Screening for lipophilic marine toxins and their potential producers in coastal waters of Weihai in autumn, 2020*
Huixia GENG , Hongxiao SUN , Chao LIU , Fanzhou KONG ,Qingchun ZHANG , Tian YAN , Rencheng YU ,
1 CAS Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences,Qingdao 266071, China
2 Laboratory for Marine Ecology and Environmental Science, Pilot National Laboratory for Marine Science and Technology(Qingdao), Qingdao 266237, China
3 University of Chinese Academy of Sciences, Beijing 100049, China
4 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
Abstract Lipophilic marine toxins (LMTs) produced by some microalgae in the sea could accumulate in shellf ish and pose potential threats to the health of seafood consumers. Phytoplankton and shellf ish samples were collected from coastal waters of Weihai in Shandong Peninsula, China in autumn, 2020, and screened for lipophilic marine toxins and their potential producers using liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis and high throughput sequencing of partial DNA (V4 region of the 18S rRNA gene) extracted from phytoplankton. Pectenotoxin-2 (PTX2), trace amounts of azaspiracid (AZA1 or AZA40), and 13-desmethyl spirolide C (13-DesMe-C) were detected in phytoplankton samples, while PTX2 and gymnodimine (GYM) were detected in shellf ish samples. The toxin content in shellf ish samples was much lower than the regulatory limit or values reported previously. Results suggest that lipophilic marine toxins should have low risk in coastal waters of Weihai in autumn. Based on the data of high throughput sequencing, the OTUs were assigned to 5 identif ied species of Alexandrium, including A. ostenfeldii capable of producing 13-DesMe-C and GYM. Two OTUs were found closely related to the toxic species in genus Dinophysis, but it is impossible to assign them to any identif ied species due to the low resolving power of the V4 region for Dinophysis. The OTUs could not be assigned to any identif ied species in the genus Azadinium,suggesting the existence of unidentif ied species in this region.
Keyword: harmful algal blooms; phytoplankton; shellf ish; lipophilic marine toxins (LMTs); Yellow Sea;liquid chromatography-tandem mass spectrometry
1 INTRODUCTION
Some microalgae in the sea can produce potent phycotoxins as secondary metabolites, which give the producers competitive advantages against other microalgae and protect them from predators (Cembella,2003; Ianora et al., 2011). Such phycotoxins could be transferred via marine food chains, and result in mortality of marine animals, damaging f isheries and aquaculture, and threatening public health (Berdalet et al., 2016; Li et al., 2021). The negative impacts of marine phycotoxins are often associated with harmful algal blooms (HABs) formed by toxic microalgae(Shumway, 1990; Landsberg, 2002). The apparent increase of HABs around the world due to climate change, eutrophication, anthropogenic activities,or increasing awareness of HABs (Gobler, 2020;Hallegraeff et al., 2021), prompted the establishment of monitoring and research on HABs and phycotoxins(Howard et al., 2021; Otero and Silva, 2022).
Marine phycotoxins could be classif ied into eight major groups based on the chemical structure:okadaic acid (OA) and its derivatives, azaspiracids(AZAs), yessotoxins (YTXs), brevetoxins (BTXs),cyclic imines (CIs), pectenotoxins (PTXs), saxitoxins(STXs), and domoic acid (DA) (Farabegoli et al.,2018). Most of the marine phycotoxins, except for STXs and DA, are lipophilic compounds, which could be collectively named as lipophilic marine toxins and detected simultaneously with liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS). Thousands of human poisoning events related to lipophilic marine toxins occurred around the world every year, particularly in Europe, Asia, and America(De Schrijver et al., 2002; Garcêa et al., 2005; Lin et al., 2015; Nicolas et al., 2017).
In China, poisoning episodes associated lipophilic marine toxins were reported occasionally (Li et al.,2012; Liang et al., 2022). Investigations on lipophilic marine toxins started from the late 1990s (Zhou et al., 1999). The early investigations on lipophilic marine toxins mainly focused on those accumulated in wild or cultured edible shellf ish, using the method of mouse bioassay (MBA). This method, however,was gradually given up due to a high proportion of false-positive result (Chapela et al., 2008; Bodero et al., 2018), and other ethical or technical concerns.The methods based on high performance liquid chromatography coupled with mass spectrometry(LC-MS) gradually take the place of MBA for detection of lipophilic marine toxins. Recently, the regulatory limit (GB 2733-2015) and offi cial method based on LC-MS/MS (GB 5009.212-2016) have been established for OA and its derivatives in shellf ish in China, but there are still no regulatory limits and methods for other lipophilic marine toxins. Using the state-of-the-art methods of LC-MS/MS, many investigations have been carried out to investigate the composition, distribution, and seasonal dynamics of lipophilic marine toxins during the last decade in the Bohai Sea (Liu et al., 2017, 2019a; He et al., 2020),Yellow Sea (Li et al., 2010; Wang et al., 2015; Chen et al., 2018; Wu et al., 2019, 2020; Liu et al. 2021a),East China Sea (He et al., 2019; Wu et al., 2020), and South China Sea (Jiang et al., 2017; Liu et al., 2019b;2020, 2021b; Ji et al., 2022). However, lipophilic marine toxins are still not well studied, particularly in some important aquaculture zones near the coast.
Weihai is a coastal city located at the east end of Shandong peninsula, and has important aquaculture industries of seaweed and oysters, scallops, and clams.Shellf ish production occupied over 60% of the total aquaculture production in Weihai (Hou et al, 2018).Despite the signif icance of aquaculture industry, there was little investigation of marine toxins in this region.However, previous investigations in the Bohai Sea and Yellow Sea indicated that this region was likely aff ected by lipophilic marine toxins, such as PTXs(Chen et al., 2018). Therefore, phytoplankton and shellf ish samples were collected in this study during the cruise along the coast of Weihai in 2020 to detect lipophilic marine toxins using LC-MS/MS, and the potential producers of toxins were resolved based on microscopy observation and high throughput sequencing analyses of phytoplankton samples.
