First observation of domoic acid and its isomers in shellf ish samples from Shandong Province, China*
Guanchao ZHENG , Haiyan WU , Mengmeng GUO , Jixing PENG , Yuxiu ZHAI ,2,3,Zhijun TAN ,2,3,**
1 Key Laboratory of Testing and Evaluation for Aquatic Product Safety and Quality, Ministry of Agriculture and Rural Aff airs,Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao 266071, China
2 Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao 266237, China
3 Collaborative Innovation Center of Seafood Deep Processing, Dalian Polytechnic University, Dalian 116034, China
Abstract A total of 133 shellf ish samples were collected in seven cities of Shandong Province, China, from May to October, 2019. The domoic acid (DA) concentrations were determined by liquid chromatographytandem mass spectrometry (LC-MS/MS), and their distribution characteristics were investigated. DA concentration was detected high in over 1/3 (36.1%) of the samples of four kinds of shellf ish in all three seasons in range from 0 to 102 μg/kg. The highest DA concentrations were 102, 101, 36.7, and 10.2 μg/kg in Crassostrea gigas, Chlamys farreri, Mactra veneriformis, and Mytilus edulis, respectively. Geographically,Yantai (22.0 μg/kg) and Weihai (16.9 μg/kg) showed relatively high concentrations of DA, whereas Rizhao and Dongying presented only 0.85- and 1.76-μg/kg DA, respectively. DA concentrations in the shellf ish samples were strongly related to seasonal changes, being signif icantly higher in autumn and summer than that in spring. The DA risk exposure assessments indicate that dietary seafood consumption did not pose a health threat to general human population. In addition, three isomers (isoA, isoD, isoE) and 5ʹ-epimer DA were detected in 3.00%–15.80% of the shellf ish samples. This study is the f irst to observe DA and its isomers in shellf ish samples of Shandong Province. The results demonstrate that DA contamination is very common and should be continuously monitored.
Keyword: domoic acid; shellf ish; liquid chromatography-tandem mass spectrometry (LC-MS/MS);Shandong Province
1 INTRODUCTION
With rapid economic development of China in the past 30 years, water eutrophication has become a serious issue, with frequent occurrence of harmful algal bloom (HAB) (Lu et al., 2014). At least 60 HAB events have been annually recorded in China(Li et al., 2017).Pseudo-nitzschiaspp. are pennate chain-forming diatoms that are widely distributed in coastal waters around the world, and a number of these species have been reported to cause HAB(Bates et al., 2018). Domoic acid (DA), a HAB toxin produced byPseudo-nitzschiaspp., has attracted global attention owing to its frequent cause of amnesic shellf ish poisoning (ASP) (Gibble et al.,2021). The first ASP incident was recorded in 1987 in Prince Edward Island, Canada, with at least 4 deaths and 143 hospitalizations (Bates et al., 1989). DA can accumulate in some marine animals such as shellf ish,and can cause potential threat to seafood safety and human health through the food chain. To protect consumers from symptomatic acute exposure to DA,Canada f irst established a 20 000-μg/kg safety limit standard for DA in shellf ish after the first ASP event,which has been adopted by other countries, such as European Union, USA, and New Zealand (Lefebvre and Robertson, 2010). In addition, some isomers have been found in shellf ish in recent years (Regueiro et al.,2011; Ben Haddouch et al., 2016), which exhibited varying toxicity (Sawant et al., 2008, 2010).
China is the largest producer of seafood in the world (Szuwalski et al., 2020). Shellf ish are f ilter feeders that can easily accumulate a variety of pollutants, including DA. Although no ASP event has been recorded in China, several studies have shown that long-term low dose DA exposure could also cause potential harm to human health. It has been demonstrated that chronic low level DA exposure could cause cognitive def icits in mice without any visible histopathological lesions in brain (Lefebvre et al., 2017). Besides, low dose DA exposure has also been noted to signif icantly alter zebraf ish gene expression and disrupt myelin structure (Panlilio et al., 2020).
Shandong Province, located in the eastern coast of China, extends to the Bohai Sea (BS) and Yellow Sea(YS), with a coastline of approximately 3 345 km,covering one-sixth of China’s coastline (Fu et al.,2018). With rapid industrialization and urbanization in these areas, many pollutants, including toxic metal pollutants (Zhou et al., 2020), perf luorinated alkyl substances (Guo et al., 2019), microplastics (Zhang et al., 2020), pesticides (Yin et al., 2015), as well as paralytic shellf ish toxins (PSTs), diarrhetic shellf ish toxins (DSTs) and DA (Chen et al., 2019; Gu et al.,2022; Liang et al., 2022), have been detected. Some investigations have found potential DA-producing algae such asPseudo-nitzschiagalaxiae(Xu and Li,2015),Pseudo-nitzschiapungensvar.averiensis, andPseudo-nitzschiapungensvar.pungens(Dong et al.,2018) in the coastal areas of Shandong Province. In recent years, DA-contaminated shellf ish have been continuously detected in the areas around Shandong Province, such as Liaoning and Zhejiang Province(Li et al., 2017; Chen et al., 2019). Although the concentration of DA in shellf ish had been reported to be relatively low (<20 000 μg/kg), contamination and HAB incidences pose potential health risk to humans.Hence, knowledge on the distribution characteristics of DA in shellfish and health risk of human exposure to DA in Shandong Province is crucial.
