Relationship between toxic and harmful microalgae and environmental factors in typical mariculture areas of East China Sea*
Anqi ZHANG , Honghan LIU , Chenhong LI , Changping CHEN ,3, Junrong LIANG ,3,Lin SUN , Yahui GAO ,2,3,**
1 School of Life Sciences, Xiamen University, Xiamen 361102, China
2 State Key Laboratory of Marine Environmental Science, Xiamen University, Xiamen 361102, China
3 Key Laboratory of Ministry of Education for Coastal and Wetland Ecosystems, Xiamen University, Xiamen 361102, China
Abstract Toxic and harmful algal blooms are usually more frequent in mariculture areas due to the abundant trophic conditions. To investigate the relationship between toxic and harmful microalgae and environmental factors, we set up 12 stations near three mariculture regions (Gouqi Island, Sandu Bay, and Dongshan Bay)in the East China Sea. We collected samples from all four seasons starting from May 2020 to March 2021.We identif ied 199 species belonging to 70 genera, of which 38 species were toxic and harmful, including 24 species of Dinophyceae, 13 species of Bacillariophyceae, and 1 species of Raphidophyceae. The species composition of toxic and harmful microalgae showed a predominance of diatoms in the summer (August),and dinof lagellates in the spring (May), autumn (November), and winter (March). The cell densities of toxic and harmful microalgae were higher in summer (with an average value of 15.34×10 3 cells/L) than in other seasons, 3.53×10 3 cells/L in spring, 1.82×10 3 cells/L in winter, and 1.0×10 3 cells/L in autumn. Pseudonitzschia pungens, Prorocentrum minimum, Paralia sulcata, and Prorocentrum micans were the dominant species and were available at all 12 stations in the three mariculture areas. We selected 10 toxic and harmful microalgal species with frequency >6 at the survey stations for the redundancy analysis (RDA), and the results show that NO 3 ˉ, water temperature (WT), pH, DO, and NO 2 ˉ were the main factors on distribution of toxic and harmful microalgae. We concluded that the rich nutrient conditions in the East China Sea mariculture areas increased the potential for the risk of toxic and harmful microalgal bloom outbreaks.
Keyword: phytoplankton; toxic and harmful microalgae; environmental factors; mariculture areas; East China Sea (ECS)
1 INTRODUCTION
Harmful algal blooms (HABs) are usually caused by the rapid proliferation and accumulation of toxic and harmful microalgae in the ocean. They can inf luence the structure and function of the ecosystem and have a negative impact on the ecological environment and human society (Berdalet et al., 2017; Chen et al., 2021b). Microalgae are an important part of the marine ecosystem, however, some toxic and harmful microalgae form algal blooms that can have a serious impact on f isheries and aquaculture (Berdalet et al.,2016). Some HAB species can produce powerful toxins that contaminate seafood and can accumulate in the food chain, leading to mass mortality in wild and farmed f ish, shellf ish, and even poisoning humans(Anderson et al., 2002; Wang, 2008). Similarly, nontoxic HABs can also be harmful to the environment,as they can lead to signif icant reductions in oxygen concentrations in coastal sheltered areas (Brandenburg et al., 2019), which can have consequential human or ecological health impacts and damage to local aquaculture and economic development. In recent years, HAB events have occurred more frequently and lasted longer in more locations than in past decades(Furuya et al., 2018). Due to the recent widespread and stable events of HAB that have adversely aff ected human health and aquaculture, there is a much greater need for society to recognize these phenomena than in the past (Zohdi and Abbaspour, 2019).
Mariculture is well developed in China, ranking f irst in the world in terms of area and production (Li et al.,2014). As a traditional industry, marine aquaculture plays an important role in promoting the economic development of coastal areas (Liao and Yang, 2019),but the existing problems associated with HABs are becoming increasingly prominent. The coastal areas of the East China Sea are well-known “hot spots” for HABs in China (Chen et al., 2021c), and the East China Sea has become an area of frequent HAB occurrence.Fujian and Zhejiang are major marine provinces in China, with vast coastal areas, rich marine resources,and important mariculture areas, and are also the areas with frequent incidences of red tide (Huang et al., 2020). Gouqi Island is located in the eastern part of Shengsi Island, which is located in the subtropical marginal sea area (Zhang et al., 2007), and mussel aquaculture is the main economic industry of Gouqi Island (Chen et al., 2012; Bao et al., 2020). Sandu Bay is located in the northeastern part of Fujian Province,it is a typical semi-enclosed harbor and the only inlet spawning ground ofPseudosciaenacroceain China(Zheng, 2007), mainly aquaculture ofPseudosciaenacroceaand abalone (Lin, 2013). Dongshan Bay is the largest bay in southern Fujian Province (Zheng,2009), and it is also an important mariculture area in Fujian Province, where the main products are abalone and scallops (Zhang et al., 2010). However, in recent years, eutrophication and HABs have been frequent in the mariculture areas, and these phenomena have a signif icant impact on the ecosystem health (Li,2000; Chai et al., 2013) and aff ect the marine culture industry.
HAB events are usually associated with rapid abnormal proliferation or high biomass accumulation of toxic or harmful microalgae on the sea surface or in water bodies (Anderson et al., 2012), and many HABs are associated with increasing eutrophication(Glibert and Burkholder, 2011). Understanding the key characteristics of HAB species diversity and population dynamics is essential (Zhou and Zhu,2006), and this understanding will serve as the basis for improved monitoring (Zingone and Enevoldsen,2000). Therefore, the investigation of the diversity of toxic and harmful microalgae in mariculture areas and their relationship with environmental factors is of great signif icance for the prediction and prevention of harmful algal blooms. There have been various studies on the phytoplankton community structure in the East China Sea (Tang et al., 2013; Zhou et al., 2018), but there are few studies focusing on the distribution of toxic and harmful microalgal species and their relationship with environmental factors in the aquaculture areas. Hence the aim of this essay is to investigate the distribution of toxic and harmful microalgae diversity in typical mariculture areas of the East China Sea (Gouqi Island, Sandu Bay, and Dongshan Bay) and to reveal the relationship between toxic and harmful microalgae and environmental factors in the mariculture areas. Understanding the baseline species composition could give insight to better anticipate and prevent HABs in mariculture areas of this region.