2 MATERIAL AND METHOD
2.1 Chemical reagents and standards
HPLC-grade solvents acetonitrile and methanol were purchased from Merck, Germany, and ammonium hydroxide used in this study was from Sigma-Aldrich, Germany. Ultrapure water of 18.2 MΩ·cm prepared with the Milli-Q water purif ication system (Milli-Q Reference, Millipore Ltd., USA) was used for toxin analysis and molecular experiments.Certif ied reference materials of lipophilic marine toxins, including OA, dinophysistoxins-1 (DTX1),yessotoxin (YTX), homoyessotoxin (homo-YTX),azaspiracid 1–3 (AZA1–3), 13-desmethyl spirolide C (13-DesMe-C), gymnodimine (GYM), and pectenotoxin-2 (PTX2) were obtained from the Institute for Marine Biosciences, National Research Council of Canada (Halifax, Canada). All the standards were stored at -20 °C in the dark.
2.2 Collection of phytoplankton and shellf ish samples
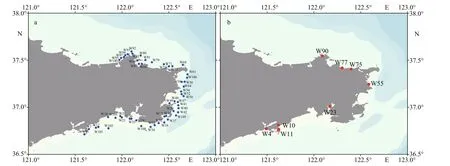
Fig.1 Illustration of the sampling sites for phytoplankton (a) and shellf ish (b) in coastal waters of Weihai in autumn, 2020
Phytoplankton samples for lipophilic marine toxin analysis and high throughput sequencing analysis were collected, following the protocols described by Liu et al. (2017) and Chen et al. (2019).Phytoplankton samples for lipophilic marine toxin analysis were collected from 53 sampling sites in 27 bays along the coast of Weihai (121.6°E–122.7°E,36.7°N–37.6°N), Shandong province, from October to November in 2020 (Fig.1). A total of 1 000-L seawater was pumped from the surface and concentrated with a phytoplankton net (pore size 20 μ m). The net-concentrated phytoplankton sample was f iltered by a sieve (pore size 200 μ m) to remove zooplankton and other organisms, then made up to a volume of 850 mL. A fraction of 50-mL concentrated phytoplankton sample was f ixed with Lugol’s iodine solution (f inal concentration ca. 2%) and preserved at room temperature for morphological observation.Another 200 mL of concentrated phytoplankton sample was f iltered onto a f iberglass membrane(GF/C, Whatman, nominal pore size 1.2 μ m),which was preserved in a freezer at -20 °C until analysis of lipophilic marine toxins with LC-MS/MS. For high throughput sequencing analysis, 2-L seawater was collected from the surface and passed through a sieve with pore size of 200 μm to remove zooplankton and other organisms, then f iltered on a polycarbonate membrane (Millipore, USA, nominal pore size 0.4 μ m) under 30-kPa vacuum to collect phytoplankton. The membranes were stored at -80 °C until analysis.
A total of 11 shellf ish samples, including 4 species of clams (Ruditapes philippinarum,Anadara kagoshimensis(=Scapharca subcrenata),Meretrix lusoria, andCetoconcha hyaline), 1 species of scallop(Argopecten irradians), 1 species of mussel (Mytilus galloprovincialis), and 1 species of oyster (Magallana gigas(=Crassostrea gigas)), were collected from 8 sites (Table 1) along the coast of Weihai (Fig.1). For each species of shellf ish, about 2 kg f lesh tissues were collected and preserved in a freezer at -20 °C until analysis of lipophilic marine toxins with LC-MS/MS.
2.3 Extraction and analysis of lipophilic marine toxins
The extraction of lipophilic marine toxins from net-concentrated phytoplankton samples and shellf ish samples referred to the protocols described in Liu et al. (2017, 2019a). Brief ly, the membrane containingphytoplankton, or homogenized f lesh tissues of shellf ish (1 g), were extracted 3 times, each with 3-mL methanol. The supernatants were then combined and made up to 10 mL with methanol in a volumetric f lask. A fraction of raw extract from phytoplankton(5 mL) or shellf ish (1 mL) in methanol was diluted with ultrapure water to around 30% and loaded into a solid phase extraction (SPE) cartridge (strata-X,3 cm3, 60 mg, Phenomenex) previously conditioned by alternative treatment with 3-mL methanol and 3-mL deionized water. The cartridge was f lushed with 3 mL of methanol/water (20꞉80, v/v), and dried under mild vacuum. Toxins in the cartridge were then eluted with 1-mL methanol containing 0.3% (v/v) ammonium hydroxide. The eluent was f iltered through a 0.22-μm syringe membrane f ilter, and preserved in a freezer at-80 °C prior to the analysis with HPLC-MS/MS.
