan et al., 2020).
Nutrient concentration and structure are other important factors aff ecting the species composition and biomass of phytoplankton in the marine environment(Zhang et al., 2004; Xu et al., 2010; Pei et al., 2019).Given that diff erent phytoplankton taxa have diff erent nutrient demands and uptake capabilities, nutrient imbalance or limitation is an important reason for the poverty of some phytoplankton taxa or the changes in community composition (Xu et al., 2010). Usually,nutrient-enriched coastal waters support the growth of large phytoplankton, e.g., diatoms and dinof lagellates(Sarthou et al., 2005; Lionard et al., 2008). Similarly,our results showed that diatoms and dinof lagellates were the main phytoplankton assemblages when DIN and DIP were high on April 19 (Figs.4 & 5). This result was further conf irmed by RDA, which revealed a positive correlation between dinof lagellates(microscopy counts and CHEMTAX estimates) and diatoms (CHEMTAX estimates), as well as DIN and DIP concentrations (Fig.8). Prasinophytes have a broad capability to respond to nutrient variations (Not et al., 2004). In this study, the negative association of prasinophyte biomass with DIN and DIP (Fig.8)suggested that nutrient concentration was not the factor that stimulated their increase in the study area. By contrast, the positive correlation of the CHEMTAX-estimated biomasses of chlorophytes and cryptophytes with DIN implied that DIN played an important role in the growth of these taxa. Moreover,although DSi concentration is an important factor that aff ects the growth of diatoms in some coastal areas(Drira et al., 2014; Erga et al., 2014), in this study,diatom biomass was not positively related to DSi concentration (Fig.8). This result indicated that DSi concentration was not a limiting factor of the diatoms in this sea area.
5 CONCLUSION
This study investigated the occurrence of the lowdensity blooms ofAlexandriumspp. in the Qinhuangdao coastal area in spring of 2019. The highest abundance ofAlexandriumspp. was 3.3×103cells/L and was observed at approximately 10 °C.Alexandriumspp. density gradually decreased starting on April 19 upon DIP limitation. It is important to note that even at low density and low Chl-aconcentration,Alexandiumbloom could cause serious harmfulness,which might be ignored in HAB monitoring. The temporal succession of the phytoplankton community during the period ofAlexandriumblooms was revealed by using microscopy and HPLC. In addition to the predominant diatoms and dinof lagellates,small nondiatoms (i.e., cryptophytes, cyanobacteria,and prasinophytes) substantially contributed to the total Chl-apool during the investigation. This study further demonstrated the importance of the combination of microscopy cell counting and CHEMTAX estimation for the investigation of phytoplankton communities. The temporal variations in phytoplankton assemblages were most likely inf luenced by temperature and nutrient availability.Increases in temperature promoted the growth of prasinophytes and haptophytes. The concentrations of DIN, of which NH4+was the most inf luential, and DIP aff ected the succession of diatoms and dinof lagellates.Consequently, further attention should be paid to the short-term changes in phytoplankton communities to improve the understanding of the laws governing the temporal variabilities of phytoplankton assemblages in the Bohai Sea.
6 DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENT
The authors would like to thank Mr. Liqiang FAN and Mrs. Qianqian GENG from Key Laboratory of Testing and Evaluation for Aquatic Product Safety and Quality, Ministry of Agriculture and Rural Aff airs, Yellow Sea Fisheries Research Institute,Chinese Academy of Fishery Sciences, Qingdao 266071, China, for assistance in f ield investigation and sample analysis.
 Journal of Oceanology and Limnology2022年6期
Journal of Oceanology and Limnology2022年6期
- Journal of Oceanology and Limnology的其它文章
- Overview of harmful algal blooms (red tides) in Hong Kong during 1975–2021
- Information standardization for typical toxic and harmful algae in China’s coastal waters—a case study of Karenia mikimotoi*
- Biochemical composition of the brown tide causative species Aureococcus anophageff erens cultivated in diff erent nitrogen sources*
- Identif ication of paralytic shellf ish toxin-producing microalgae using machine learning and deep learning methods*
- Screening for lipophilic marine toxins and their potential producers in coastal waters of Weihai in autumn, 2020*
- First observation of domoic acid and its isomers in shellf ish samples from Shandong Province, China*