2 MATERIAL AND METHOD
2.1 Sample collection
According to the distribution of mariculture areas, a total of 12 sampling stations were set up in the typical mariculture areas of Gouqi Island (122°44ʹ57″E–122°49ʹ27″E, 30°42ʹ34″N–30°43ʹ51″N), Sandu Bay (119°44ʹ58″E–119°54ʹ19″E,26°22ʹ13″N–26°37ʹ17″N), and Dongshan Bay(117°20ʹ6″E–117°42ʹ8″E, 23°36ʹ18″N–23°56ʹ31″N),and 4 stations were set up in each mariculture area(Fig.1).
Four f ield sampling surveys were conducted in May 2020, August 2020, November 2020, and March 2021. At each sampling site, water samples were collected from the surface layer at a depth of 2 m with a Plexiglas water collector, and 1 L of water was f ixed with 15-mL Lugol’s solution. To determine of the species composition and cell density of phytoplankton and toxic and harmful microalgae, we f iltered 15 L of water through a 20-μm pore size phytoplankton net and collected 100 mL. Then, this subsample was f ixed in 1.5 mL of formaldehyde.
2.2 Phytoplankton and environmental factors
The water and net samples were brought to the laboratory and concentrated to 10 mL and 50 mL.After sedimentation for 24 h, the supernatant was discarded with a siphon tube. Phytoplankton were identif ied and counted using a 0.1-mL phytoplankton counting chamber under a light microscope (Olympus BX51, Olympus Corporation, Tokyo, Japan) at a magnif ication of 200–1 000× (Jiang et al., 2019).
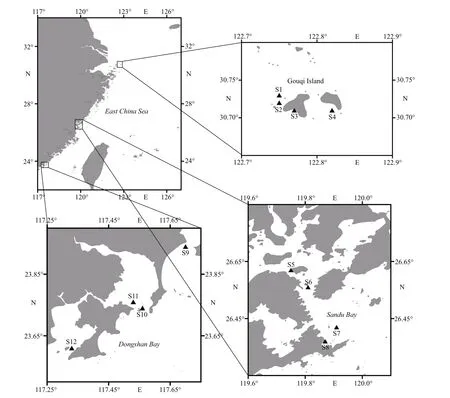
Fig.1 The sampling stations in typical mariculture areas of the East China Sea
For nutrient analysis, water samples for nutrient measurements were collected using a 0.45-μm pore size cellulose acetate membrane f ilter for orderly f iltration of seawater samples. Water temperature(WT), salinity (Sal), pH, and dissolved oxygen(DO) were measured with a U-50 multi-parameter water quality monitor (HORIBA, Japan) on site.Concentrations of phosphate (PO43ˉ), nitrate (NO3ˉ),nitrite (NO2ˉ), and silicate (SiO32ˉ) were measured and analyzed with an AA3 Nutrient Salt Automatic Analyzer (SEAL, Germany).
2.3 Data analysis
The phytoplankton and environmental data were analyzed using SPSS 28.0 software. One-way ANOVA was conducted to evaluate the diff erences in the phytoplankton or toxic and harmful microalgal cell densities. Diff erence in seasonal variation of environmental factors at 12 sampling sites was analyzed by two-way ANOVA (Cui et al., 2018). The statistical signif icance was set atP< 0.05. Dominance was calculated by the species cell density/total cell density to def ine dominant species (Chen et al., 2016).
The analysis of species data and environmental factors was performed using the “ggplot2” package(Wickham et al., 2020) and the “vegan” package(Oksanen et al., 2020) in RStudio 1.4.1717.Redundancy analysis (RDA) was used to characterize the correlation between environmental factors and toxic and harmful microalgae. Since our toxic and harmful algae data were zero-inf lated, we employed the Helliger transformation to our species andenvironmental variables (Legendre and Gallagher,2001). The signif icance of environmental factors in relation to species community composition was assessed using Monte Carlo permutation tests (Yu et al., 2016). All other graphing and analyses were performed in RStudio 1.4.1717 and Microsoft Offi ce Excel 2019.