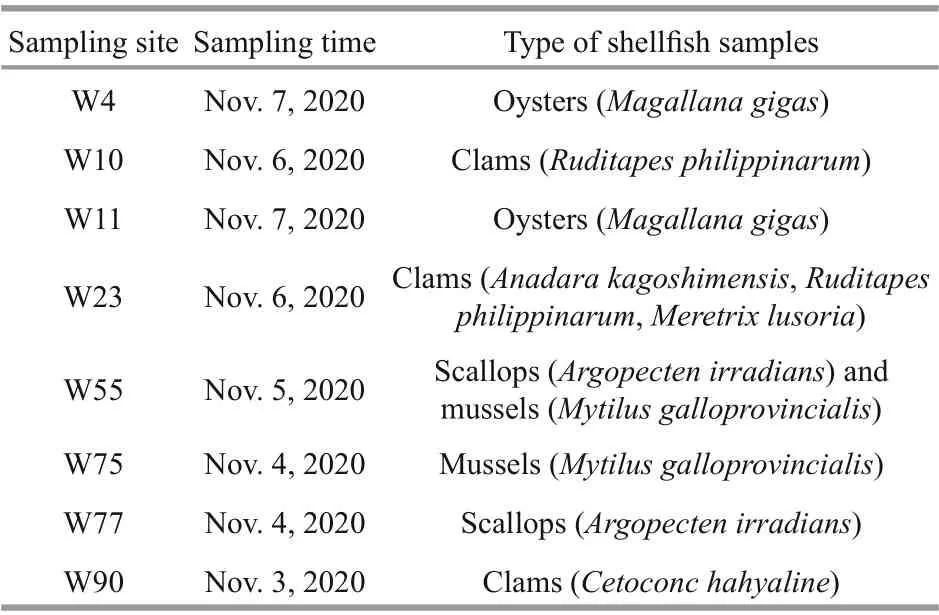
Table 1 List of shellf ish samples collected from the coastal waters of Weihai
Lipophilic marine toxins in phytoplankton and shellf ish were analyzed using a LC-MS/MS method described in Liu et al. (2017, 2019a). Brief ly, a Thermo Fisher UltiMate 3000 HPLC system was used for chromatographic separation of lipophilic marine toxins with a Waters X-Bridge C18 column(150 mm× 3 mm, 3.5 mm) and a guard column(10 mm× 2.1 mm, 3.5 mm). Mobile phases A(acetonitrile/water, 10꞉90, v/v) and B (acetonitrile/water, 90꞉10, v/v), both containing 6.7-mmol/L ammonium hydroxide (pH 11), were used to elute lipophilic marine toxins using a gradient program as described in Liu et al. (2017). A mass spectrometer AB-SCIEX-4500 Q-Trap (Applied Biosystems,Darmstadt, Germany) equipped with a TurboSpray®interface was used to detect lipophilic marine toxins.The mass spectrometer was operated in both negative and positive electrospray ionization (ESI) and in multiple reaction monitoring (MRM) mode for three diff erent retention time windows (Liu et al., 2017).
Instrument response to lipophilic marine toxins were determined with detection limit of 1.42 ng/mL and quantif ication limit of 4.27 ng/mL (Liu, 2017).The matrix eff ects, recovery and precision of the method have been examined by Liu et al. (2019a).The results showed that the clean-up procedure using Strata-X cartridges could signif icantly reduce matrix eff ects. The method had good performances,with recoveries ranged from 83% to 126% for phytoplankton samples and 83% to 132% for shellf ish samples. Relative standard deviation (RSD) was less than 10% for phytoplankton samples and less than 14% for shellf ish samples (Liu, 2017). The shellf ish samples were treated in duplicate and single phytoplankton samples collected from each site were analyzed in this study.
2.4 High throughput sequencing analysis of phytoplankton samples
In this study, high-throughput sequencing data for phytoplankton samples were used to screen for potential producers of lipophilic marine toxins in the coastal waters of Weihai. Protocol for sample preparation and analysis refers to Chen et al. (2019). Genomic DNA of phytoplankton samples was extracted following the protocol of Winnepenninckx et al. (1993). A primer pair for eukaryotes (forward primer D514,5ʹ-TCCAGCTCCAATAGCGTA-3ʹ and reverse primer B706R, 5ʹ-AATCCRAGAATTTCACCTCT-3ʹ) was used to amplify the V4 region of the 18S rRNA gene(18S rDNA) (Cheung et al., 2010; Zimmermann et al., 2011). The NEB Next®Ultra™ DNA Library Prep Kit for Illumina (Illumina Inc., USA) was used to construct the sequence libraries, which were sequenced on the Illumina HiSeq2500 platform.Reads were truncated and assembled using FLASH(Version 1.2.7), and QIIME (Version 1.7.0) was used to generate high-quality clean tags. After removing singletons from the dataset (Flynn et al., 2015), and sequences were clustered into operational taxonomic units (OTUs, similarity above 97%) using UPARSE software (Version 7.0.1001) (Edgar, 2013; Flynn et al., 2015). Taxonomic assignment of the OTUs related toAlexandriumspp.,Dinophysisspp. andAzadiniumspp. were completed using both SILVA Database (Release 119, https://www.arb-silva.de/documentation/release-119/) and Nucleotide Database in NCBI (https://blast.ncbi.nlm.nih.gov/).
A total of 35 representative sequences of the 18S rDNA V4 region were selected for the species in genusAlexandriumand downloaded from GenBank.Together with the sequences of 6 OTUs assigned toAlexandriumspp., a maximum-likelihood tree was established using Mega 10 (https://megasoftware.net/) variance estimation method with bootstrap replications of 10 000 and the best-f itting nucleotide substitution model of Tamura 3-parameter+G(Kumar et al., 2018). The sequence ofProrocentrumdonghaiensewas used as the outside group. The OTUs ofDinophysisspp. andAzadiniumspp. identif ied in the study area were analyzed using the same protocol,with 22 representative sequences of the genusDinophysis, and 25 representative sequences of the genusAzadinium.
2.5 Microscopy observation of phytoplankton samples
Phytoplankton samples f ixed with Lugol’s iodine solution were settled for 24 h to 200 mL following the protocol of the Utermöhl method (Sournia, 1978), and a fraction of 0.2 mL was observed under an inverted light microscope (Primo Vert, Zeiss, Germany).Alexandriumspp.,Dinophysisspp. andAzadiniumspp. were identif ied and counted under the microscope.
2.6 Data processing
Date acquisition of phycotoxins with LC-MS/MS was performed using software Multiquant 3.0. All the data were processed with Microsoft Excel (Microsoft,USA).