Accordingly, the present study aimed to investigate the (1) general characterization of DA and its isomers concentrations in shellf ish samples by employing liquid chromatography-tandem mass spectrometry(LC-MS/MS); (2) spatial and species variations of DA in shellf ish samples; (3) relationships between DA concentrations and seasonal variation; and (4) health risk of human exposure to DA through aquatic food consumption. The results of this study could provide background data on DA contamination in Shandong Province.
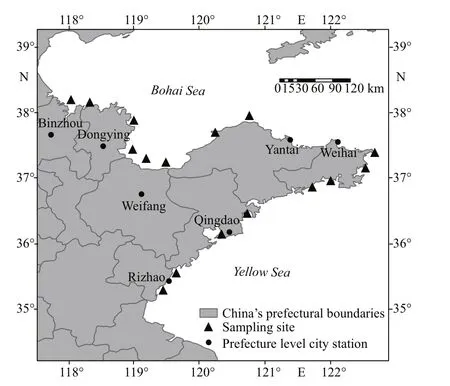
Fig.1 Sampling stations along the Shandong Province in 2019
2 MATERIAL AND METHOD
2.1 Sample collection
Shellf ish samples were collected from May to October 2019 from shellf ish farms in the following cities along the coastal areas of the Shandong Province:Binzhou (BZ), Dongying (DY), Weifang (WF), Yantai(YT), Weihai (WH), Qingdao (QD), and Rizhao (RZ)(Fig.1). A total of 133 shellf ish samples were collected,which consisted of the following main cultured categories: 64 clams (Ruditapesphilippinarum,Cyclinasinensis,Meretrix, andMactraveneriformis),nine mussels (Mytilusedulis), 34 scallops (Chlamysfarreri), and 26 oysters (Crassostreagigas). The shellf ish samples were collected randomly from the local shellf ish culture area and sorted by location and type of specimens, washed with clean water, stored in a portable refrigerator at <4 °C, and transported to the laboratory within 48 h. The whole soft body tissue of the shellf ish samples was homogenized with a T18 basic Ultra-Turrax mixer at 24 000 r/min(IKA, Germany), and the homogenates were stored at-20 °C until LC-MS/MS analysis.
2.2 Sample analysis
Sample extraction and cleanup were conducted according to a previously described method with minor modif ications (Furey et al., 2001). A total of 5.00±0.02 g of homogenized shellf ish tissue were added to a 50-mL polypropylene centrifuge tube,and extracted with 12.0 mL of 50% MeOH. Then,the sample was vortexed for 1 min, ultrasonicated for 10 min, vortexed for 1 min, and centrifuged at 4 000 r/min for 10 min. The resulting supernatant was transferred into a new 50-mL polypropylene tube and the pellet was re-extracted with 5.0 mL of 50%MeOH. The volume of the combined supernatants was adjusted to 25.0 mL with the extracting solvent,vortexed for 1 min, and centrifuged for 15 min at 10 000 r/min.
The Bond Elut SAX cartridge (3 mL, 500 mg,Agilent Technologies, USA) was preconditioned prior to sample loading with MeOH (6.0 mL), H2O(3.0 mL), and 50% MeOH (3.0 mL), respectively,and 5.0 mL of the sample extract were loaded on the cartridge. Then, the cartridge was washed with 5.0 mL of 10% v/v acetonitrile and eluted with 4.0 mL of 0.3% v/v formic acid at a constant f low rate of 2 mL/min. The eluate was adjusted to 4.0 mL with eluting solvent, f iltered through a 0.22-μm mixed cellulose f iltration membrane (Agela Technologie,China), and transferred into autosampler vials prior to LC-MS/MS analysis.
LC-MS/MS analysis was performed using an U3000 HPLC system (Thermo Scientif ic, USA)linked to a Thermo TSQ Endura mass spectrometer equipped with an ESI source operated in positive ion mode. The chromatographic system was equipped with a binary pump and an autosampler with a 100-μL sample loop. By employing f low injection analysis at 10 μL/min, the mass spectrometer was tuned using CRM-DA-f DA standard solution (1.0 μg/mL),and the f inal ion source conditions were as follows:spray voltage of +3.50 kV, sheath gas f low of 25 arb,auxiliary gas f low of 15 arb, sweep gas f low of 0 arb,ion transfer tube temperature of 300 °C, and vaporizer temperature of 250 °C. Full-scan MS in the positive ion mode produced predominant peak at [M+H]+m/z312.0, which was selected as the precursor ion. The SRM transitions from the protonated DA ion werem/z312.0 >266.0 ([M-HCOOH+H]+) at a collision energy (CE) of 15 V used for quantitative analysis,and conf irmatory transition wasm/z312.0 >248.0([M−HCOOH–H2O+H]+) at CE of 16 V, under the conditions of RF lens of 142 V, dwell time of 100 ms,and collision-induced dissociation gas setting of 1.5 mTorr.