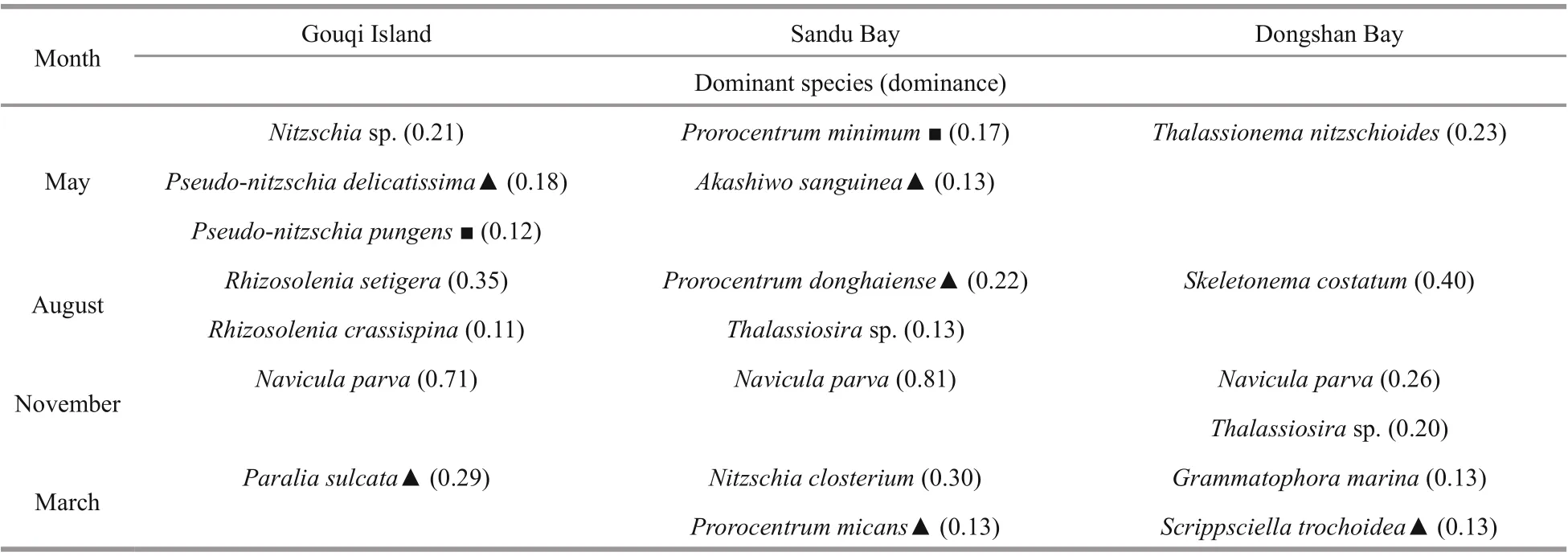
Table 1 Dominant species of phytoplankton in typical mariculture areas of the East China Sea
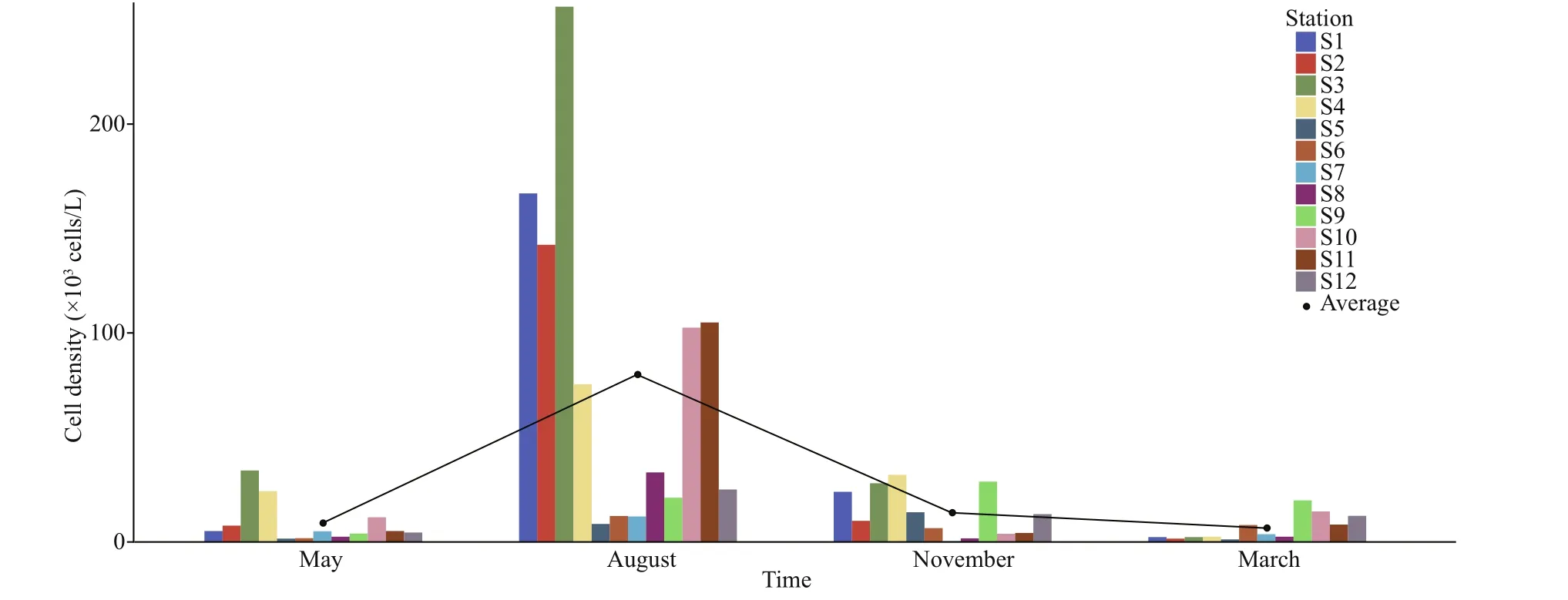
Fig.2 Temporal variation of phytoplankton cell density in typical mariculture areas of the East China Sea
3 RESULT
3.1 Phytoplankton assemblage
Across the 12 stations, we identif ied 199 phytoplankton species belonging to 70 genera, of which 145 species were Bacillariophyceae (72.9%),49 species from Dinophyceae (24.6%), 2 from Cyanophyceae (1%), 2 from Chrysophyceae (1%),and 1 from Raphidophyceae (0.5%). Of the 17 dominant species, we found eight harmful and toxic microalgal species (Table 1).
