Stress regulation of photosynthetic system of Phaeocystis globosa and their hemolytic activity*
Ni WU , Suping FU , Xinru SONG , Mengmeng TONG ,**, Tianjiu JIANG ,**
1 Key Laboratory of Aquatic Eutrophication and Control of Harmful Algal Blooms of Guangdong Higher Education Institute,Research Center of Hydrobiology, Jinan University, Guangzhou 510632, China
2 South China Sea Institute of Planning and Environmental Research, State Oceanic Administration, Guangzhou 510300, China
3 Ocean College, Zhejiang University, Zhoushan 316021, China
Abstract Blooms of Phaeocystis globosa have been reported accountable for massive f ish mortality worldwide. The toxigenic mechanisms of P. globosa, however, remain largely unclear due to the multiple structures and/or synergistic or antagonistic eff ects of hemolytic compounds. External stressors could lead to the regulation of photoprotective or antioxidative defense system, as well as the potential hemolytic activity. Therefore, the light-induced photosynthetic system, including the accessory photosynthetic growth,the relative electron transfer rate (ETR), photosynthetic effi ciency ( F v/ F m), quantum yield of photosystem II(Yield), together with the hemolytic activity of P. globosa were investigated under variable environmental conditions in the present study. Results conf irmed that hemolytic activity of P. globosa was initiated by the light, but inhibited by low temperature (16 °C), high light intensity (>100 μmol/(m 2·s)), and iron-limited conditions. Interestingly, the hemolytic activity was not impacted by photosynthetic electron inhibitors(Diuron, atrazine, paraquat, and dibromothymoquinone), which signif icantly inhibited the photosynthetic activity of P. globosa. The correlated response of hemolytic and photosynthetic activity of P. globosa under those environmental factors suggested that the hemolytic compounds of P. globosa would be involved in the photosynthetic process but not in the electron transfer chain of P. globosa.
Keyword: Phaeocystis globosa; hemolytic activity; photosynthetic system
1 INTRODUCTION
The genusPhaeocystiswere globally distributed and extensive blooms were reported mostly along the European and Chinese coast (Schoemann et al., 2005). TenPhaeocystisspecies were identif ied morphologically and molecularly includingP.globosa,P.antarctica,P.poucheti,P.cordata,P.brucei,P.jahnii,P.amoeboidea,P.giraudii,P.scrobiculata, andP.sphaeroides(Shen et al.,2018; Wang et al., 2021), among which,P.globosa(He et al., 1999) andP.poucheti(Hansen et al., 2004)were reported toxic.P.globosagiant colony were f irst reported in coastal China since 1997 (Chen et al.,1999), Viet Nam since 2002 (Qi et al., 2004; Hai et al., 2010), and the Arabian Sea (Madhupratap et al.,2000), causing massive f ish death (Liu et al., 2015),mussel mortalities (Peperzak and Poelman, 2008),power plant closure (Gong et al., 2018; Liu and Zhou,2018), and tourism closure (Nejstgaard et al., 2007;Blauw et al., 2010). The estimated economic loss ofP.globosareached ca. 35 million US dollars in South China Sea in 1997 (Qi et al., 2004), and ca.0.65 million US dollars in Phan Ri Bay of Vietnam in 2002 (Doan et al., 2003).
The potential eff ects ofPhaeocystisblooms include the production of hemolytic substance (He et al.,1999), dimethylsulfoniopropionate (DMSP), reactive oxygen species (ROS), and other polyunsaturated fatty acids (PUFA) (Van Leeuwe and Stefels, 2007;Sheehan and Petrou, 2020). Blooming ofP.globosacan transform DMSP into dimethyl sulf ide (DMS)with the production of ~0.5 Tmol/a (Mohapatra et al.,2013). Polyunsaturated aldehydes (PUAs), converted from PUFA with the presence of ROS, may lead to the blood apoptosis of sea urchin or human cytotoxicity(Pohnert, 2005). Hemolytic activity ofP.globosaposed the most threat to the environment and f isheries(Yang et al., 2009; Liu et al., 2010). In addition, the giant colony ofP.globosa, clogging the cooling system of power plant (Kang et al., 2020; Xu et al.,2020) or forming mucilage to make gelatinous foam(Karlson et al., 2021) were recently reported as the major ecological impact in Asian waters. Meanwhile,P.globosablooms have inf luenced the viscosity change of seawater and leaded to the variation of marine ecological environment (Peperzak and Gäbler-Schwarz, 2012; Sheik et al., 2014). In terms of hemolytic toxins, the known components are mainly glycolipids, unsaturated fatty acids, fatty acid amides and porphyrins (Hiraga et al., 2008; Henrikson et al.,2010; Bertin et al., 2012). Hemolytic substance from one strain ofP.globosawas similar with the structure of digitonin (He et al., 1999). Those hemolysins function similarly as the detergent solubilization,digtonin or Triton X-100, which caused acute toxicities to aquatic animals, i.e.,Artemiasalina,Brachionusplicatilis, and other zooplanktons (Yang et al., 2009). In additon, the synergetic eff ect of hemolytic toxins and ROS may show a critical role in f ish killing during the blooms (Twiner and Trick,2000; Ling and Trick, 2010).
