A two-year (2020–2021) observation of marine phycotoxins in phytoplankton in typical mariculture areas of East China Sea*
Xiaoqing TIAN , Chengqi FAN , Yunyu TANG , Haiyan ZHANG , Wei KANG ,Sha CHEN , Chongbin LI , Ya’nan LU ,**
1 Key Laboratory of East China Sea & Oceanic Fishery Resources Exploitation and Utilization, Ministry of Agriculture and Rural Aff airs, East China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Shanghai 200090, China
2 Shanghai Ocean University, Shanghai 201306, China
Abstract To learn the marine phycotoxins (MPTs) contamination status in mariculture areas in the East China Sea (ECS), from May 2020 to October 2021, 80 net-concentrated phytoplankton samples were collected from 12 stations in three typical areas with high incidence of red tides, namely, Gouqi Island,Sandu’ao-Lianjiang, and Zhangzhou-Dongshan Island in ECS, and MPTs of the samples were detected. Six types of toxins were detected in 24 samples from 9 stations. Lipophilic marine toxins (LMTs) were more common and diverse in these areas. Pectenotoxin 2 (PTX2) was the main lipophilic marine toxin (LMT) in the concentrated phytoplankton samples and the occurrence showed seasonal diff erences from north to south.According to the potential risks of pectenotoxin (PTXs) to seafood safety, it is suggested to execute regular monitoring on PTXs in ECS and a mandatory standard should be formulated based on the comprehensive analysis of in-situ monitoring and lab research. Meanwhile, contamination risks of cyclic imine toxins in the north and domoic acid (DA) in the south of ECS should also be paid with attention to. Only 2 paralytic shellf ish toxins (PSTs), N-sulfocarbamoyl toxin C2 (C2), and decarbamoyl gonyatoxin 3 (dcGTX3), were found from spring samples in the north of ECS. As the biggest mussel culture county of China, Gouqi Island showed higher toxin diversity and the toxin detection rate was higher than the other two areas. In Gouqi Island area, PSTs were a serious potential threat in spring, and LMTs instead of PSTs became the main risk in summer-autumn seasons. To ensure the safety of seafood and marine environmental health, it is recommended to conduct long-term targeted tracking and monitoring of MPTs in this and similar mariculture areas.
Keyword: marine phycotoxins (MPTs); pectenotoxin 2 (PTX2); mariculture areas; East China Sea; seafood safety
1 INTRODUCTION
Marine phycotoxins (MPTs), mainly produced by harmful marine microalgae, frequently accumulate in bivalves which make great losses in aquaculture and cause serious safety issues to human health (Grienke et al., 2014; Liang et al., 2022). East China Sea (ECS)is one of the most important aquaculture areas in China. An increasing number of harmful algal blooms(HABs) events have been recorded during the last two decades in the coastal waters of ECS. From 2002 to 2017, the cumulative size aff ected by HABs in ECS was 103 776 km2; from 2011 to 2017, there were 212 HABs in ECS; Zhejiang coastal area showed the most frequent HABs and was the most aff ected area by HABs (Zhang et al., 2020). On 20thcentury, the dominant microalgae species of HABs in ECS were diatoms. However, during the past 20 years of the 21thcentury, dinof lagellates, includingProrocentrumdonghaiense,Kareniamikimotoi, andAlexandriumspp., have become the majority species of large-scale HABs (Zhou et al., 2008, 2022; Yu et al., 2017, 2018).For example, in the spring of 2005, a red tide caused by dinof lagellates was recorded which the size was more than 10 000 km2and the economic loss was over 30 million RMB in the Changjiang (Yangtze)River estuary and adjacent waters; in 2012, a red tide caused byK.mikimotoiin Fujian Province resulted in at least 2 billion RMB losses (Yu et al., 2017).Pessimistically, researches showed that changes in nutrient composition of the Changjiang River discharge and global climate warming may make dinof lagellate HABs in ECS more frequently in future(Wang et al., 2021; Zhou et al., 2022).
Comprehensive and eff ective monitoring and early warning is an important method to prevent and control the contamination of MPTs in the mariculture areas. In China, research on MPTs in phytoplankton or seawater was mainly concentrated in the bays of the Yellow Sea and the Bohai Sea, while research on the East China Sea (ECS) was relative rare (Chen et al., 2019; Liang et al., 2022). In Changjiang River estuary and adjacent sea areas, according to a study of phytoplankton from the end of March to the end of May in 2011, N-sulfocarbamoyl toxins C1 and C2 were the dominated toxins accounting for 94%of the total toxins (Chen, 2013). A research about paralytic shellf ish toxins (PSTs) in phytoplankton showed that May and June were the high-risk period of PSTs contamination (Liu et al., 2020). Another correlation analysis from results of three surveys in spring-autumn season during 2017–2018 showed that the lipophilic marine toxins (LMTs) levels in seawater were positively correlated with dissolved oxygen and salinity, but negatively correlated with temperature and nutrients (He et al., 2019). In Nanji Island, middle coast of ECS, Qu et al. (2016) studied LMTs in the mussels along coast of Nanji Island with solid phase adsorption toxin tracking (SPATT)technology and 7 types of LMTs were detected. In three locations of the south coast of ECS, Xiamen,Jinjiang, and Dongshan, results showed a quite low detection level (0.03–0.2 ng/L) of LMTs (Zhang et al., 2018).