3 RESULT
3.1 Lipophilic marine toxins in phytoplankton and shellf ish in coastal waters of Weihai
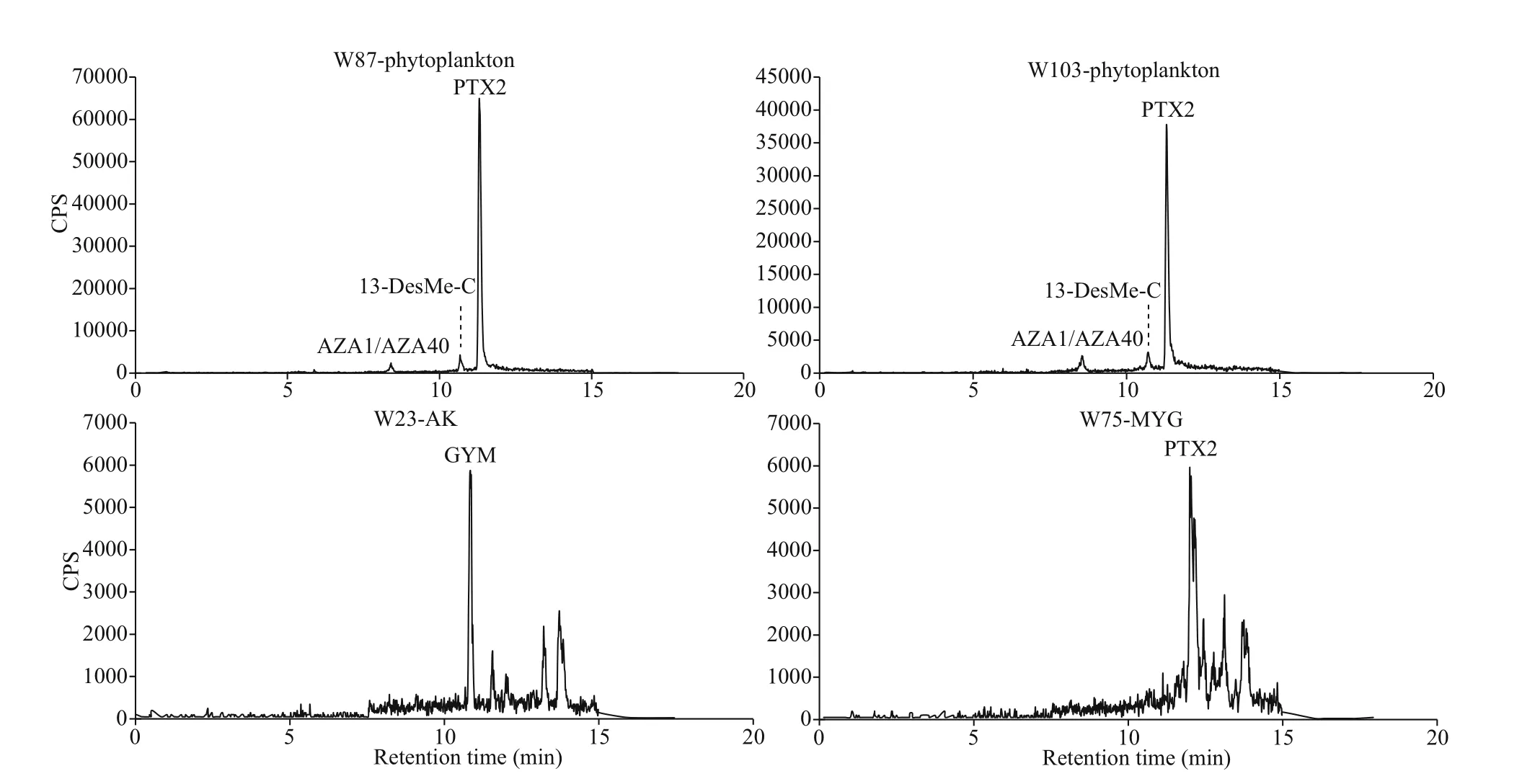
Fig.2 The HPLC-MS/MS chromatograms for lipophilic marine toxins in phytoplankton and shellf ish samples collected from coastal waters of Weihai
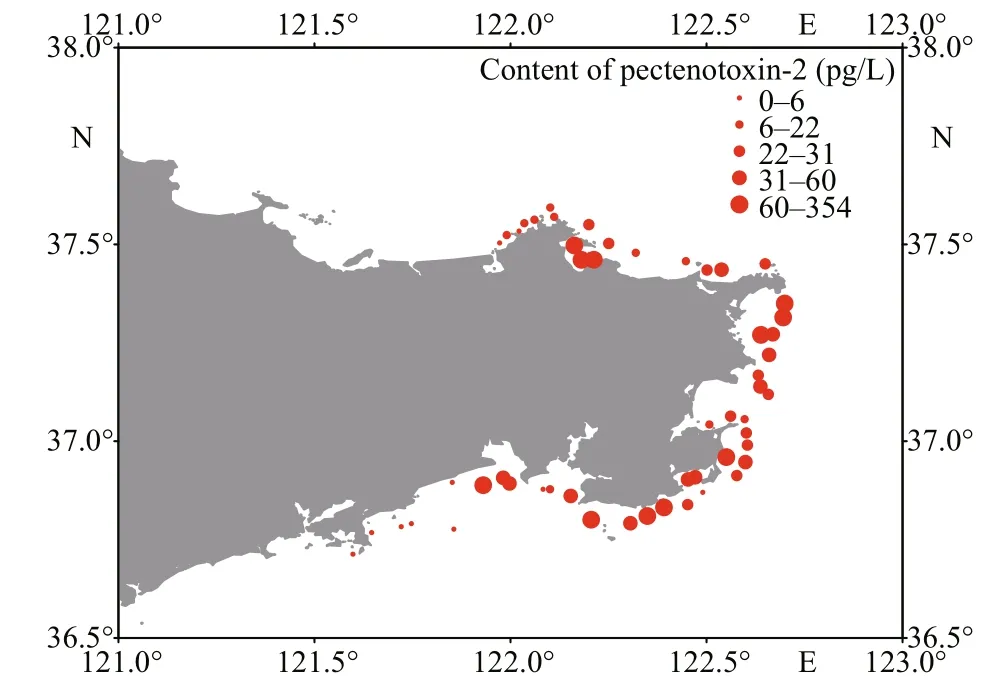
Fig.3 Distribution and content of pectenotoxin-2 (pg/L) in the phytoplankton samples collected from coastal waters of Weihai
Three lipophilic marine toxins, namely AZA1/AZA40, 13-DesMe-C, and PTX2, were detected in net-concentrated phytoplankton samples collected from coastal waters of Weihai. Among the three toxins, PTX2 was the ubiquitous toxin detected in 47 phytoplankton samples (Figs.2 & 3). The maximum content of PTX2 was 354 pg/L in a sample collected from the Weihai Bay north to the city. PTX2 content also had a high value of 192 pg/L in a region close to the Rushan Bay south to Weihai. Besides, on the east coast of Weihai, the PTX2 content also had relatively higher values. Trace amount of AZA1 or AZA40 was detected in 28 phytoplankton samples, which were widely distributed along the coast of Weihai (Figs.2& 4). However, the amount of toxin was too low to determine the content quantitatively. Moreover, it is hard to discriminate between AZA1 and AZA40 since the two analogues had the same molecular weight and similar retention time. 13-DesMe-C was detected in 16 phytoplankton samples, which were mainly distributed in the northern and eastern coastal waters of Weihai. The content of 13-DesMe-C was also very low and it is impossible to make quantitative determination.
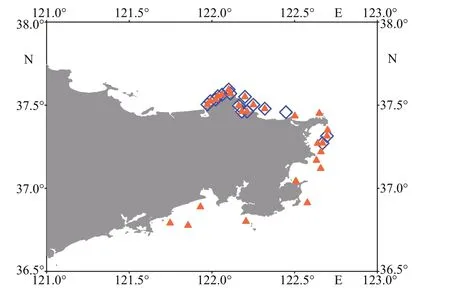
Fig.4 Distribution of azaspirolide-1 (orange triangle) and 13-desmethyl spirolide C (blue diamond) in the phytoplankton samples collected from coastal waters of Weihai
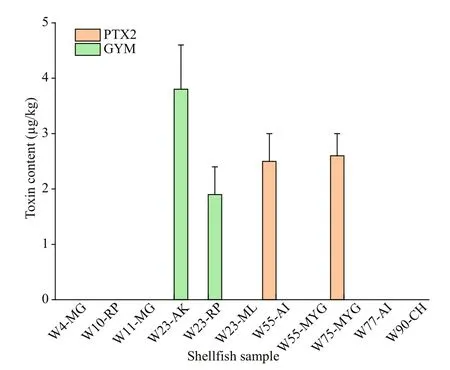
Fig.5 Component and content of lipophilic marine toxins in the shellf ish collected from coastal waters of Weihai
Only two lipophilic marine toxins, namely GYM and PTX2, were detected in shellf ish samples collected along the coast of Weihai (Figs.2 & 5).PTX2 were detected in a scallop sample collected form site W55 and a mussel sample from site W75.Content of PTX2 in the scallops and mussels were 2.45±0.5 and 2.63±0.4 μg/kg, respectively. GYM was detected in the two clam samples ofR.philippinarumandA.kagoshimensisfrom the sampling site W23,and the contents were 1.87±0.5 and 3.77±0.8 μg/kg,respectively.