Now the question becomes that what would be the driver(s) of the toxicity ofP.globosa. Previous studies indicated that light (Moisan et al., 2006; Guo et al.,2007; Masotti et al., 2010), temperature (Selleslagh and Amara, 2008; Cao et al., 2015), and algicidal compounds (Yang et al., 2015; Zhang et al., 2017)could impact the hemolytic activity ofP.globosa.The highest level of hemolytic activity was observed at 25 °C with the irradiance of 100 μmol/(m2·s)and the N꞉P ratio of 16꞉1 (Cao et., 2015). With the increasing salinity (19–29), temperature (15–25 °C),and irradiance (14–38 μmol/(m2·s), the production of CH3Cl and CH3Br increased accordingly (Yan et al., 2019). Superf luous ROS ofP.globosacan be triggered by the algicidal compounds, such as cyclo-(Pro-Gly), prodigiosin and combination of urocanic acid, N-acetylhistamine, and L-histidine (Zhuang et al., 2018). The production of DMSP inP.globosawas found associated with low temperature (20 °C), high salinity (40) (Shen et al., 2011), acidic pH (pH=5)(Mohapatra et al., 2014), low ratio of N꞉P, and high concentrations of iron (Zhu et al., 2013).
Therefore, how would it possible that the hemolytic compounds ofP.globosabe driven by the external stresses? In this case, a serious experiment was designed to investigate the response of photosynthetic system and hemolytic activity ofP.globosaunder the external stresses of light, temperature, iron, and algicidal compounds.
2 MATERIAL AND METHOD
2.1 Algae and culture condition
The strain ofP.globosawas isolated from the coast of Shantou, South China Sea in 1997 and obtained from Research Center of Harmful Algae and Marine Biology, Jinan University, China. All cultures were incubated in f/2-Si medium (salinity of 28 and pH 8)under a light꞉dark (L꞉D) cycle of 12 h꞉12 h at 25 °C with an irradiance of 90 μmol/(m2·s).
Cell number was estimated using a hemacytometer every two days and growth rate was calculated by the diff erence in cell number during the exponential growth using the equation given by Guillard (1975).

whereN2andN1are the cell numbers at timet2andt1.
2.2 Physical environment stress
The stress experiments were carried out at three temperatures (16, 22, and 28 °C), f ive light intensities (30, 60, 100, 180, and 270 μmol/(m2·s))and three diff erent FeCl3concentrations (0, 10-5, and 10-7μmol/L). Artif icial seawater was used instead of natural seawater (Harrison et al., 1980) for two generations to preculture the algae in f/2-Si medium.The exponential growth phase ofP.globosawas inoculated into fresh f/2-Si medium to ensure the same initial density and cultured under designed conditions as shown in Table 1. The position of bottles was changed every day to reduce the eff ects of uneven lighting or temperature. All treatments were conducted in triplicate. Subsamples were collected every two days to determine cell density,photosynthetic f luorescence, and hemolytic activity.

Table 1 The maximum growth rates (μ max) of P. globosa during exponential growth under designed environmental conditions
2.3 Photoperiod experiments
A stock culture ofP.globosawas grown in a f/2-Si medium at 25 °C under 90 μmol/(m2·s) of coolwhite fluorescent illumination on a 12-h꞉12-h L꞉D cycle (light period 09꞉00–21꞉00). Subsamples were collected every four hours to determine cell density,photosynthetic f luorescence and hemolytic activity.The experiment last for 24 h.