Phytoplankton cell densities were highest during the summer and relatively lower during the autumn, spring,and winter (Fig.2). The phytoplankton cell density was signif icantly higher during the summer than during the other seasons (ANOVA, df=3,F=9.437,P< 0.001), with an average of (80.03±77.81)×103cells/L in summer(August), (13.87±11.49)×103cells/L in autumn(November), (8.90±10.08)×103cells/L in spring (May),and (6.53±6.13)×103cells/L in winter (March).
3.2 Toxic and harmful microalgae assemblage and seasonal succession
A total of 38 toxic and harmful microalgal species belonged to 21 genera were found among the identif ied phytoplankton species (Table 2). With 24 species belonging to Dinophyceae and 13 species belonged to Bacillariophyceae, and 1 species belonged to Raphidophyceae (Yuan et al., 2009).
The species numbers (ANOVA, df=2,F=20.047,P<0.001) and cell density (ANOVA, df=2,F=1.221,P< 0.05) of toxic and harmful microalgae were signif icantly higher at the Gouqi Island stations(Fig.3a–b). There were 10 species of toxic and harmful microalgae with higher occurrence frequency( > 6) at each station (Fig.3a),P.pungens,P.sulcata,P.minimum, andP.micanswere found at all stations during the investigation (Fig.3b). Light microscopic photos of the high frequency toxic and harmful microalgal species are shown in Fig.4.
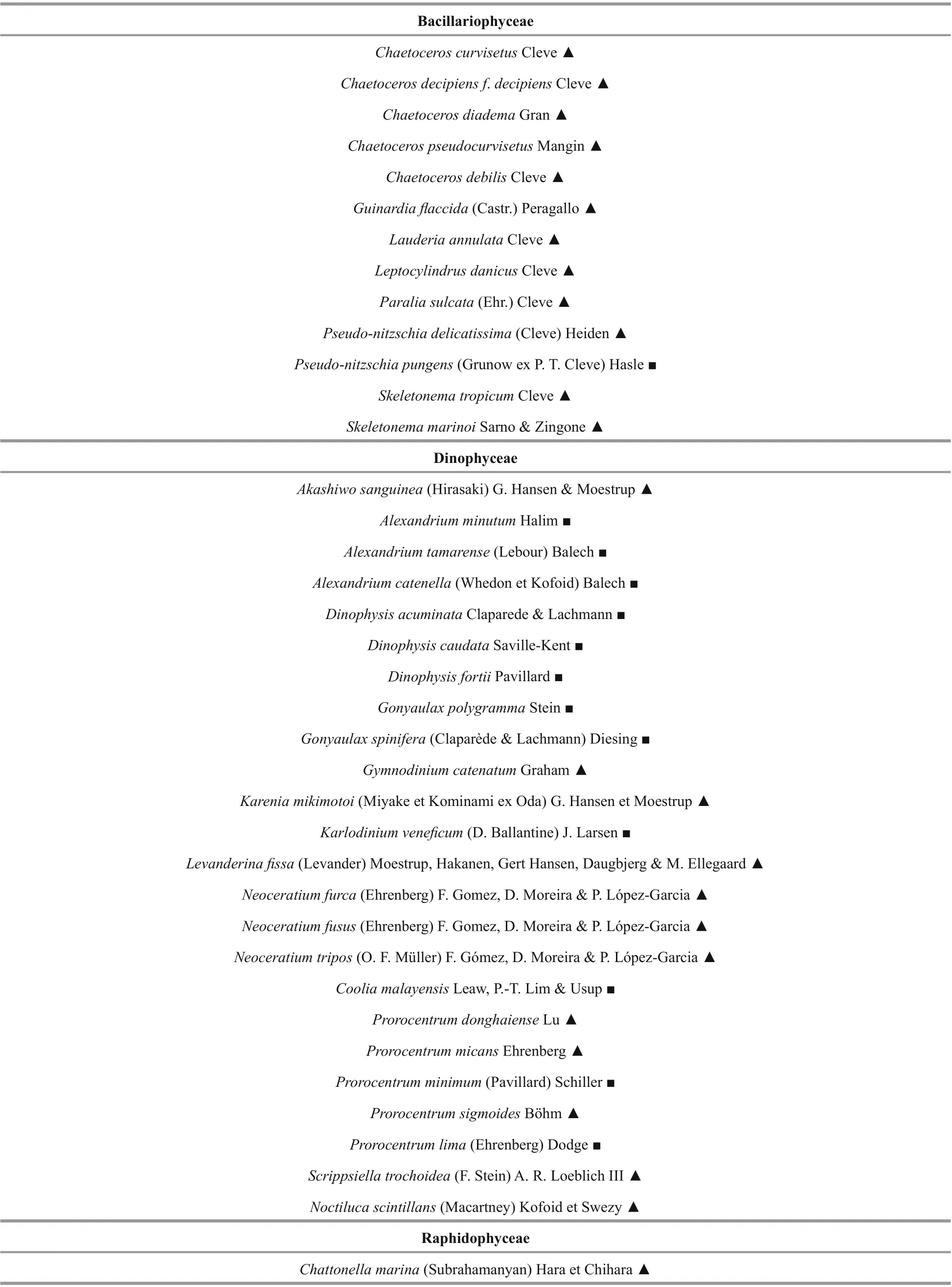
Table 2 List of toxic and harmful microalgal species in typical mariculture areas of the East China Sea
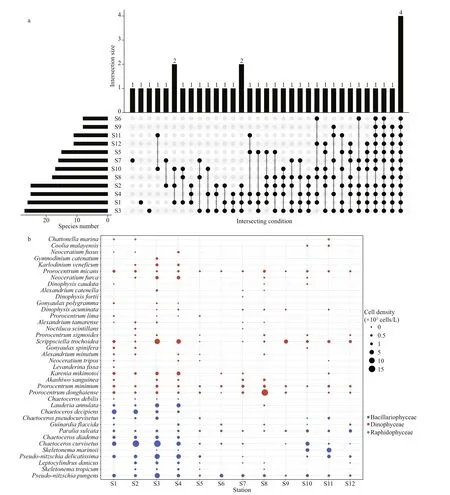
Fig.3 Composition and distribution of toxic and harmful microalgae in typical mariculture areas of the East China Sea
Fig.4 Light microscope photos of toxic and harmful microalgal species in typical mariculture areas of the East China Sea(frequency > 6)
During the sampling period, the distribution of toxic and harmful microalgae in the study regions was dominated by dinof lagellates in winter (March),spring (May), and autumn (November) when the temperature was relatively low. In summer (August),when the temperature was higher, diatoms were dominant (Fig.5a). We did not observe any toxic and harmful microalgal species in autumn at Stations S6 and S8 located in Sandu Bay (Fig.5a).