A total of 10 μL of the sample were injected into a LC Kinetex C18column (2.1×100 mm, 2.6 μm,Phenomenex, USA). Before the detection of isomers,we f irst used a fast gradient method to screen the total DA in the shellf ish samples. The system was operated under the following gradient elution program: solution A (2-mmol/L ammonium formate in H2O) and solution B (100% MeOH) at a f low rate of 0.3 mL/min: 0–1 min, 20% B; 1–3 min, 20%–90%B; 3.0–3.1 min, 90%–20% B; and 3.1–8.0 min, 20%B. The DA retention time was around 2.94 min. If it was a positive sample (detection limit = 0.08 μg/kg),the isomers were detected. The system was operated under the following gradient elution program:solution A (0.1% formic acid in H2O) and solution B (0.1% formic acid in acetonitrile) at a f low rate of 0.3 mL/min: 0–1 min, 5% B; 1–20 min, 5%–20%B; 20–22 min, 20%–5% B; 22–25 min, 5% B. Both the certif ied calibration solution (CRM-DA-f) and mussel tissue reference material (CRM-ASP-Mus-b)for DA were purchased from Certif ied Reference Materials Program, National Research Council(Halifax, NS, Canada). Individual stock solutions of DA were prepared in acetonitrile/water (1꞉9, v/v)and stored in amber glass vials at -20 °C. Further dilutions and mixtures were prepared by following appropriate procedures. The average recovery of DA from CRM-ASP-Mus-b was 90% (n=3). Figure 2 shows a typical chromatogram of total DA standard solution (10.0 ng/mL) and real oyster samples with fast gradient method, respectively.
2.3 Dietary intake and risk assessment
To evaluate the health risk of human exposure to DA following consumption of diff erent shellf ish produced in coastal Shandong Province, Estimated Daily Intake (EDI, ng/(kg·d)) and Hazard Index(HR) were determined based on the measured DA concentration in shellf ish and oral reference dose(RfD). A HR <1.0 indicated acceptable risk and HR >1.0 represented potential health risk of human exposure to DA. The EDI and HR were calculated as follows (Wang et al., 2021):
EDI=(C×Q)/BW,
HR=EDI/RfD,
whereCis the mean DA concentration (μg/kg, wet weight) in shellf ish samples,Qis the average daily consumption of aquatic food (g/d), and BW is the average body weight (kg) of Chinese in diff erent age and gender groups. An RfD of 30 μg DA/kg body weight was considered in accordance with the European Food Safety Authority (EFSA, 2009).
Fig.2 Chromatogram of total DA standard solution (10.0 ng/mL) (a) and real oyster samples (b)
3 RESULT
3.1 General characterization of DA concentrations in shellf ish samples
A total of 133 shellf ish samples, including seven shellf ish species, were collected from the coastal areas of Shandong Province. Among them, 48 shellf ish samples contained detectable DA. The DA detection rate was relatively high (36.1% of the samples),which indicated widespread DA contamination in Shandong Province. The mean DA concentration in all the shellf ish samples was 7.33 μg/ kg and ranged from 0 to 102 μg/ kg.
3.2 Spatial variation of DA in shellf ish samples
In this study, shellf ish samples were collected from shellf ish culture areas in seven cities along the coastline of Shandong Province. The number of samples collected from WH, QD, DY, WF, YT, RZ,and BZ was 30, 26, 25, 20, 12, 12, and 8, respectively.At least one sample was contaminated with DA in each city, and the DA concentrations varied among diff erent locations (Fig.3). The DA detection rate in diff erent cities presented the following trend: WH(50.0%), BZ (50.0%)>DY (44.0%)>WF (40.0%)>YT (33.3%)>QD (19.2%)>RZ (8.33%). Although the highest mean DA concentration was found in shellf ish samples from YT (22.0 μg/ kg), the maximum DA concentration (102 μg/ kg) was noted in shellf ish samples from WH. Besides, shellf ish samples from BZ (mean DA concentration, 8.85 μg/ kg) and DY(mean DA concentration, 1.76 μg/ kg) showed relatively high DA detection rate; however, their mean DA concentrations were comparatively low.The lowest mean DA concentration and detection rate were observed in RZ (10.2 μg/ kg, DA was detected in only one shellf ish sample in this city).