2.4 Photosynthetic electron inhibitors stress
Diuron, atrazine, dibromothymoquinone (DBMIB),and paraquat were chosen as four photosynthetic electron inhibitors. Certain amount of these inhibitors,0.1, 0.15, 0.1, and 4.5 mg/L for diuron, atrazine,DBMIB, and paraquat, respectively, were added into 100 mL of exponential growth culture ofP.globosa.Then samples were mixed and placed under 25 °C with an irradiance of 100 μmol/(m2·s). Photosynthetic effi ciency and hemolytic activity were detected after one hour.
2.5 Data analysis
2.5.1 Photosynthetic f luorescence
Photosynthetic activity of samples was determined by measuring in-vivo chlorophyll f luorescence of photosystem II (PS II) using a Phyto-pulse amplitude modulation (PAM) (Walz, Eff eltrich, Germany).Subsamples were taken and treated in darkness at 25 °C for 5 min each. The temperature experiment was conducted in darkness under the corresponding temperature conditions. The photosynthetic effi ciency(Fv/Fm), the relative electron transfer rate (ETR),and quantum yield of photosystem II (Yield) were determined by Phyto-PAM.
2.5.2 Hemolytic activity assay
The hemolytic activity ofP.globosawas evaluated by rabbit erythrocytes described in Eschbach et al. (2001) and Ling and Trick (2010). The rabbit erythrocytes were taken from the auricular veins of New Zealand rabbits and washed twice in the erythrocyte lysis assay (ELA) buff er. For the hemolytic activity, algae subsamples were placed into 10-mL tubes and centrifuged at 6 000×gfor 10 min. Then the pallets were re-suspended in 400-μL ELA buff er and stored at -20 °C. Before detecting the hemolytic activity, the samples were crushed in an ice bath by an ultrasonic crusher under the conditions of 10% power(650 W), 50 s (pulse on 2 s, pulse off 1 s) to obtain the hemolytic toxin extract. One hundred and f ifty microlitre ofP.globosahemolysin extract and 150 μL of 0.6% rabbit erythrocytes were mixed in a 1.5-mL centrifuge tube (As). After centrifugation of the same volume of hemolysin buff er and rabbit erythrocytes,the absorbance of the supernatant was measured to eliminate the background value of erythrocytes (Aa).The absorbance of the supernatant was measured as the negative control (Ab) after centrifugation of the same volume of hemolysin extract and erythrocytes buff er. The positive control was the absorbance of the supernatant of completely lysed rabbit erythrocytes(Ac). All samples were well-mixed and placed under 25 °C with an irradiance of 100 μmol/(m2·s) for 5 h, then centrifuged at 3 000 r/min for 10 min. The supernatant was used to measure the absorbance at 414 nm in Microplate Reader (Biotek Synergy HT,USA).
The hemolytic activity was calculated according to the following formula (Ling and Trick, 2010):
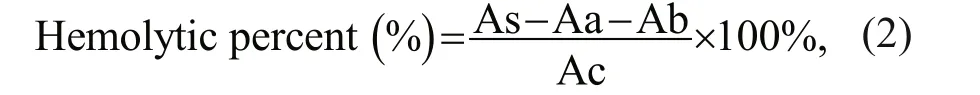
where As is samples, Aa is algae background value,Ab is the background of erythrocytes (negative control), and Ac is the lysed rabbit erythrocytes(positive control).
The half eff ect concentration (EC50) is the concentration of algae cells that lyse half of the rabbit’s red blood cells within 5 h. The EC50was determined as the reaction algae density for subsequent experiments. The algae were gradually diluted into seven concentrations, 1.0, 2.0, 4.0, 8.0,20.0, 40.0, and 80.0×105cells/mL. The EC50ofP.globosawas then determined as 1×106cells/mL.
2.5.3 Pigment analysis
The pigment samples were extracted every two days under light experiment as described in Buff an-Dubau and Carman (2000) and Zapata and Garrido(1991). Fifty milliliter of subsamples were f iltered through Waterman GF/F glass f iber f ilters (0.7-μm nominal pore size, 25-mm diameter). Then the f ilter membranes were cut into pieces and incubated in 3 mL of 95% methanol (v꞉v). Then the samples were centrifuged at 4 000×gfor 5 min. The supernatant was f iltered into the chromatographic bottle for pigment detection. Pigment were determined using high performance liquid chromatography (HPLC)(Agilent 1200) with C8 column (4.6 mm×150 mm, 3.5 μm) and UV-detector as described in Zapata et al. (2000). The mobile phase used in this study were eluent A: Milli-Q water, eluent B: acetonitrile, eluent C: methanolacetonitrile-acetone (volume ratio: 1꞉1꞉3), eluent D:methanol-acetonitrile-acetic acid pyridine (2꞉1꞉1).Elution time was 40 min and f low rate was 1 mL/min.