Seasonal diff erences in the density of toxic and harmful microalgae were evident (higher in spring and summer, lower in autumn and winter). The cell densities of toxic and harmful microalgae at each station averaged of (3.53±5.42)×103cells/L in spring, (15.34±15.28)×103cells/L in summer,(1.00±1.08)×103cells/L in autumn, and (1.82±1.44)×103cells/L in winter (Fig.5b).
3.3 Environmental factors
According to the environmental factors of the 12 stations (Fig.6), the trophic conditions in the East China Sea mariculture area during the four seasons ranged from 0.125 to 4.429 μmol/L for PO43ˉ (Fig.6a), 1.507 to 33.672 μmol/L for NO3ˉ (Fig.6b), 0.083 to 5.313 μmol/L for NO2ˉ (Fig.6c), and 1.768–32.160 μmol/L for SiO32ˉ(Fig.6d). There were no signif icant diff erences in the nutrient parameters of PO43ˉ, NO2ˉ, and SiO32ˉ (twoway ANOVA,P> 0.05) with seasonal variation at each station, but signif icant diff erences were found for NO3ˉ(two-way ANOVA,P< 0.05).
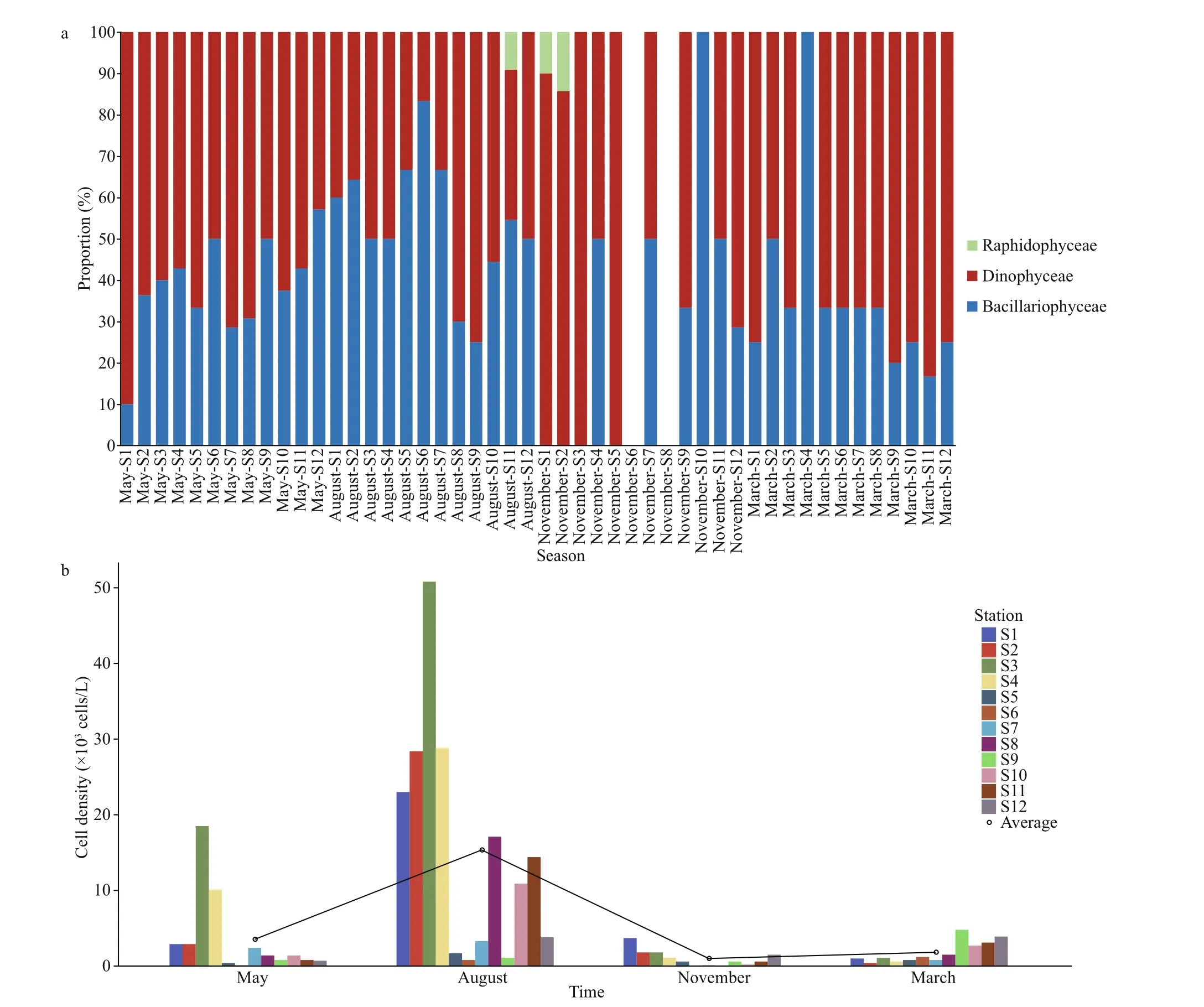
Fig.5 Seasonal variations in species composition and density distribution of toxic and harmful microalgae in typical mariculture areas of the East China Sea
In terms of physical-chemical parameters, water temperature (WT) varied signif icantly with the season, ranging from 11.74 to 32.16 °C (Fig.6e), and salinity (Sal) varied from 21.9 to 33.9 (Fig.6f), pH varied over a wide range from 4.90–9.67 (Fig.6g), and dissolved oxygen (DO) varied from 2.70–8.56 mg/L(Fig.6h). Signif icant diff erences were found in water temperature and dissolved oxygen with season (twoway ANOVA,P< 0.001), but no signif icant diff erences were found for salinity and pH (two-way ANOVA,P> 0.05).