3.3 Species variation of DA in shellf ish samples
The DA concentrations varied among diff erent shellf ish species (Fig.4). Among the samples presenting detectable DA concentration, the highest DA concentration was noted inC.gigas(102 μg/ kg),whereas the lowest DA concentration was found inM.veneriformis(0.15 μg/ kg). As indicated in Fig.4,oysters showed higher mean DA concentration than the other shellf ish species examined. The mean DA concentration in diff erent shellf ish species exhibited the following trend: oysters (24.0 μg/ kg)>scallops (5.89 μg/ kg) >clams (2.20 μg/ kg) >mussels(1.14 μg/ kg). Oysters presented the highest DA detection rate (80.8%, 21 of 26 shellf ish samples),followed by clams (35.9%, 23 of 64 shellf ish samples), mussels (11.1%, one of nine shellf ish samples), and scallops (8.82%, three of 34 shellf ish samples). Although scallops showed a relatively low DA detection rate, the DA concentrations in twoC.farrerisamples (101 and 96.1 μg / kg, respectively)were higher than that in most of the shellf ish samples,close to the highest DA concentration in oysters.In contrast, DA contamination in mussels was not serious, and was detected in only oneM.edulissample.
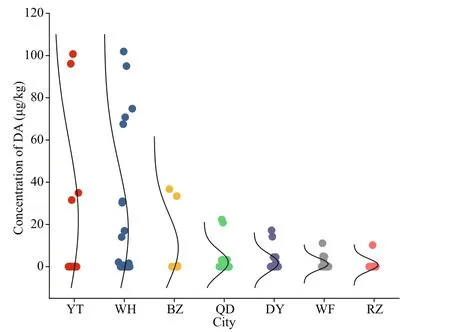
Fig.3 Spatial variation of DA in shellf ish samples collected from seven cities along the Shandong Province
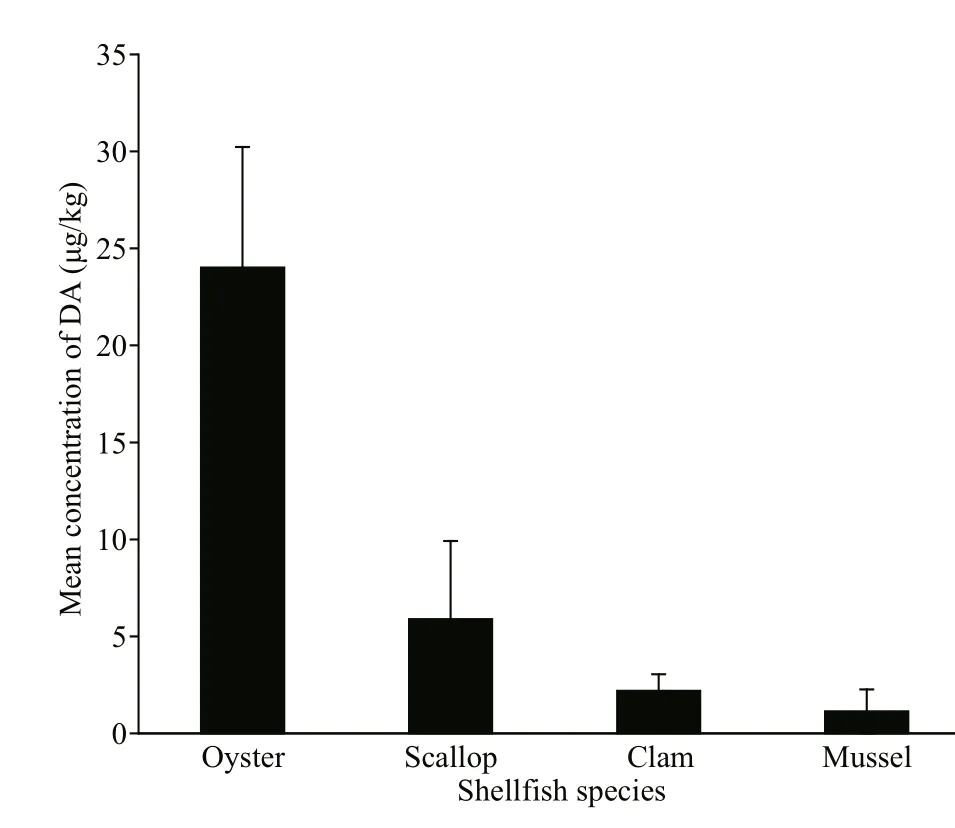