To learn the status and regular pattern of MPTs contamination in the East China Sea, the netconcentrated phytoplankton samples from three typical mariculture areas in ECS were collected in two consecutive years (2020–2021), and the toxins were detected by high performance liquid chromatographymass spectrometry (HPLC-MS) method. The research results will be helpful in learning knowledge of the outbreaks characteristics of marine microalgae toxins in ECS. Moreover, it will also be benef icial to protect marine ecological environment and ensure the safety of aquaculture seafood.
2 MATERIAL AND METHOD
2.1 Reagent and material
Thirteen PSTs certif ied reference standards(saxitoxin (STX), decarbamoyl saxitoxin (dcSTX),neosaxitoxin (NEO), decarbamoyl neosaxitoxin(dcNEO), gonyatoxins 1–5 (GTX1–5), decarbamoyl gonyatoxins 2–3 (dcGTX2–3), N-sulfocarbamoyl toxins C1–2 (C1–2)), 10 LMTs certif ied reference standards (okadaic acid (OA), dinophysistoxins 1–2(DTX1–2), pectenotoxin 2 (PTX2), yessotoxin (YTX),13-desmethyl spirolide C (SPX1), gymnodimine(GYM) and azaspiracids 1–3 (AZA1–3)) and domoic acid (DA) were purchased from the National Research Council (NRC) of Canada. Formic acid (>98%),ammonium acetate (>97%), acetonitrile, and methanol(absolute, hypergrade) were purchased from J. T. Bake(USA). Deionized water was produced by a Milli-Q water purif ication system (Millipore, Billerica, MA,USA). Solid phase extraction (SPE) column Strata-X(60 mg3/mL) was purchased from Phenomenex (USA).Columns for HPLC were TSK-Amide-80 (3 μm,2 mm×150 mm, TSKgel, Japan) and Kinetex XB-C18(2.6 μm, 2.1 mm×100 mm, Phenomenex, America),respectively. GF/C f ilter membrane (Φ47 mm) was from Sangon Biotech (China). Temperature, salinity,chlorophylla(Chla), pH, and dissolved oxygen(DO) were measured with U-50 water quality monitor(HORIBA, Japan).
2.2 Sampling stations and procedures
Twelve stations were selected in three typical coastal aquaculture areas (4 stations for each)covering the East China Sea (Fig.1). Phytoplankton samples were collected in May, August, November of 2020, and March, May, August, October of 2021.From each station, 200-L surface seawater was pumped and f iltered through 20-μm mesh in length of 100 cm, then rinsed with seawater and diluted to 1 L for phytoplankton collection. An amount of 300-mL concentrated liquid was f iltered with GF/C f ilter membrane (Φ47 mm) and the membranes were frozen at -20 ℃.
2.3 Extraction of toxins from the phytoplankton
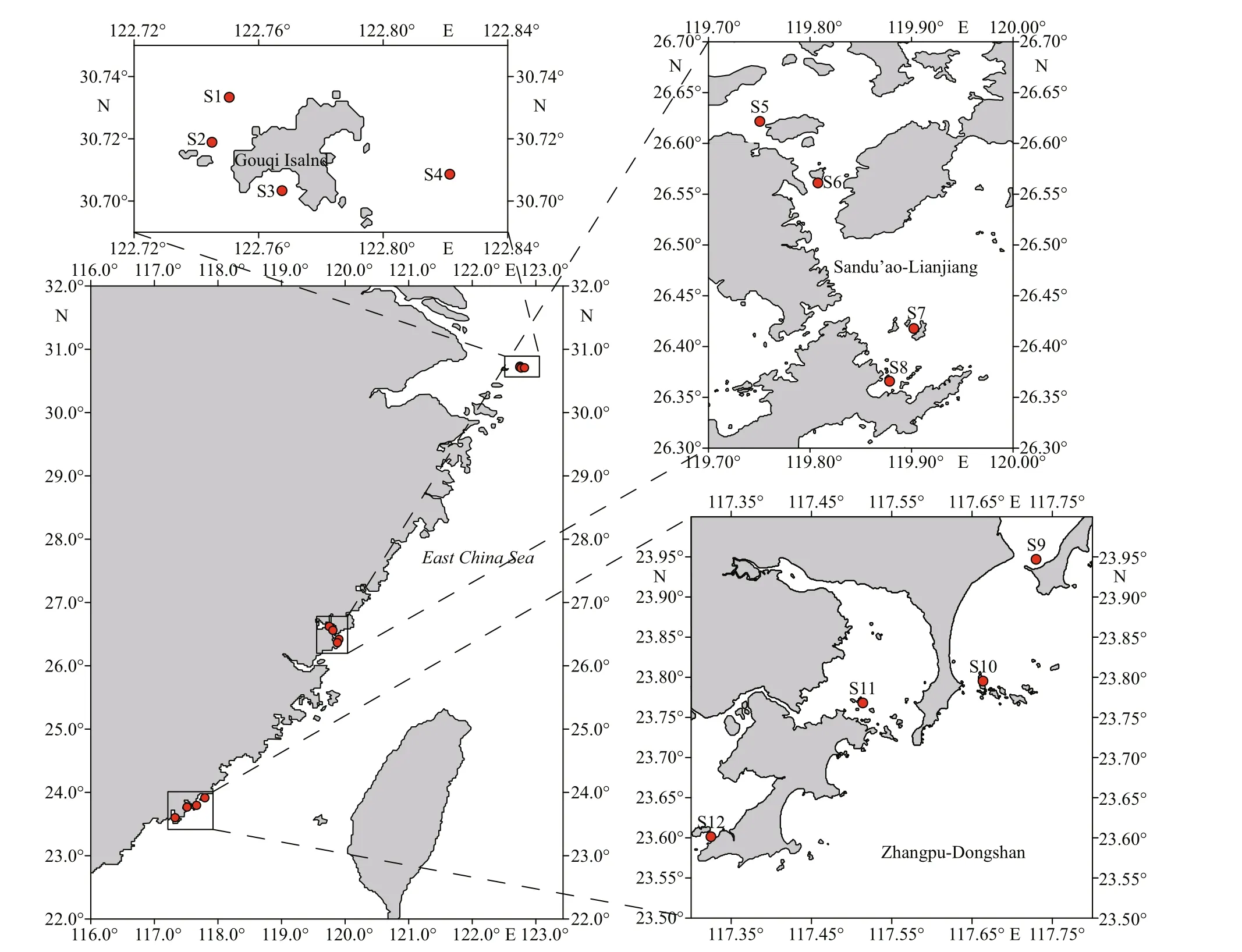
Fig.1 Sampling locations in three typical mariculture areas of East China Sea