3.4 Eff ects of environmental factors on toxic and harmful microalgae
We investigated the spatial and seasonal distribution of environmental factors on toxic and harmful microalgal communities in typical mariculture areas of the East China Sea. According to the RDA results, toxic and harmful microalgae showed signif icant seasonal changes during the whole year of sampling (Fig.7a). During the spring,toxic and harmful microalgae were mainly inf luenced by DO (Fig.7a). During the transition period from spring to summer, the toxic and harmful microalgae were limited by nitrate NO3ˉ. Then, in autumn, the growth of toxic and harmful microalgae was mainly inf luenced by pH, while in winter the main limiting environmental factor was WT. The results indicate that the succession of toxic and harmful microalgae was mainly driven by the diff erent environmental factors during the sampling year.
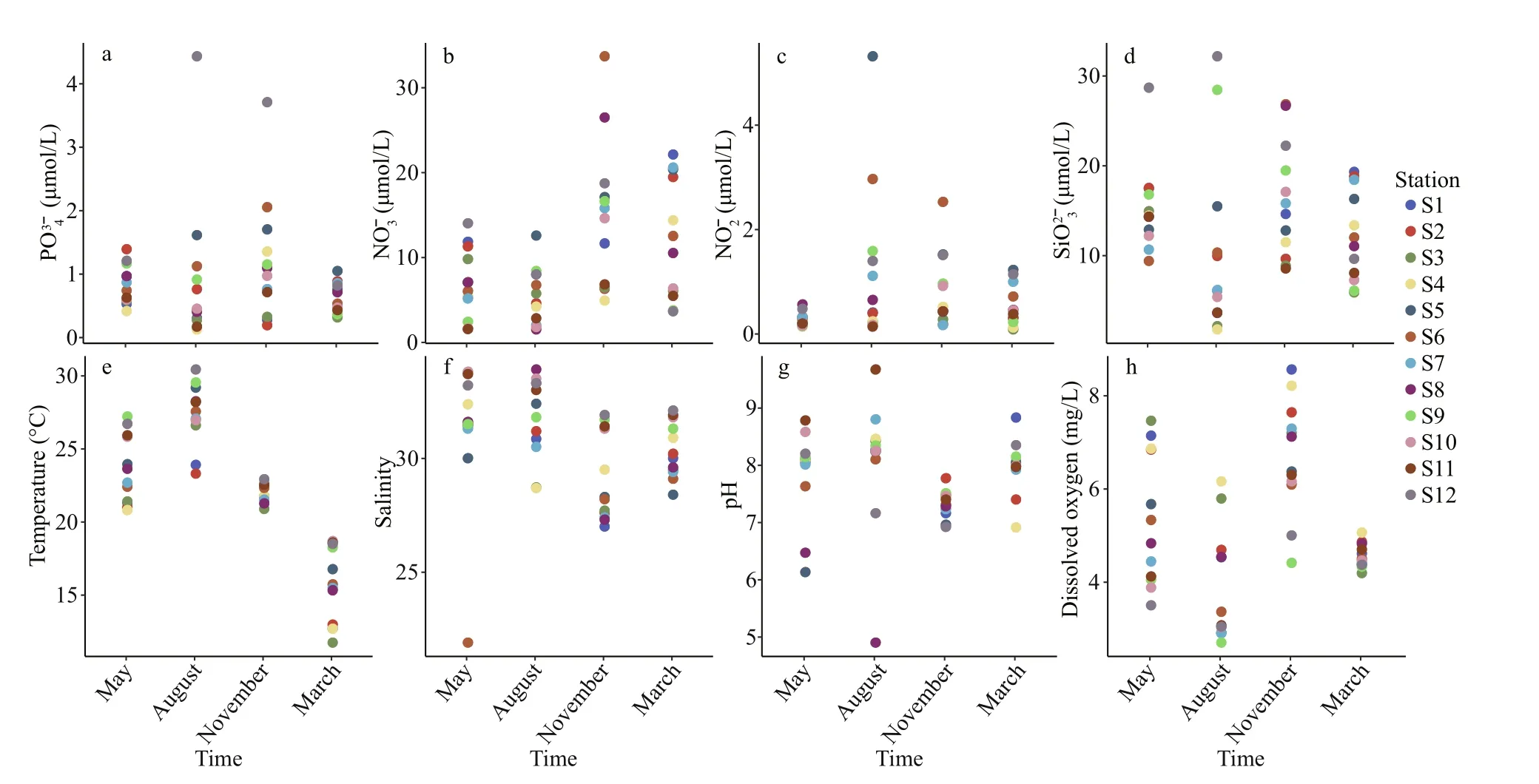
Fig.6 Temporal and spatial variations of the environmental factors
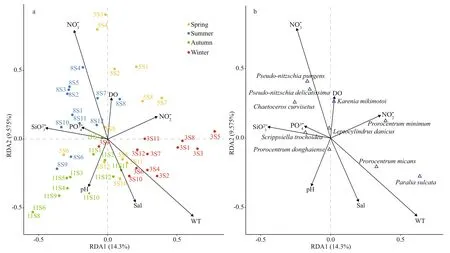
Fig.7 Redundancy analysis (RDA) of environmental factors and toxic and harmful microalgae data from all sampling stations during the four seasons
Among all samples, 10 species with a wide distribution (frequency > 6) were selected as the species data matrix (Fig.7b). The variation of toxic and harmful microalgal assemblages between stations and seasons was mainly aff ected by NO3ˉ, WT, pH, DO,and NO2ˉ.P.pungens,Pseudo-nitzschiadelicatissima,andChaetoceroscurvisetusshowed a preference for NO3ˉ.P.sulcataandP.micanswere characterized by high WT.Prorocentrumdonghaiensehad a particular pH.Kareniamikimotoiwas positively correlated with DO.P.minimumshowed particular favor to high NO2ˉ.