Fig.4 Contamination of DA in diff erence shellf ish species
3.4 Relationships between DA concentrations in shellf ish samples and seasonal variation
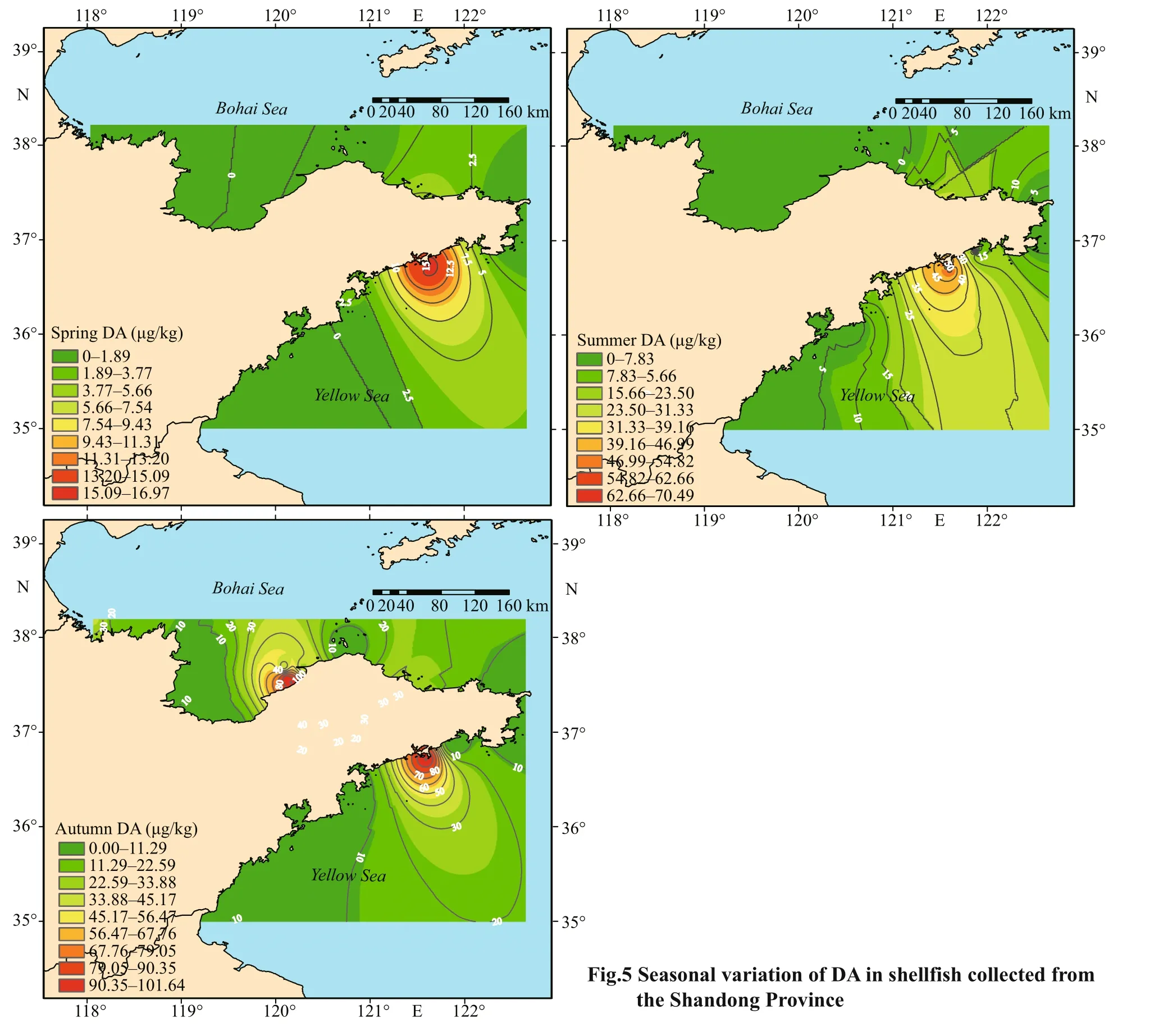
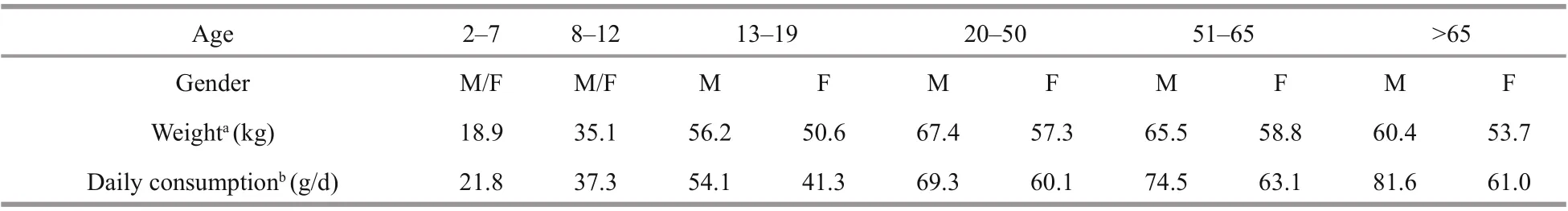
Table 1 Daily aquatic food consumption for diff erent age and gender groups

Fig.6 Ratio of DA and isomers in shellf ish collected from the Shandong Province
A total of 42, 54, and 37 shellf ish samples were collected from the coastline of Shandong Province in 2019 spring (March–May), summer (June–August),and autumn (September–November), respectively.The DA concentrations in the shellf ish samples were strongly related to seasonal variation (Fig.5). The DA detection rate and mean concentration in shellf ish samples increased with seasonal variation and the toxin was detected in 14.3%, 40.7%, and 54.0% of the shellf ish samples collected in spring, summer,and autumn, respectively. Shellf ish samples collected in autumn (mean DA concentration, 16.5 μg/ kg)and summer (mean DA concentration, 6.05 μg/ kg)presented higher DA concentrations in the range of 0–102 and 0–74.8 μg/ kg, respectively, when compared with those collected in spring (mean DA concentration, 0.90 μg/ kg). In contrast, low DA levels were detected in shellf ish samples collected in spring,with DA concentrations ranging from 0 to 17.0 μg/ kg.