Frozen membranes with phytoplankton cells mentioned in Section 2.2 were cut into small pieces and put in the centrifuge tubes. For PSTs detection,the tubes were ultrasonically extracted for 5 min with 5-mL 1% acetic acid solution in ice bath, 2-mL extractions was centrifuged for 10 min at 10 000 r/min and f iltered through 0.22-μm f ilter, then kept at -80 ℃for further analysis. For LMTs and DA detection,tubes were ultrasonically extracted for 5 min with 5-mL methanol in ice bath, then were centrifuged and f iltered as PSTs procedures and kept in the same conditions before analysis.
2.4 Liquid chromatography tandem mass spectrometry (LC-MS/MS) analysis
2.4.1 Detection of Extraction of PSTs
For common analogues of PSTs, multiple reaction monitoring (MRM) was used. The Shimadzu DGU-20A5R HPLC (Shimadzu Corporation, Japan) was coupled to the Sciex Qtrap 5500 tandem quadrupole mass spectrometer (Danaher Corporation, USA)with an electrospray ionization interface. The chromatographic separation was performed on a TSK-gel Amide-80®HILIC column (150 mm×2 mm i.d., 3 μm) (Tosoh Bioscience LLC, Montgomeryville,PA, USA) using a f low rate of 1.2 mL/min at 40 °C.The binary mobile phase were water (solvent A) and 95% acetonitrile (solvent B), each of them containing 2-mmol/L ammonium formate and 50-mmol/L formic acid. The gradient ran from 90% B to 80% B over 3.6 min, decreased to 60% B over an additional 2.4 min, held for 1.5 min at 60% B, increased to 90%B over an additional 1 min, held for 1.5 min at 90% B before re-equilibration for the next run. High resolution mass spectrometry conditions included spray voltage of 4.5 kV and -4.5 kV for positive and negative ion,respectively, curtain gas (CUR) pressure of 20 psi,ion source gas1 (GS1) and gas2 (GS2) pressure of 30 psi, ion source temperature of 550 °C and collision gas (CAD) of medium. Common analogues were scanned using the selective reaction monitoring (SRM)transitions shown in Table 1.
2.4.2 Detection of Extraction of LMTs and DA
Toxins were analyzed by LC-MS/MS systemcomposed of a Thermo Scientif ic HPLC system and a Thermo TSQ Quantum Ultra triple-quadrupole mass spectrometer with electrospray ionization (ESI) source(Thermo Fisher Scientif ic, Waltham, MA USA). The chromatographic separation was performed on a KinetexXB-C-18 column (2.6 μm, 2.1 mm×100 mm,Phenomenex, America) at flow rate of 1.2 mL/min at 40 ℃. The binary mobile phases were water (solvent A) and 95% acetonitrile (solvent B), both mixed with 2-mmol/L ammonium formate and 50-mmol/L formic acid. From start to 7.00 min, the gradient shifted from 20% B to 90% B, and was kept at this proportion for 3 min, then recovered to 20% B in 1/10 min and was maintained for 2 min for re-equilibration for the next run.