3.2 Potential producers of lipophilic marine toxins in coastal waters of Weihai
Based on the results of high throughput sequencing data of phytoplankton samples collected from coastal waters of Weihai, a total of 5 300 101 tags were acquired (76 112/sample on average), and 1 703 OTUs were assigned to eukaryotic microalgae. According to the BLASTing results of the NCBI nucleic acid database, 6 OTUs were assigned to the species in genusAlexandrium, and 2 OTUs were assigned to the species in genusDinophysis, and 2 OTUs were assigned to the species in genusAzadinium.
Although vegetative cells ofAlexandriumspp.were observed in net-concentrated phytoplankton samples, it is diffi cult to identify them to the species level. Based on the data of high throughput sequencing and phylogenetic results, 6 OTUs related toAlexandriumwere assigned toA.pacif icum,A.affi ne,A.fraterculus,A.leeiandA.ostenfeldii/A.andersoni(Fig.6). It is impossible to discriminateA.ostenfeldiifromA.andersonisince the two species have the same sequence in the V4 region. All the OTUs had a high identity to their corresponding species (>99%),except for the OTU 1315 assigned toA.fraterculus(96.3%).
Vegetative cells ofDinophysiswere observed in net-concentrated phytoplankton samples, and the species could be identif ied primarily asD.caudata.Based on the phylogenetic analysis of V4 sequences forDinophysisspp., they could be roughly divided into two groups (Fig.7). Toxin-producing species,such asD.acuminata,D.acuta,D.caudata,D.fortii,D.infundibulus,D.norvegica, andD.triposwere grouped in the same cluster. Two OTUs derived from high throughput sequencing in this study were grouped together within this cluster, with high identity(>98%). However, it is impossible to further identify the species based on the high throughput sequencing results using V4 region of the LSU.
No vegetative cells ofAzadiniumwere observed in net-concentrated phytoplankton samples. However,two OTUs derived from high throughput sequencing were found closely related to the genusAzadiniumbased on the BLASTing results. However, the phylogenetic analysis did not support the identif ication of these OTUs to any known species in the genusAzadinium(Fig.8).