3.5 Risk assessment evaluation
Table 1 shows the daily aquatic food consumption by Chinese residents in diff erent age and sex groups based on the monitoring report on nutrition and health status of Chinese residents (2010–2013) (Piao et al.,2019) and the f ifth China total diet study (Wu et al.,2018). Aquatic food consumption was divided into 10 age/sex groups and ranged from 21.8 to 81.6 g/d.The average weight of males was higher than that of females in the age group of >13 years old. The EDI and HR were calculated based on the average weight,daily aquatic food consumption for each group and RfD of DA. The highest EDI (9.90 ng/(kg·d)) was noted in the age group of >65 years, whereas the lowest EDI (5.98 ng/(kg·d)) was determined in the 13–19 years old female group (Table 2). The HR of DA exposure through aquatic food consumption fordiff erent age and gender groups ranged from 0.20‰to 0.33‰, which were lower than the risk value of 1.
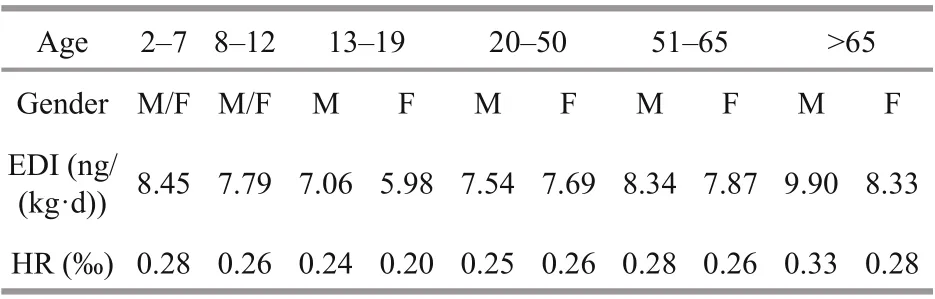
Table 2 Hazard index of DA exposure through aquatic food consumption for diff erent age and gender groups
3.6 Composition characteristics of DA and its isomers concentrations in shellf ish samples
The detection rates of isoE, isoD, isoA, and epi-DA in all collected shellf ish samples were calculated to be 15.8%, 15.8%, 14.3%, and 3.00%, respectively.The ratio of isomers to DA was around 18.0% (Fig.6).Figure 7a shows the LC-MS/MS chromatogram of DA and its isomers standard solution (DA, 1 007 ng/mL;isoA, 5.2 ng/mL; isoD, 4.1 ng/mL; isoE, 0.13 ng/mL;and epi-DA, 11 ng/mL) with slow gradient method.One large peak and several smaller peaks were observed, whose retention times coincided with those of isoE (7.93 min), isoD (8.28 min), isoA (8.75 min),DA (9.65 min), and epi-DA (10.22 min) of the standard(CRM-DA-f). Although the isomers concentrations were low, f ive DA and isomers were also detected in some scallop and oyster samples (Fig.7b). No DA or isomers were detected inC. sinensisandR.philippinarum, and small amounts of DA and isomers were found in other bivalve species collected from diff erent cities. The mean isoE (1.35 μg/kg) and isoD (1.27 μg/kg) concentrations in shellf ish samples were higher than mean isoA (0.63 μg/kg) and epi-DA (0.12 μg/kg). The ratio of isomers in oysters was similar to that in scallops and clams.
4 DISCUSSION
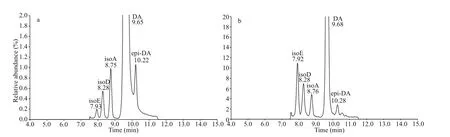
Fig.7 Chromatogram of DA and its isomers standard solution (a) and real oyster samples (b)

Table 3 DA reported in Chinese marine organisms
To accomplish high-quality protein production in China, mariculture has become one of the fastest growing industries since the 21stcentury (Yu and Han,2020). Shandong Province is an important region of China in aquaculture and f ishery with a gross ocean product of >1 220 billion CNY (Chinese Yuan) in 2015, ranking the second among all provinces and accounting for 17% of China’s gross ocean product(Su and Yang, 2018). Although there are no relevant studies on DA in shellf ish along the coastal areas of Shandong Province, relatively low or negligible DA concentrations have been detected in several shellfish in nearby provinces or sea areas, such as in scallop (C.farreri) in Liaoning Province, clam(R.philippinarum) in Bohai Sea, and oyster (O strearivularisGould) in Shantou (Chen et al., 2001, 2019).The highest DA concentration (14 900 μg/kg) was noted in shellf ish collected from a local market in Hulu Island, Liaoning Province in 2017, which is higher than that detected in this study (100 μg/ kg). Besides,DA has also been detected in other marine organisms,such as crab (Thalamitacrenata, 18 200 μg/kg) (Ji et al., 2011). Although the DA concentrations detected in shellf ish and other marine organisms in China have not exceeded the safety limit of 20 000 μg/kg and only few samples have been found to contain relatively high DA concentrations, more attention must be paid to DA contamination (Table 3).