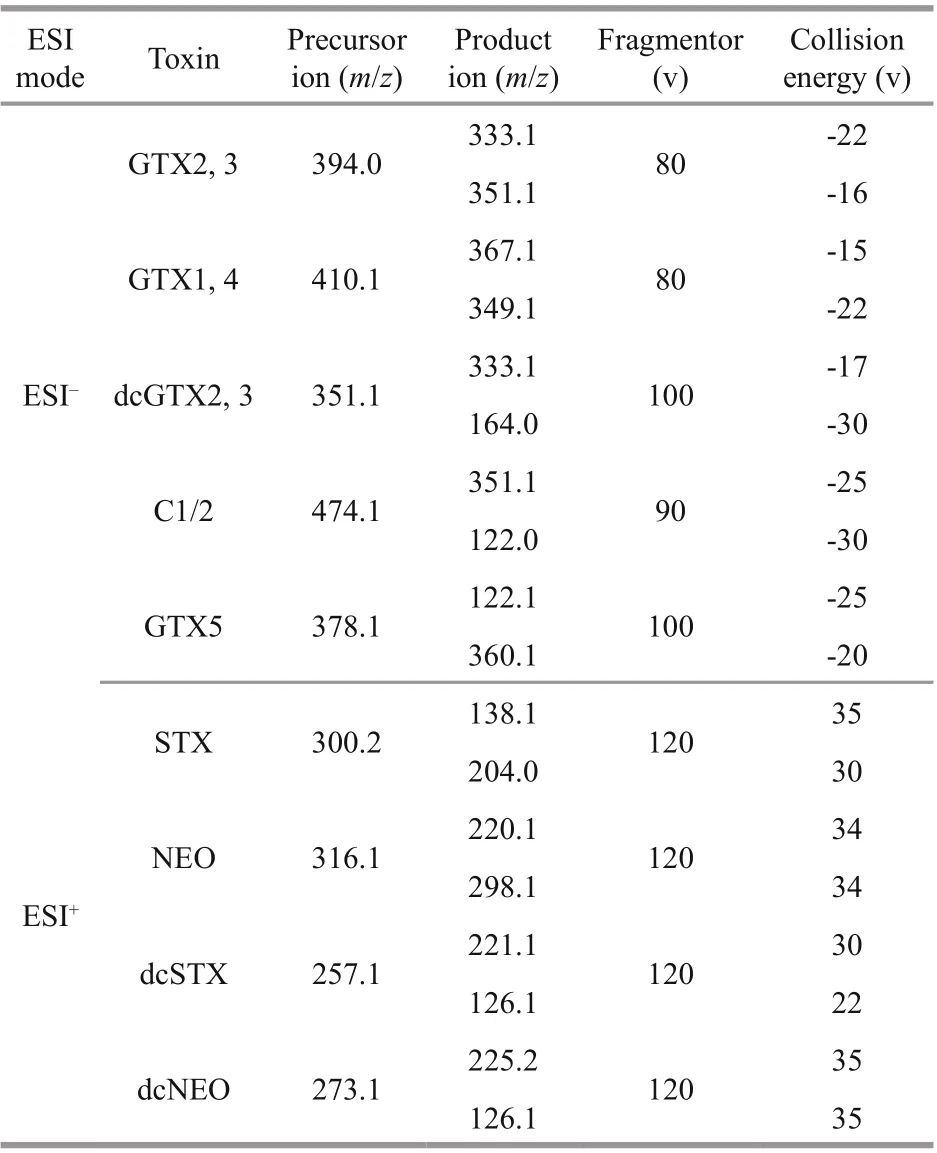
Table 1 Acquisition parameters of MRM mode scanning for PSTs
The ESI source was set on the positive and negative ion mode with 3 500-V spray voltage and 350-℃capillary temperature. The sheath gas and auxiliary gas f low rates were 30 and 10 psi, respectively. The MS parameters of retention time, optimized ion transitions, and collision energies are listed in Table 2.
2.5 Statistical analysis

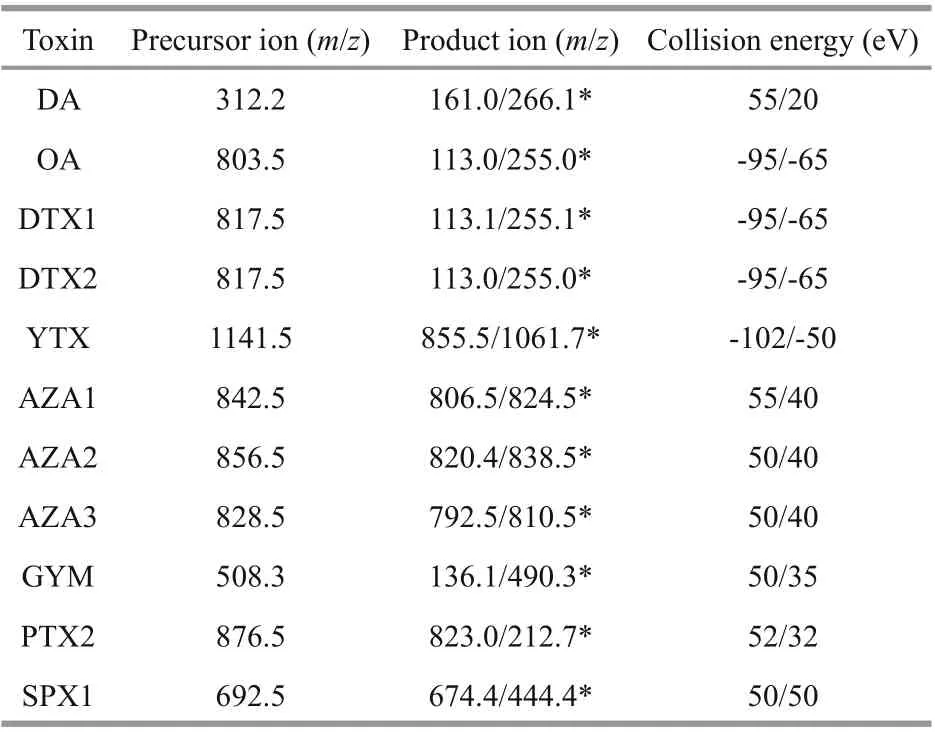
Table 2 The mass spectrometry parameters for LMTs and DA
Concentration of toxins in phytoplankton samples was calculated with Eq.1.in whichXi: toxin concentration in phytoplankton samplei, unit: ng/L,imeans the certain sample in 80 samples,i=1, 2, ∙∙∙, 80;Ci: the corresponding toxin concentration of sampleishown on the standard curve, unit: μg/mL;Vd: dilution volume, unit: mL;Vs:volume of f iltered seawater, unit: L.