4 DISCUSSION
4.1 Lipophilic marine toxins and their risks in coastal waters of Weihai
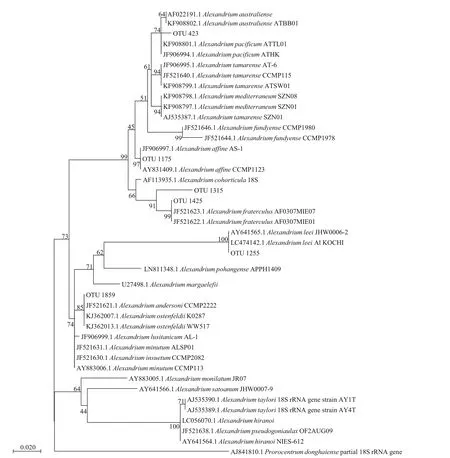
Fig.6 Phylogenetic tree established for species in genus Alexandrium and the OTUs derived from high throughput sequencing results of phytoplankton samples in coastal waters of Weihai based on the V4 region of the LSU
The coastal waters of Weihai, which host nearly 30 bays, is an important mariculture zone in the Yellow Sea. However, there is little information on potential risks of phycotoxins in this region. In this study, although results of single phytoplankton samples at each station were provided, it should not have signif icant impact on the risk assessment of lipophilic marine toxins in coastal waters of Weihai.Based on the investigation, 4 typical lipophilic marine phycotoxins, namely AZA1/AZA40, 13-DesMe-C,PTX2, and GYM, were detected in phytoplankton and shellf ish samples. Among the toxins, PTX2 was detected in both phytoplankton and shellf ish samples, and widely distributed in coastal waters of Weihai in autumn. In China, PTX2 was detected for the f irst time in oysterMagallanagigasfrom the northern Yellow Sea, and in clamMactrachinensisand musselMytilusgalloprovincialisfrom the East China Sea in 2007 (Liu et al., 2011). The following investigations also demonstrated the importance of PTX2 in the Bohai Sea (Wang et al., 2015; Liu et al.,2017, 2019a) and the South China Sea (Jiang et al.,2017; Liu et al., 2021b). In the Yellow Sea, PTX2 was widely detected in shellf ish, phytoplankton, seawater,and sediment (Luo, 2011; Wang et al., 2015; Chen et al., 2017). In most of the investigations, PTX2 was found as the dominant toxin component (Luo, 2011;Liu et al., 2017, 2020, 2021b; Chen et al., 2018).The investigations indicated that PTX2 content in shellf ish or phytoplankton samples had notable seasonal variations. An investigation in the coastal waters of Qinhuangdao found that content of PTX2 in phytoplankton reached the maximum from April to May (Sun et al., in press). In Jiaozhou Bay of the Yellow Sea, PTX2 content in phytoplankton had the maximum value in June and July (Luo, 2011),which was basically in accordance with the variation pattern of the PTX2 in seawater (Li et al., 2014; Wu et al., 2019) and in shellf ish (Wang et al., 2016). In Daya Bay of the South China Sea, PTX2 in net-haul phytoplankton samples reached the highest level during the autumn and winter (Jiang et al., 2017).Such diff erent seasonal patterns of PTX2 may ref lect diverse producers of PTX2, or various seasonal dynamics of the PTX2 producers.
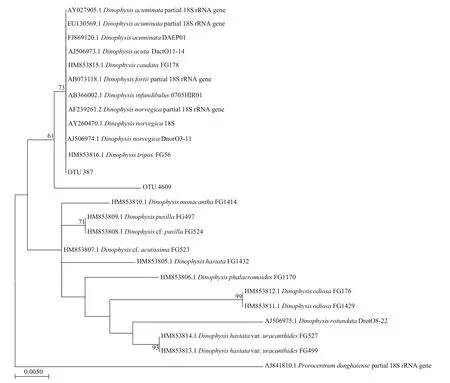
Fig.7 Phylogenetic tree established for species in genus Dinophysis and the OTUs derived from high throughput sequencing results of phytoplankton samples in coastal waters of Weihai based on the V4 region of the LSU
Trace amounts of AZA1/AZA40 was detected in a part of phytoplankton samples in the coastal waters of Weihai. Analogues of AZA toxins, mainly AZA2,have been detected in shellf ish (Wu et al., 2018; Liu et al., 2019a), phytoplankton (Li et al., 2017), and suspended particle material (Chen et al., 2018) in the coastal waters of China. AZA1 was also detected in shellf ish samples collected from the northern Yellow Sea and the South China Sea (Yao et al., 2010), and in phytoplankton and suspended particle materials in the Bohai Sea and the Yellow Sea (Liu et al., 2017;Chen et al., 2018). However, Krock et al. (2014)has indicated that AZA1 detected in coastal waters of China might be a misidentif ication of AZA40, an isomer of AZA1 identif ied in strains ofAzadiniumpoporumisolated from China. AZA1 is the major toxin component produced byAzadiniumspinosumin European waters, but it has not been detected in the toxic isolates ofAzadiniumfrom the China Seas. So far, there is little information available on the seasonal variation patterns of AZAs in the coastal waters of China. An investigation in the northern Yellow Sea indicated that the highest content of AZA-2 (21.5 μg/kg)was detected in June in Zhangzidao island (Wu et al.,2018).