For each analysis, 30-μL standard pigment solution,90-μL methanol, and 40-mL Milli-Q water were mixed as standard working solution. All treatments were performed under low light. Standard pigments were obtained from DHI Inc. (Denmark)
2.5.4 Statistical analysis
One-way ANOVA and correlation analysis were used to test the diff erences of photosynthetic f luorescence value, hemolytic activity, growth, and pigment under diff erent conditions. All statistical analysis were conducted using SPSS 19.0 software.Origin 8.0 and Sigmaplot 12.5 were used for graphic rendering. Signif icant diff erences were determined using simplet-test (P<0.05).
3 RESULT
3.1 Eff ect of temperature
Low temperature inhibited the growth ofP.globosa. The maximum growth rate (μmax) ofP.globosaincreased with the increasing temperature,reaching 0.21, 0.33, and 0.44 (n=3) at 16, 22, and 28 °C, respectively (Table 1). The exponential growth lasted 10 days in higher temperature, whereas 8 days in low temperature, resulting to the highest cell concentration of 1.70×106and 1.89×106cells/mL in 22 °C and 28 °C (Fig.1a).
The temperature stress on photosynthetic system II(PSII) ofP.globosawere also pronounced (Fig.1b).Signif icant low values ofFv/Fm(circle), Yield (bar)and ETR (square) cells were observed in 16 °C(Fig.1b), indicating the temperature inhibition of photosynthetic activity ofP.globosa. Photosynthetic effi ciency (Fv/Fm) ofP.globosakept the maximum level of 0.64±0.03 (n=18) on average during the entire growth at 28 °C (Fig.1b), on the other hand,dramatically (One Way ANOVA,P<0.05) decreasedFv/Fmfrom 0.63 at exponential growth to 0.47 when cell aged at 22 °C, and from 0.57±0.01 to 0.37±0.01 at 16 °C (Fig.1b). Similar trends were observed in the eff ective quantum yield of PSII (Yield) and relative electron transport rate (ETR). The maximum Yield and ETR ofP.globosawere 0.58±0.01 and 34±0.6 at exponential growth ofP.globosaat 28 °C. The colder (16 °C) and older (day 11) cells ofP.globosawere under great stress, with Yield of 0.30 and ETR of 14.33, respectively.
The response of hemolytic activity ofP.globosawas signif icant as the varied temperatures (Fig.1c–d)and cell ages (Fig.1c). The cells cultured under low temperature, had low hemolytic activity during the entire growth phase. In contrast, hemolytic activity ofP.globosasignif icantly increased (One Way ANOVA,P<0.05) at day 4 in 22 °C and 28 °C, which varied from 23% to 51% and 63% on average, respectively(Fig.1c). In general, hemolytic activity ofP.globosaincreased with increasing temperature (Fig.1d).To evaluate the response of hemolytic activity to the photosynthetic system ofP.globosaunder the temperature treatments, the correlations between hemolytic activity andFv/Fm, Yield, and ETR were analyzed in Fig.1e. The clear positive correlations were found between hemolytic activity (HA) andFv/Fm, Yield and ETR, with theR2of 0.45, 0.43, and 0.44, respectively.
3.2 Eff ect of iron
Iron signif icantly aff ected the physiological and toxinological characteristics ofP.globosa. Growth response ofP.globosaunder the exposure of iron are presented in Fig.2a.P.globosadid not grow without iron, but with small amount of iron, i.e., 10-7μmol/L,cells growth rapidly, reaching 0.49/d (Fig.2a). And the maximum growth rate was found in 10-5μmol/L of FeCl3with 0.62/d.