3 RESULT
3.1 Toxin detection rate and diversity
Toxins were detected in 24 samples from 9 stations,which accounted for 30.0% of the total 80 samples and 75.0% of total 12 stations (Fig.2 and Supplementary Fig.S1). The majority of samples were found only one type of toxin except for 2 samples for having 2 diff erent toxins (8.3% of total samples with toxins).Stations 1 and 7 showed the highest toxin detection rate (57.1%) among all stations. Stations 5, 9, and 12 showed no toxins in the two years. In three mariculture areas, Gouqi Island showed the highest toxin detection rate (39.3%), in which toxins were detected from 11 samples of the total 28 samples. Meanwhile, the samples with 2 toxins were all in Gouqi Island area.Toxin detection rate in Sandu’ao-Lianjiang area was 28.6%. Although the toxin detection rate in Zhangpu-Dongshan area was the lowest at 20.8%, it did not present that this area was better than the other two because there was a lack of data for August in 2021.
Six types of toxins were detected, including 2 PSTs (C2 and dcGTX3) and 4 LMTs (PTX2, SPX1,GYM, and DA). The toxin with the highest detection rate was PTX2, which was detected in 20 samples(25.0%). The highest concentration was 0.117 ng/L at S10 station in October 2021. The other three LMTs were only detected once, respectively, and toxin detection rate was all 0.01%. Regarding to PSTs, C2 was detected twice while dcGTX3 was only once.
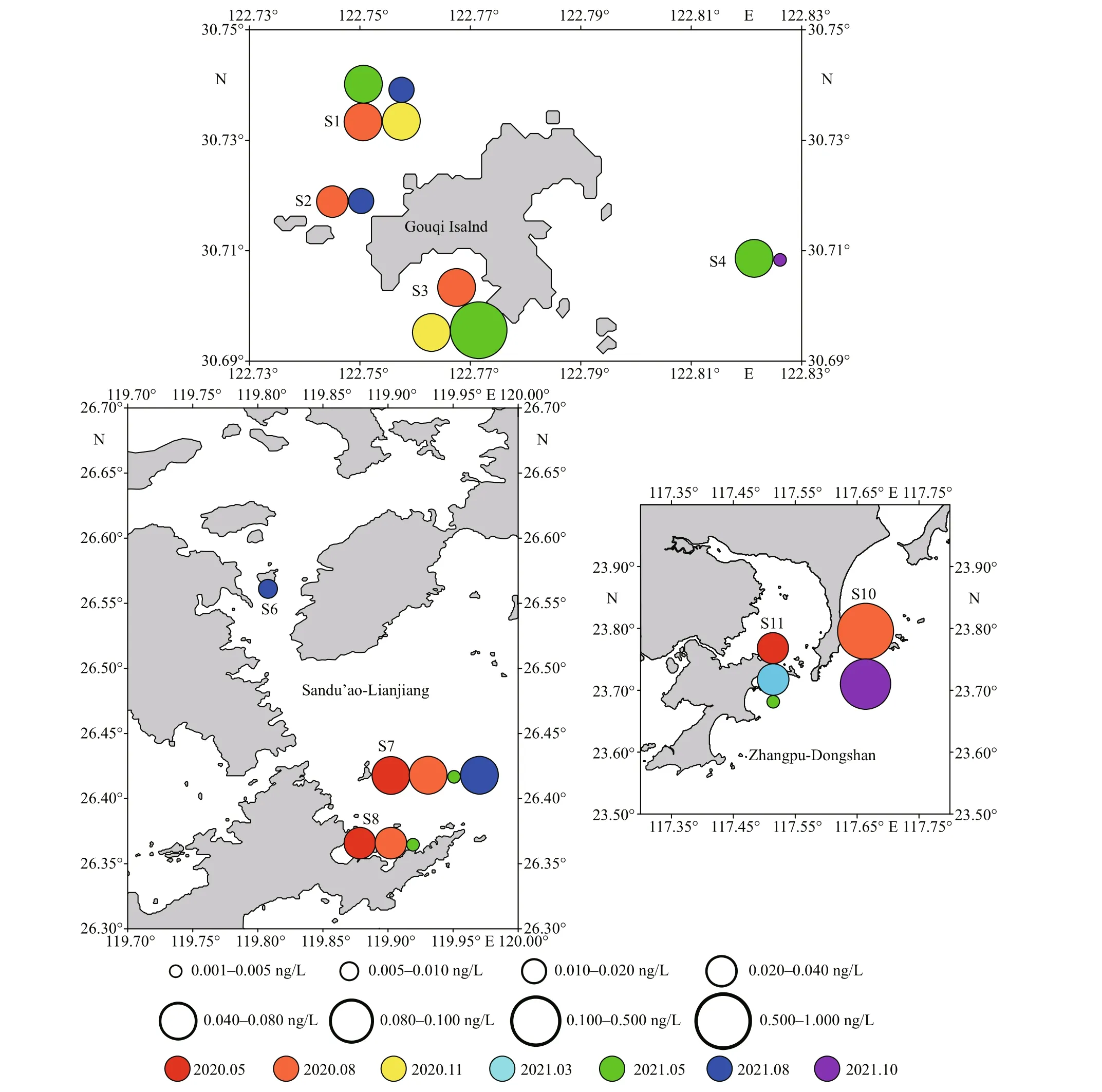
Fig.2 Distribution of MPTs in three mariculture areas of ECS
The toxin diversity in Gouqi Island areas was the highest where 2 PSTs and 3 LMTs were detected. In Sandu’ao-Lianjiang area, only PTX2 was detected. In Zhangpu-Dongshan area, except for PTX2, DA had been detected from sample of S10 in August 2020 with a high concentration of 0.991 ng/L.