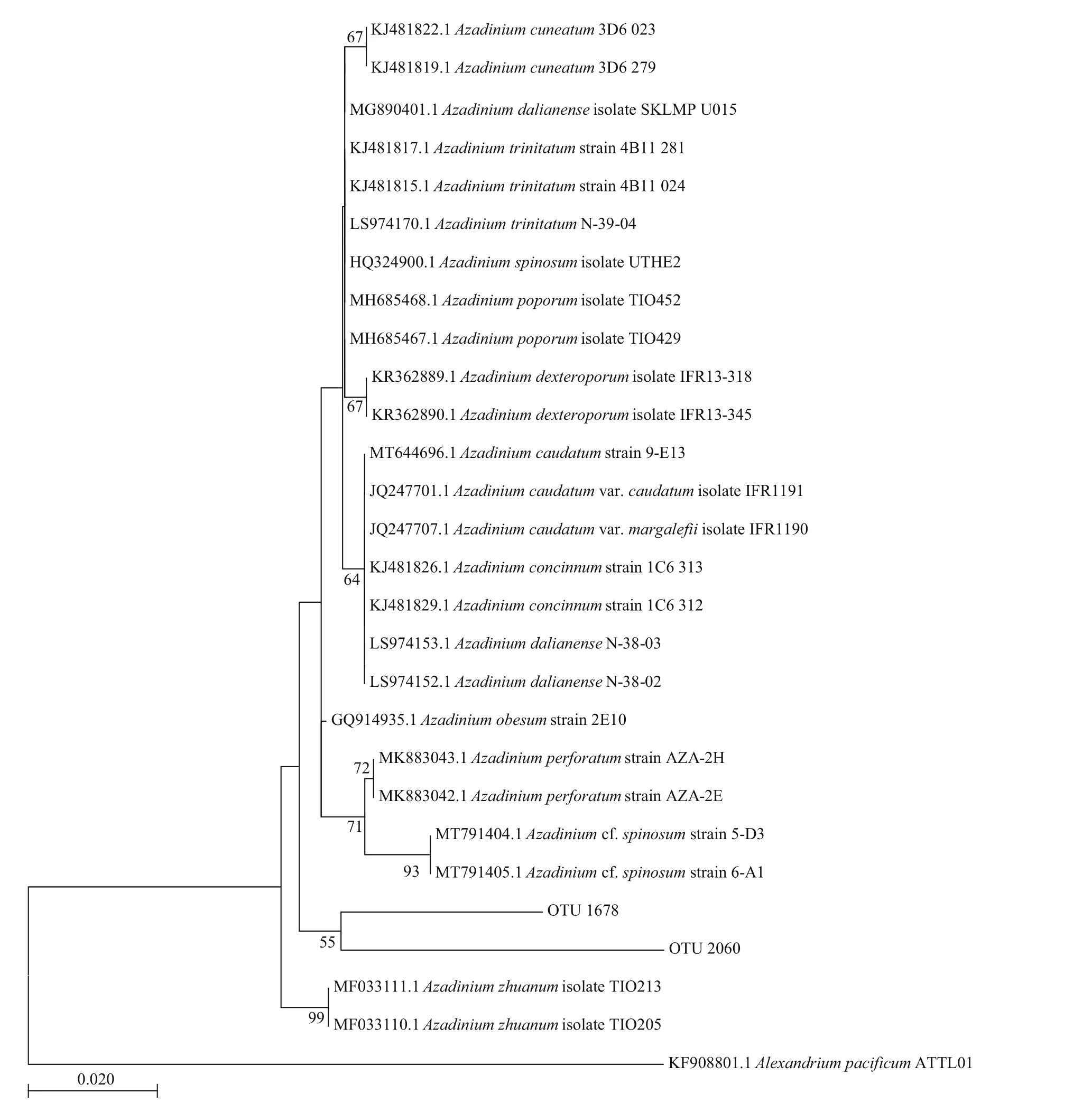
Fig.8 Phylogenetic tree established for species in genus Azadinium and the OTUs derived from high throughput sequencing results of phytoplankton samples in coastal waters of Weihai based on the V4 region of the LSU
Both 13-DesMe-C and GYM belong to CIs, a group of toxins encompassing a macrocyclic ring with a conserved cyclic imine group. In this study, trace amounts of 13-DesMe-C and GYM were detected in phytoplankton and shellf ish samples respectively. Ji et al. (2018) also detected 13-DesMe-C and GYM in shellf ish samples cultured in Rongcheng, Weihai. In previous studies, 13-DesMe-C and GYM have been detected in phytoplankton, seawater, and shellf ish samples in the Bohai Sea, Yellow Sea, and South China Sea (Yao et al., 2010; Liu et al., 2017; Ji et al.,2018, 2022; Wu et al., 2019; Liang et al., 2022), and the contents of the two toxins in the Yellow Sea and the Bohai Sea were much lower than the South China Sea and the East China Sea.
In the shellf ish samples collected from coastal waters of Weihai in this study, only PTX2 and GYM were detected, and toxin content was very low.Content of PTX2 in the scallop and mussel samples was only 2.63±0.4 μg/kg, which is far below the regulatory limit for seafood safety of the European Union (160 μg/kg). Content of GYM (3.77±0.8 μg/kg)is also much lower than those previously reported in this region (for example, 20.23 μg/kg in an oyster sample from Rongcheng) (Ji et al., 2018). Lipophilic marine toxins with low content in coastal waters of Weihai would not lead to potent risks of seafood consumers. Moreover, PTXs have been removed from the standards for live bivalves in Commission Delegated Regulation (EU) 2021/1374, since the European Food Safety Authority (EFSA) has stated that there is no evidence of adverse eff ects linked with PTXs (Otero and Silva, 2022). Although CIs have potent neurotoxicity, they are not regulated in most of the countries now. Therefore, it can be addressed that lipophilic marine phycotoxins in coastal waters of Weihai in autumn have little risk to the health of human beings. It should be noted, however, the investigation was only carried out in autumn, while the abundance of toxic algae and content of phycotoxins could be much higher in spring and summer. Therefore, the f inding of this study is not enough to ref lect the potential risks of lipophilic marine toxins in a comprehensive way,and more time-series investigations are still needed.