4 DISCUSSION
4.1 Spatial and temporal variation of toxic and harmful microalgae
The cell densities of toxic and harmful microalgae from the three mariculture regions displayed strong seasonal variation with the highest variation in summer (August) (Fig.5b). The cell densities of phytoplankton and toxic and harmful microalgae in Sandu Bay and Dongshan Bay were both at low levels during the spring (Figs.2 & 5b), which diff ered from past studies (Xu and Hou, 2006; Li, 2012). Previous studies showed that the high occurrence period of red tide in Fujian coastal waters was in May (Huang et al., 2020); however, in this study, the density of phytoplankton and toxic and harmful microalgae in the mariculture area of Fujian coastal waters remained at a low level in May, especially at Stations S5 and S6 (Figs.2 & 5b). The occurrence of red tide near the study stations (including Stations S5 and S6)before the timeframe of our sampling period could be responsible for the low levels of low phytoplankton cell density observed. Phytoplankton cell densities tend to sharply decrease after red tides (Xu et al.,1992; Mu et al., 2015), which is what we observed in our study.
In recent years, there seems to be a shift in dominant taxa of algal blooms from non-toxic diatoms to toxic dinof lagellates (Glibert and Burkholder, 2011), and an increase in red tide species in the East China Sea(Zhou et al., 2001). Ouyang et al. (1993) found a total of 6 species of toxic red tide species, including 5 species of toxic dinof lagellates, in the mariculture area of Shengsi Island, Zhejiang Province, and Xu et al. (2010) identif ied 6 species of toxic red tide species including 5 species of toxic dinof lagellates,in Fujian coast water. In this study, 13 species of toxic microalgae were found, including 12 species of toxic dinof lagellates (Table 2), and there was a signif icant increase in the number of toxic dinof lagellate species. At the same time, it can be seen that the species of toxic and harmful microalgae were mostly dominated by dinof lagellates in the yearly investigation (Fig.5a).
Four species of toxic and harmful microalgae were identif ied at all stations (Fig.3a). Among them,P.pungensandP.micansare widely distributed in the East China Sea as generalist species (Wang et al.,2001; Chen et al., 2021a), andP.pungenshas a strong nitrogen absorption ability (Zhang and Zhou, 1997).Glibert et al. (2008) indicated thatP.minimumis a type of species that expands with the nutrient level of the marine environment, andP.sulcata, as an indicator species of coastal environments, can be a good indicator of eutrophication in seawater (McQuoid and Nordberg, 2003), so its wide distribution may ref lect the eutrophication of the mariculture area.
4.2 Relationship between toxic and harmful microalgae and environmental factors
The occurrence of harmful algal blooms caused by toxic and harmful microalgae requires a specif ic environment (Liu et al., 1999), such as hydrological conditions, nutrients and biological environments(Chen et al., 2001). As shown in Fig.7a–b, the optimal environmental factors that inf luenced the toxic and harmful microalgae community included NO3ˉ, WT,pH, DO, and NO2ˉ. Water temperature determines the types of harmful algal blooms that occur, which is also necessary for the germination and reproduction of some toxic and harmful microalgal cysts (Pan and Jiang, 2004). In this study, the maximum WT(Fig.6e) occurred in August, and the peak growth of both phytoplankton and toxic microalgae occurred in this month (Figs.2 & 5b). According to the RDA results,P.sulcataandP.micansshowed a positive correlation with WT in the mariculture area (Fig.7b),which is consistent with the results of Yang et al. (2016) and Wang et al. (2001).P.sulcataandP.micansare eurythermal species, while the East China Sea has a subtropical and temperate climate.The suitable temperature is an important reason for their wide distribution. Dissolved oxygen is a major factor in determining the growth of algae in the water column and is also the primary key factor in determining the success or failure of aquaculture (Yao and Miao, 2021). We found a positive correlation with DO and a negative correlation with Sal forK.mikimotoi(Fig.7b), which is in clear agreement with the results of Zhao et al. (2020) and Su et al.(2020). pH not only has an important inf luence on the growth of phytoplankton, but also aff ects the toxicity eff ect of toxic algae producing toxins to other marine ecosystems (Schmidt and Hansen, 2001).P.donghaienseis widely distributed in the East China Sea (Chen et al., 2021a), and Yoo (1991) suggested that pH is the main factor aff ecting the growth ofP.donghaiense, and that its population growth has obvious requirements on the pH range (Dai et al.,2011). In this study, we also investigated that pH is the growth limiting factor ofP.donghaiensein the East China Sea (Fig.7b).