Although there was less data, it showed trends that the types of toxins were diff erent according to seasons in diff erent areas. In Gouqi Island area, PSP toxins only occurred in spring-summer season, while LMTs were detected mainly in summer and autumn-winter(October and November). In Sandu’ao-Lianjiang area,toxins were mainly detected in May and August. In Zhangpu-Dongshan area, because the data of August 2021 was lost, it was not easy to summarize the trend.
3.2 Trends of PTX2
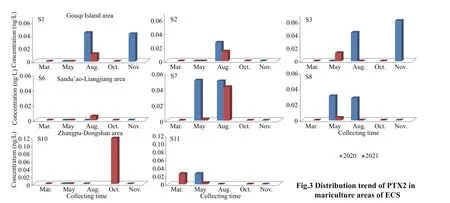
In this research, only PTX2 was found in all three mariculture areas. The concentration in 2021 was higher than that in 2020 (Fig.3). However, the highest concentration was detected in S10 in October 2021 with 0.117 ng/L, which was almost double than the second highest concentration which detected in S3 in November 2020 (0.062 ng/L).
Pectenotoxin 2 was the only toxin detected from the 28 samples in Sandu’ao-Lianjiang area and showed obvious seasonal trend, which was detected only in May and August. Among them, S7 and S8 showed worse status than the other two stations. In 2020, the concentrations of samples from S7 in both May (0.051 ng/L) and August (0.050 ng/L) were obviously higher than those from S8 (0.031 and 0.028 ng/L, respectively). In 2021, the concentrations in May were all quite low (S7 with 0.002 ng/L and S8 with 0.003 ng/L), but in August, the concentration of S7 increased rapidly to 0.042 ng/L, while the toxin disappeared in S8. PTX2 was found only once with low concentration at 0.006 ng/L at S6 in August 2021,while it was never found at S5.
In Zhangpu-Dongshan area, PTX2 was not found at S9 and S12. At S11, PTX2 was f irst detected from samples collected in March with a concentration at 0.026 ng/L, and then the concentration went down to 0.003 ng/L in May. The highest concentration(0.117 ng/L) was recorded at S10 in October 2021,and because the data of August in the same year was missing, it was diffi cult to know whether it happened coincidentally.
4 DISCUSSION
4.1 Contamination risks of PSTs in mariculture areas in ECS
Paralytic shellf ish toxin contamination is common in gastropods and bivalves of ECS, and showed the trends that the toxicity of samples collected from off shore waters was generally higher than that of samples from inshore, as well as that PSTs concentration was typically the highest in spring of all bivalves (Liang et al., 2022). Liu et al. (2020)found that April to June was the peak season of PSTs in Zhoushan Islands, where C1 and C2 showed the highest concentration in this period. Meanwhile,low concentration of GTXs, dcGTX, and NEO were also detected in the same period. In our research,the detection of high concentration of C2 (highest at 1.409 ng/L) and low concentration of dcGTX3 in Gouqi Island (part of Zhoushan Islands) in May showed the same tendency as Liu’s research.
Dinof lagellate of genusAlexandriumwas the common microalgae that could produce PSTs in coastal areas of China. From 2002 to 2017, 24Alexandriumblooms were reported in China, among which 14 occurred in the East China Sea, and most of them were complex ofAlexandriumspp. andProrocentrumdonghaiens(Liang et al., 2019). A research about the diversity ofAlexandriumin China showed that Atama complex Group IV (A.pacif icum) was the major reason corresponding to PSTs producing in ECS area,and C1and C2 were predominant in almost all strains(Gu et al., 2013; Zou et al., 2014). In the north ECS area, spring was a season with more dinof lagellate blooms (Liu et al., 2020). Therefore, in mariculture area of the north ECS,Alexandriummicroalgae blooms and PSTs contamination monitoring should be enhanced.
4.2 Pectenotoxins contamination risks and suggested countermeasures
Pectenotoxin 2 (PTX2) was the main toxin that could be detected in ECS mariculture areas. In Gouqi Island, the north of ECS, PTX2 was found in samples from August and November; In Sandu’ao-Lianjiang,the middle coast of ECS, PTX2 was found in samples of May and August. In Zhangpu-Dongshan, the south coast of ECS, PTX2 was found f irst time in March. In general, the outbreak time of PTX2 showed a trend of gradually advancing from north to south, which may be attributed to that the seawater temperature was higher gradually from the north to the south.