To the best of our knowledge, there is no study on DA contamination of shellf ish in Shandong Province.The DA concentration in shellf ish (Table 3) collected from the coast of China’s other provinces was found consistent with that we observed in this study. Besides,investigation of the DA concentration in diff erent shellf ish in Zhoushan City, located in East China Sea,revealed 54%–69% DA detection rate from diff erent sampling batches (Wang, 2011), which is higher than that determined in shellf ish samples from all the cities examined in the present study. However, it must be noted that most of the DA concentrations in shellf ish from Zhoushan City were <1 000 μg/kg, which is only slightly higher than those detected in this study.Pseudo-nitzschiaspp. are distributed worldwide in marine waters, and are known to produce the toxin,DA. According to a recent research, 58Pseudonitzschiaspp. have been identif ied around the world,of which 31 species have been reported in Chinese coastal waters, including the coastal region of Shandong Province (Chen et al., 2021). Since the identif ication of the f irst toxigenic diatom species(Pseudo-nitzschiasimulanssp. nov.) in Chinese waters (Li et al., 2017), several other ChinesePseudonitzschiastrains have been conf irmed to produce DA (Huang et al., 2017, 2019; Pang et al., 2018).In the present study, shellf ish products from each of the examined city were found to be contaminated with DA, although the toxin concentration widely varied among the cities. In particular,P.galaxiae,P.pungensvar.averiensis, andP.pungensvar.pungenshave been detected in Qingdao, located in the coast of Shandong Province (Xu et al., 2015; Dong et al.,2018), which are potentially toxic diatom species that can produce DA under suitable growth conditions(Bates et al., 2018). The spatial variation of DA accumulation in the seven cities examined may be related to diff erent DA-producing species. Although this was expected in nature, it must also be emphasized that the sampling was opportunistic, and diff erences might have coincided with the sampling date. While most of the previous studies on DA contamination of shellf ish in China have focused on the Chinese southeast coastal waters (Huang et al., 2019; Chen et al., 2021) and have detected manyPseudo-nitzschiaspp., only a relatively few studies have investigated onPseudo-nitzschiaspp. occurrence in Shandong Province, which requires more attention.
It has been reported that the DA depuration rates obviously varied among diff erent shellf ish species.Many bivalve species, including mussels such asMytilusgalloprovicialis(Blanco et al., 2002)andM.edulis(Novaczek et al., 1992), depurate DA from their bodies within a few hours or days.However, scallops (Placopectenmagellanicus)require a few weeks to depurate DA from their tissues(Douglas et al., 1997), while razor clams (Siliquapatula) depurate DA over many months (Trainer and Bill, 2004). Similarly, in the present study, the accumulated DA concentrations in diff erent shellf ish samples signif icantly varied. Although many studies have shown that DA accumulation and elimination rates widely vary in diff erent shellf ish species,the detoxif ication mechanism is still unknown.Nevertheless, a previous study has demonstrated that Pacif ic razor clams (S.patula) may contain more than one subtype of glutamate receptor, and could tolerate high concentrations of DA (Trainer and Bill, 2004). DA is a hydrophilic molecule with three carboxyl groups and an amine group (Shum et al.,2018), and mainly exists in a water-soluble state in shellf ish cells and is excreted from the body without being metabolized (Novaczek et al., 1991). Analysis of the digestive gland transcriptional response of the musselM.galloprovicialisto DA exposure indicated overexpression of several membrane transporters that belong to the family of solute carriers (Pazos et al.,2017). These membrane transporters may be effi cient transmembrane transporters that can depurate DA outside the tissues; thus, the slow depuration of DA in some shellf ish species may be owing to the lack of an effi cient effl ux transporter.
Seasonal variation and levels of toxins in shellf ish are strongly related (Dursun, 2021).Pseudo-nitzschiaspp. have a wide range of growth temperature from 8.4 °C to 23.5 °C, and the highest DA production byP.australisin laboratory culture was observed between 13.5 °C and 18.6 °C (Thorel et al., 2014).Besides, seawater surface temperatures (14–17 °C)have been found to have a positive correlation with the abundance ofPseudo-nitzschiaspp. (Walz et al.,1994). It has been reported that autumn and summer seawater surface temperatures are more suitable forPseudo-nitzschiagrowth, with the highest DA concentration of 2 671 ng/net tow observed in September phytoplankton samples andPseudonitzschiacell density of >6.0×105cells/net tow noted in August and November in Daya Bay, South China Sea (Jiang et al., 2017). Similarly, in the present study, the DA detection rate and mean concentration were also higher in autumn and summer than those in spring. In China, HABs caused byPseudonitzschiaspp. have been reported in Dalian (Liaoning Province) and Qingdao (Shandong Province), and may occur throughout the year, especially in summer and autumn (Lu et al., 2014). It has been reported that monsoon climate could signif icantly inf luence the hydrodynamic environment in these areas around Shandong Province and cause substantial seasonal changes (Yuan et al., 2020). A large amount of freshwater from Huanghe (Yellow) River and Changjiang (Yangtze) River gets discharged into these areas during the summer monsoon season,which leads to lower salinity and higher nutrients,thus signif icantly aff ecting the marine ecosystem,especially, phytoplankton growth in these areas (Yang et al., 2015).