Nutrients are the main limiting factor for the growth of natural phytoplankton populations in the marine environment, and some studies have suggested that eutrophication may be a key factor in the occurrence of HABs (Yang et al., 2019). Dinof lagellates can rapidly develop blooms in aquatic environments.This process requires large amounts of nutrients,especially nitrogen (Abassi and Ki, 2022). We found a positive correlation betweenP.minimumand NO2ˉ,which coincides with earlier studies (Fan and Glibert,2005; Glibert et al., 2008). The abundance of nitrogen nutrients is the material basis for red tides (Berdalet et al., 1996). Previous studies have shown that changes in nitrogen in the water column are important in inf luencing diatom growth (Chen et al., 2002; Fei and Jiang, 2008).P.pungenshad a high correlation with NO3ˉ in Fig.7b, which is consistent with the results of Lü et al. (2006). Therefore, we believe that nutrients,such as NO3ˉ and NO2ˉ, also have major inf luences on the growth of toxic and harmful microalgae in the mariculture areas.
4.3 Relationship between toxic and harmful microalgae and mariculture areas
The environment of the mariculture area and the feeding eff ect of the mariculture products will inf luence the composition of toxic and harmful microalgae.Previous studies have indicated that shellf ish have a greater selective tendency to feed on dinof lagellates than diatoms (Rouillon et al., 2005; Zhang et al.,2008). We found that dinof lagellate species among toxic and harmful microalgae were more abundant than diatoms in all three mariculture areas, but the density remained relatively low (Fig.3b). Mussels are the main mariculture product, which feed selectively on phytoplankton (Kiørboe et al., 1980) and less on toxin-producing and indigestible algae (Langdon and Waldock, 1981). Interestingly, when the density of algal cells is low, mussels will feed as much as possible to ensure their growth regardless of the nature of the food (Tan and Ransangan, 2017). In this study,there was a high level of distribution ofP.pungensin the Gouqi Island mariculture area (Fig.3b). Because of the dispersion ofP.pungens, mussels may selectively feed on it, and mussels in the Gouqi Island mariculture region may be exposed to amnesic shellf ish poisoning(ASP) toxin.
The nutrient conditions in the mariculture area are also an important reason for the occurrence of toxic and harmful algal blooms (Wang et al., 2008).Previous studies found eutrophication on Gouqi Island (Bao et al., 2020), Sandu Bay (Zheng, 2007),and Dongshan Bay (Li, 2000). These regions show intensif ication of eutrophication, especially NO3ˉ and PO43ˉ (Fig.6a & b). In the mariculture region of Gouqi Island,P.pungensandP.delicatissimawere correlated with NO3ˉ (Fig.7b). They were found at all four locations, and were the dominant species in the spring (Fig.3b; Table 1). NO2ˉ was high in the Sandu Bay mariculture region (Fig.6c).P.minimum(Sierra-Beltrán et al., 2005), with a preference for NO2ˉ, was not only widespread but also one of the dominant species in the spring (Fig.3b; Table 1). In Dongshan Bay, PO43ˉ was relatively high (Fig.6a). RDA showed a positive correlation betweenScrippsiellatrochoideaand PO43ˉ (Fig.7b). During the winter,S.trochoideabecame the dominat species in Dongshan Bay and was widely distributed throughout the region (Fig.3b;Table 1). Considering the wide distribution of these toxic and harmful microalgae and the intensif ication of eutrophication in the mariculture area (Bao et al.,2020; Huang et al., 2020), there is a potential risk of harmful algal blooms in typical mariculture areas in the East China Sea.
5 CONCLUSION
This study revealed the relationship between toxic and harmful microalgae and environmental factors in three typical mariculture areas in the East China Sea. During the research period, 38 species of toxic and harmful microalgae belonging to 21 algal genera were identif ied. The toxic and harmful microalgae taxa were dominated by dinof lagellates. There were 8 species of toxic and harmful microalgae that appeared as dominant species:P.pungens,P.delicatissima,P.minimum,Akashiwosanguinea,P.donghaiense,P.sulcata,P.micans, andS.trochoidea. Among them,P.pungens,P.minimum,P.sulcataandP.micanswere found at all stations. The RDA results showed that interactions between toxic and harmful microalgae and environmental factors were closely related to NO3ˉ, WT, pH, DO, and NO2ˉ.P.pungens,P.delicatissima,P.minimum, andS.trochoideawere prevalent under high-nutrient conditions. Considering the importance of East China Sea mariculture, the monitoring of nutrient conditions in the mariculture area is necessary.
6 DATA AVAILABILITY STATEMENT
The datasets generated or analyzed during the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENT
The authors would like to thank Professors Yanan LU and Chengqi FAN from the East China Sea Fisheries Research Institute for their help during sampling and data collection.
 Journal of Oceanology and Limnology2022年6期
Journal of Oceanology and Limnology2022年6期
- Journal of Oceanology and Limnology的其它文章
- Overview of harmful algal blooms (red tides) in Hong Kong during 1975–2021
- Information standardization for typical toxic and harmful algae in China’s coastal waters—a case study of Karenia mikimotoi*
- Biochemical composition of the brown tide causative species Aureococcus anophageff erens cultivated in diff erent nitrogen sources*
- Identif ication of paralytic shellf ish toxin-producing microalgae using machine learning and deep learning methods*
- Screening for lipophilic marine toxins and their potential producers in coastal waters of Weihai in autumn, 2020*
- First observation of domoic acid and its isomers in shellf ish samples from Shandong Province, China*