PTXs, a type of marine toxin with polyether lactone structures produced fromDinophysismicroalgae, was f irst found from Japanese cultured scallop (Patinopectenyessoensis) (Liu and Liang,2010; Grienke et al., 2014). Now, over 20 PTXs have been found and identif ied from shellf ish and algae all over the world (Liu and Liang, 2010; Paredes et al.,2011; Díaz et al., 2020). Some studies showed that only four PTXs, PTX2, PTX11, PTX12, and PTX13,originated from algaeDinophysis, while the others were metabolic compounds in shellf ish or derivative compounds during chemical extraction (Dominguez et al., 2010). In China,Dinophysisspecies distributed widely along coast (Gu et al., 2022),D.rotundataandD.fortiiwere reported in Dalian and Qingdao in the Yellow Sea (Luo, 2011; Gao et al., 2017) and Hongkong in South China Sea (Gu et al., 2022);D.caudatawas recorded in Hainan Province of the South China Sea (Chen and Ni, 1988) and East China Sea (ECS) (Li et al., 2015).
In microalgae samples from Qingdao (Luo, 2011)and Qinzhou Bay in the South China Sea (Xu et al.,2021), PTX2 was detected. In the East China Sea, Li et al. (2015) collected and identif iedD.caudatain Gouqi Island seawater in July and September 2013,and found PTX2 in them. In our research, PTX2 was found in phytoplankton samples in August, which was the same season as Li’s research. It might show that the summer-autumn (August and September)was the incident time for PTX2 toxin in Gouqi Island.However,Dinophysisgenus cells were not found in our research. One of the possible reasons was that the abundance ofDinophysiscells was not high enough to be collected. The other possible reason was that the cells were attached on the inner side of trawl net and could not to be washed into the sample bottle.In future research, the phytoplankton collecting time should be prolonged and the net should be rinsed for more times to ensure the sample number of microalgae.
Pectenotoxins were f irstly considered as family of diarrheal shellf ish poisons (DSP), because they were often detected with OA. However, later studies showed that PTXs did not induce diarrhea(Nicolas et al., 2017). Although PTXs was found to cause gastrointestinal disorders, for example, the middle-lower intestine eroding and gastric organs injury (Ito et al., 2008), more adverse eff ects were shown as liver, spleen, and kidneys injuries (Terao et al., 1986; Yoon and Kim, 1997; Ito et al., 2008).Moreover, some researches showed that PTX2 could inhibit the actin (Hori et al., 1999; Allingham et al.,2007). Butler et al. (2012) found that PTX2 caused a dose-dependent decrease in both rate and yield of skeletal muscle actin polymerization, but its analog,PTX2 seco acid, did not cause the same eff ect,suggesting that the intact lactone ring was necessary for bioactivity. Some research reported the in vitro hepatic biotransformation path in Wistar rats of PTX2,and found that PTX2 transferred rapidly to two major and several minor oxidized metabolites (Sandvik et al., 2020). It may explain why PTX2 seldom induces serious oral toxicity.
In view of the possible hazards of PTXs to human health, the European parliament promulgated the regulatory limits of PTXs (Regulation (EC) No.853/2004) in 2004. The limit of the total amount of OA, DTXs, and PTXs in the edible bivalve mollusks was no more than 160-μg/kg OA equivalents (Li et al., 2009; Chen et al., 2014). However, there has been no limit about PTXs in China yet. In previous toxin monitoring and analysis in phytoplankton from the Bohai Sea to the Changjiang River estuary, it was found that PTXs were common in these areas and PTX2 was the main structure of PTXs (Liu, 2017). In fact,there have been many reports on PTX2 in shellf ish in China. Guo et al. (2012) found it inChlamysferreri,Argopectensirradias, andCrassostreagigasin culture farms of Lingshan, Qingdao; Deng (2017) reported PTX2 in shells from retail markets in Qingdao. In the north Yellow Sea, PTX2 was identif ied from cultured scallopChlamysferreri(Chen, 2013) and it was considered as one of the major toxins in that area (Wu et al., 2018). In the East China Sea, PTX2 was found in oysterCrassostreagigassamples in September 2012, April, May and June 2013, and musselMytilusgalloprovincialissamples in September 2012, May and June 2013, in Gouqi Island area (Li et al., 2012,2015). A study in 2008 showed that PTX2 was the most frequently detected toxin in coastal shellf ish in China, with a detection rate of 44% and the highest value of 53.2 μg/kg (Liu et al., 2014).
“You poor child,” said the prince and princess; then they praised the crows, and said they were not angry for what they had done, but that it must not happen again, and this time they should be rewarded.
In our research, it seems that risks of PTXs pollution in the East China Sea mariculture areas are possible in specif ic seasons. The cultured shellf ish in China are in great risk of contamination by PTXs.It is recommended that PTXs detecting standards in seafood should be formulated as soon as possible.Meanwhile, the monitoring of algaeDinophysisin China coastal aquaculture area is not complete or systematic. The knowledge about the main toxicproducing species ofDinophysis, toxin types and the outbreak pattern ofDinophysisred tide is still insuffi cient. To understand the regulation of PTXs outbreaks and the cultured shellf ish situation of contamination by PTXs along China coastline,regular monitoring should be conducted on theDinophysisspecies, toxin-producing characteristics,and the PTXs detection of cultured shellf ish in future. It will provide a theoretical basis for f inding prevention and control countermeasures of PTXs contamination.