Dietary aquatic food intake is an important approach to evaluate human exposure to DA.According to the data from the f ifth China total diet study (Wu et al., 2018), signif icant diff erences in aquatic food consumption were noted among diff erent age and gender groups, ranging from 21.8 to 81.6 g/d.In the present study, the highest HR value was 0.33‰,indicating that DA exposure through diff erent shellf ish consumption had an acceptable risk for both adults and children. Males faced higher health risk through aquatic food consumption owing to their higher body weight and shellf ish consumption than females. A major limitation of the present study is evaluation of the outcomes based on a single contamination factor.It has been reported that dietary intake of aquatic food may be higher among f ish farmers and islanders, when compared with that for the general population (Wu et al., 2014). Moreover, although the average daily intake of seafood by urban residents has been found to be 42.3 g/d (Li et al., 2013), seafood consumption can also be related to the geographic location,because coastal residents generally consume more seafood. Hence, future studies must also consider other risk factors when evaluating the health risks of DA exposure through aquatic food consumption.
To date, DA, along with its isoforms A–F, its 5ʹ-epimer, and its two lactone derivatives, have been identif ied in algae or shellf ish, and have been noted to be highly water-soluble. Previous studies have shown that the generaPseudo-nitzschiaandNitzschiacould produce diff erent isomers (Bates et al., 2018), and that shellf ish can accumulate isomers from diff erent toxicproducing algae through f ilter-feeding. UV-exposed dilute DA solutions photoisomerize reversibly into isoD, isoE, and isoF, and decarboxylate irreversibly and these isomers might act as photointermediates in the photochemical degradation of DA in the ocean(Bouillon et al., 2008), which may also be the reason for the accumulation of diff erent isomers in shellf ish.Although there have been reports on DA-producing toxic algae and contaminated shellf ish along the coast of China, no further analyses on the occurrence of isomers have been conducted, which are crucial for future studies. Assessment of acute seizurogenic potencies of isomers and their binding affi nities to heterogeneous populations of both kainate and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptors via in vivo seizure induction revealed that the toxicity of diff erent isomers widely varied (Sawant et al., 2008, 2010). Although the concentrations of isomers in the present study were relatively low,accurate quantif ication of diff erent isomers allows scientif ic evaluation of the magnitude of DA toxicity and provides essential data for DA risk assessment in shellf ish.
5 CONCLUSION
The present study provided the baseline concentration of DA in marine shellf ish collected from the coastal areas of Shandong Province in China.The overall DA detection rate in the shellf ish samples was 36.1%, indicating ubiquitous DA contamination in Shandong Province. The results obtained highlight the spatial and species variation of DA concentration in shellf ish. The relationship between seasonal changes and DA concentration revealed that environmental changes could signif icantly aff ect DA accumulation in shellf ish samples. Although the highest DA concentration determined in the present study was much lower than the permitted limit of 20 000 μg/kg, DA contamination might be signif icantly underestimated owing to its high detection rate and diff erent toxic isomers. Furthermore, the lower health risk of DA exposure for the general population was found to be associated with seafood consumption in the coastal areas of Shandong Province. AsPseudonitzschiaspp. is very common in the Chinese coastal waters, they may be a potential threat to aquatic food consumers, and further studies in the coastal areas around Shandong Province could lead to the identif ication of new DA-producing algal species.Besides, analyses of the trend in DA and its isomers concentration in shellf ish in Shandong Province could provide valuable data for risk assessment and monitoring strategy in future.
6 DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
 Journal of Oceanology and Limnology2022年6期
Journal of Oceanology and Limnology2022年6期
- Journal of Oceanology and Limnology的其它文章
- Overview of harmful algal blooms (red tides) in Hong Kong during 1975–2021
- Information standardization for typical toxic and harmful algae in China’s coastal waters—a case study of Karenia mikimotoi*
- Biochemical composition of the brown tide causative species Aureococcus anophageff erens cultivated in diff erent nitrogen sources*
- Identif ication of paralytic shellf ish toxin-producing microalgae using machine learning and deep learning methods*
- Screening for lipophilic marine toxins and their potential producers in coastal waters of Weihai in autumn, 2020*
- Spatial variation of lipophilic marine algal toxins and its relationship with physicochemical parameters in spring in Laizhou Bay, China*