4.2 Potential producers of lipophilic marine toxins in coastal waters of Weihai
PTXs are produced by toxic species in genusDinophysis, which can produce both okadaates(okadaic acid and its analogues dinophysistoxins) and PTXs (Reguera et al., 2012). At present, 12 species in the generaDinophysisandPhalacromaare capable of producing okadaates or PTXs. In the Yellow Sea and the East China Sea, toxic dinof lagellatesDinophysisacuminata,D.fortii,D.caudata, andPhalacromarotundatum(=D.rotundata) are widely distributed,and some of them are capable of producing PTX2,OA, and DTX1 (Luo, 2011; Li et al., 2015, 2017; Gao et al., 2017). Therefore, OA and DTXs were often detected simultaneously with PTX2. In this study,however, no OA or DTXs were detected, probably due to the unique species ofDinophysispresent in autumn along the coast of Weihai. During the investigation,onlyD.caudatawith low abundance were observed in net-concentrated phytoplankton.Dinophysiscaudatais generally considered as a tropical or temperate species, which appear later in autumn following the bloom ofD.acuminatacomplex in spring and summer in temperate waters (Reguera et al., 2012).In the southern part of China, such as Daya Bay,D.caudatawas often present as a dominantDinophysisspecies (Jiang et al., 2017; Liu et al. 2021b), and PTX2 was detected as a major toxin component in the concentrated phytoplankton samples. Therefore,D.caudatais most likely to be the producer of PTX2 in the coastal waters of Weihai in autumn.
Dinophysisseldom dominates the phytoplankton(Reguera et al., 2012), and it is diffi cult to make accurate identif ication of theDinophysisspecies simply based on their morphological features.Therefore, some co-occurring species with similar morphological features have been termed as species complex, such as “D.acuminatacomplex”. In this study, high throughput sequencing was used to assist the identif ication of toxin producer. A total of two OTUs were assigned toDinophysisspecies. The target V4 region, however, have very low ability to resolve the diff erent species in genusDinophysis. The results of phylogenetic analysis groupedDinophysisspecies with known V4 sequences into two major groups(Sun et al., in press). Most of the toxicDinophysisspecies were grouped together, but it is diffi cult to further identify the species based on the V4 region.In previous studies, it was also found that ribosomal RNA genes are not applicable to infer taxonomic and/or phylogenetic relationships forDinophysisspp.(Reguera et al., 2012). These results suggest that the rDNA molecular markers are not appropriate to discriminateDinophysisspp., and high-resolution plastid or mitochondrial DNA markers should be tested in the future.
Dinof lagellatesKareniaselliformisandAlexandriumostenfeldii/A.peruvianumhave been implicated in the biosynthesis of CIs like GYMs and spirolides (SPXs) (Molgó et al., 2017). Dinof lagellatesA.ostenfeldiiandA.peruvianumproduce a large family of SPXs, andKareniaselliformisproduces diff erent groups of GYMs including A, B, and C. Recently, it was found thatA.ostenfeldiiandA.peruvianumalso produced GYM analogs (Molgó et al., 2017). The simultaneous occurrence of GYM and 13-DesMe-C suggest they could derive from the same toxic algae.In this study, an OTU was assigned toA.ostenfeldii/A.andersonii, suggesting thatA.ostenfeldiicould be the origin of the two CIs. However, no strains ofA.ostenfeldiiisolated from the coastal waters of China produce GYM or SPX (Gu, 2011). The origin of CIs still needs to be further conf irmed.
The Amphidomataceae is an increasingly growing family of Dinophyceae (Salas et al., 2021). AZAs were mainly produced by toxic dinof lagellates from the generaAzadinium(Aza.poporum,Aza.spinosumandAza.dexteroporum) andAmphidoma(Amph.languida). In this study, two OTUs related toAzadiniumwere grouped together to form a cluster diff erent from all the known toxic species in the genusAzadinium. The results suggest that unidentif iedAzadiniumspecies exist in this region, which may account for the AZA1/AZA40 detected in the coastal waters of Weihai.
5 CONCLUSION
In this study, LC-MS/MS and high throughput sequencing methods were applied to screen for lipophilic marine toxins and their potential producers in coastal waters of Weihai. Four typical lipophilic marine toxins, PTX2, AZA1/AZA4, 13-DesMe-C,and GYM, were detected in phytoplankton and shellf ish samples. The content of lipophilic marine toxins in shellf ish was low and has little risk to seafood consumers. Based on the microscopy and high throughput sequencing results, it can be deduced thatD.caudataandA.ostenfeldiiare likely to be the producers of PTX2 and CIs (GYM and 13-DesMe-C).The producer of AZAs could not be determined but may linked to unidentif ied species in the genusAzadinium.
6 DATA AVAILABILITY STATEMENT
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENT
The authors thank Dr. Xiaotian HAN for the information of morphological observation of phytoplankton samples.
 Journal of Oceanology and Limnology2022年6期
Journal of Oceanology and Limnology2022年6期
- Journal of Oceanology and Limnology的其它文章
- Overview of harmful algal blooms (red tides) in Hong Kong during 1975–2021
- Information standardization for typical toxic and harmful algae in China’s coastal waters—a case study of Karenia mikimotoi*
- Biochemical composition of the brown tide causative species Aureococcus anophageff erens cultivated in diff erent nitrogen sources*
- Identif ication of paralytic shellf ish toxin-producing microalgae using machine learning and deep learning methods*
- First observation of domoic acid and its isomers in shellf ish samples from Shandong Province, China*
- Spatial variation of lipophilic marine algal toxins and its relationship with physicochemical parameters in spring in Laizhou Bay, China*