4.3 Cyclic imine toxins and DA contamination risks
Gymnodimine and 13-desmethyl spirolide C were chemical characterized by a macrocycle and two conserved features that include the cyclic imine group and spiroketal ring system. In our research, these two cyclic imine toxins, GYM and SPX1 were only detected in very low concentration both at 0.001 ng/L.However, reports showed that they had been detected in shellf ish for many times in China (Liang et al.,2022). Liu et al. (2014) had found high concentration(4.2 μg/kg) of SPX1 in oysterAlectryonellaplicatulafrom the middle coast of ECS. Li et al. (2015) detected GYM in three species of shellf ish from ECS and found that GYM could be detected almost in samples of all seasons, and the highest levels occurred in winter. The highest concentration of GYM in ECS was recorded at 36.09 μg/kg inBatillariazonalis, while GYM and/or SPX1 were detected from diff erent species of bivalves and gastropods collected ECS in spring 2016 (Ji et al.,2018). From previous study, Gouqi Island was a highrisk place of GYM in ECS, where the detection rate in shellf ish samples was high to 32.3% (Li et al., 2015).In research of LMTs in surface water of Changjiang River and adjacent sea area, GYM was detected in 9 stations of total 25 stations with a detection rate at 36.0% (He et al., 2019). In our research, although GYM was only detected in very low concentration in a sample from Gouqi Island in October 2021, it still suggested the risk of cyclic imine toxins in autumn and winter season in the north ECS.
Gymnodimine was f irst isolated from dinof lagellate of genusKarenia(formerlyGymnodinium) in 1995(Seki et al., 1995), then it was found in several diff erent species of the same genus, e.g.,K.mokimotoi,K.brevisulcata,K.selliformis(Mountfort et al.,2006; Tan et al., 2013; Li et al., 2018). AlthoughK.selliformishad not been recorded in Chinese water area (Liang et al., 2022),K.mokimotoiwas conf irmed as the dominant species of HABs in ECS. Early back to 1986,K.mokimotoiwas discovered in the south of ECS (Yu et al., 2017). In 2012, even 12 HABs caused byK.mokimotoiwere recorded with a whole size of 627.7 km2and economic losses up to 2 billion RMB(Chen et al., 2015). Toxin SPX1 could be produced byAlexandriumostenfeldii(Tillmann et al., 2014),a species of toxic dinof lagellate found in Chinese water (Gu, 2011; Liang et al., 2022). Although the same microalgae has not been found in ECS area till now, the former reports about HABs of uncertain species,Alexandriumspp. (Yu et al., 2017), indicated the possible threat ofA.ostenfeldii. To avoid contamination risks of these two kinds of toxins, not only the seafood should be detected on schedule, but also the toxic dinof lagellates should be monitored in special mariculture area of the north ECS, especially in summer and autumn-winter.
Domoic acid is a kind of toxin which mainly produced by genus diatomsPseudo-nitzschia(Yu et al., 2020). Although there were 24 potentially toxicPseudo-nitzschiaspecies that had been recorded in China (Li et al., 2017), it was found that this genus of diatoms was mostly distributed in the South China Sea (SCS) of China (Yu et al., 2020). DA was not often found in culture seafood (Liang et al., 2022).In ECS, DA had ever been detected in shellf ish from Zhejiang with low concentration (Song et al., 2008;Wang, 2011). Wang et al. (2019) had found DA in seawater from ECS by using a disk-based SPE technology. In our research, high concentration DA(0.991 ng/L) was detected in samples from the south ECS, indicating that DA pollution risk could not be ignored in the south of ECS.
5 CONCLUSION
Eighty net-concentrated phytoplankton samples were collected from 12 stations in three ECS mariculture areas and 6 types of toxins were detected in 24 samples from 9 stations. LMTs were more common and diverse in these areas. PTX2 was the main LMT in the concentrated phytoplankton samples and the occurrence showed seasonal diff erences from north to south. According to the potential risks of PTXs to seafood safety, it is suggested to execute regular monitoring on PTXs in ECS and a mandatory standard should be formulated based on the comprehensive analysis of in-situ monitoring and lab research. Meanwhile, contamination risks of cyclic imine toxins in the north and DA in the south of ECS should be taken into consideration, too.
There were only 2 PSTs, C2, and dcGTX3, found from spring samples in the north of ECS, which suggested that PSTs was the main risk in spring in this area.
Gouqi Island showed higher toxin diversity in this research and the toxin detection rate was higher than the other two areas. PSTs were the serious potential threats in spring, and LMTs instead of PSTs became the main risk in summer-autumn season. As the biggest mussel culture county of China, the MPTs pollution in this area should be paid high attention to.
To ensure the safety of seafood and marine environmental health, it is recommended to have long-term targeted tracking and monitoring of MPTs in ECS mariculture areas.
6 DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed during the current study are not publicly available due to the requirement of the project but are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENT
Many thanks to Professor Lin SUN of Xiamen University and two postgraduates in her research group, Anqi ZHANG and Honghan LIU, for sample collecting and environmental analysis.
 Journal of Oceanology and Limnology2022年6期
Journal of Oceanology and Limnology2022年6期
- Journal of Oceanology and Limnology的其它文章
- Overview of harmful algal blooms (red tides) in Hong Kong during 1975–2021
- Information standardization for typical toxic and harmful algae in China’s coastal waters—a case study of Karenia mikimotoi*
- Biochemical composition of the brown tide causative species Aureococcus anophageff erens cultivated in diff erent nitrogen sources*
- Identif ication of paralytic shellf ish toxin-producing microalgae using machine learning and deep learning methods*
- Screening for lipophilic marine toxins and their potential producers in coastal waters of Weihai in autumn, 2020*
- First observation of domoic acid and its isomers in shellf ish samples from Shandong Province, China*