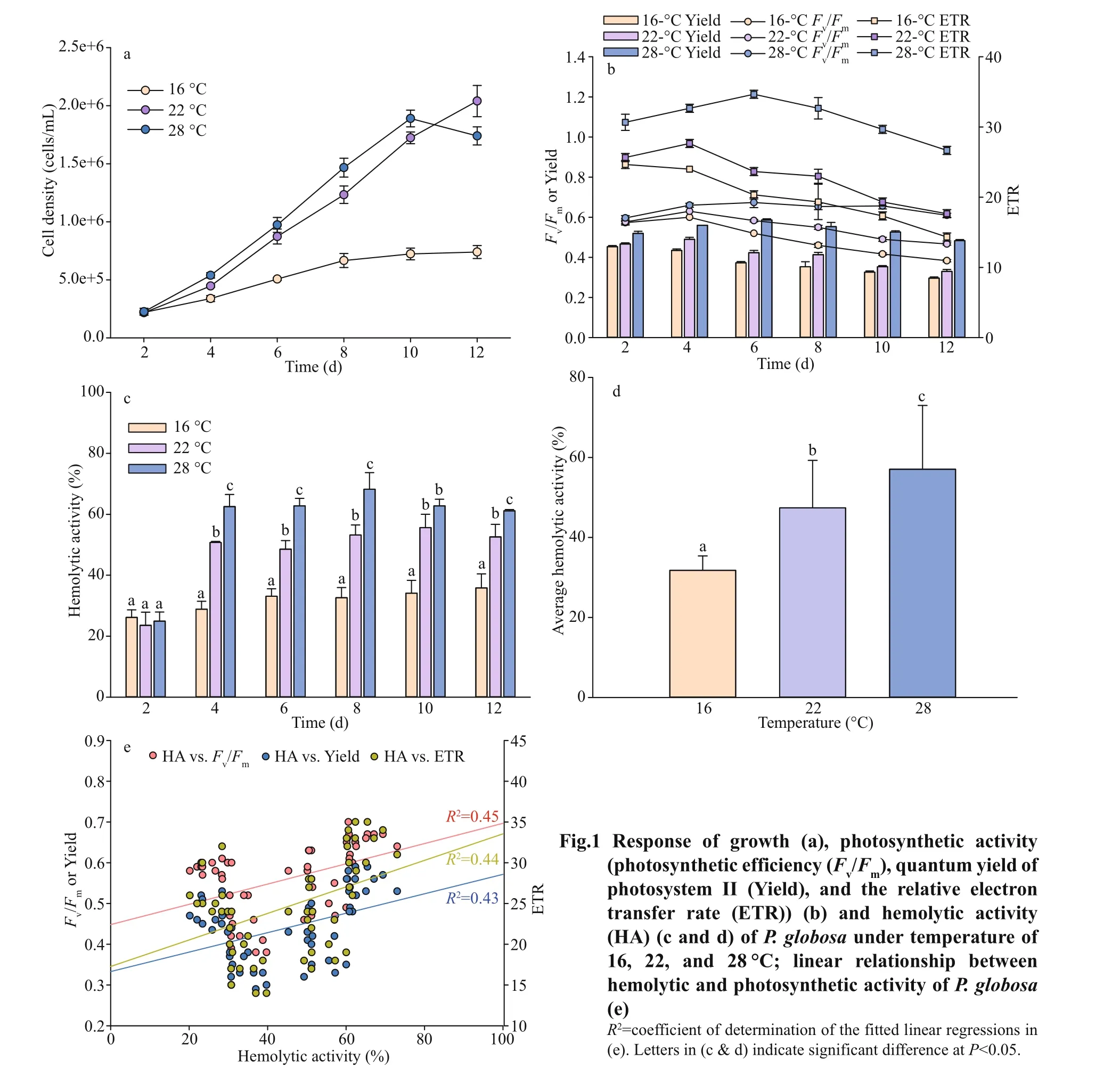
As for the response ofFv/Fm, Yield, and ETR,healthier cells ofP.globosa, higher values of PSII activity, were observed under iron suffi cient(10-5μmol/L) condition (Fig.2b), however, even the growth was not limited under iron low condition(Fig.2a), but all photosynthetic activities were declined from 0.64 to 0.38 (Fv/Fm), 0.40 to 0.25(Yield), and 26.33 to 15.67 (ETR) at day 4. TheFv/Fm,Yield, and ETR values ofP.globosareached 0.09,0.03, and 1.67 under most stressful condition when cell aged (Fig.2b).
Iron also had a great impact on hemolytic toxin production ofP.globosa. Lowest hemolytic activity was detected under iron free condition through all growth stage, with an average of 18% (n=15) per 1 million cells. On the other hand, hemolytic compounds were produced when iron was added. Higher hemolytic activity with around 43% was detected in iron suffi cient cultural condition (Fig.2c & d).
Linear relationship of photosynthetic and hemolytic activity was found in Fig.2e. Similar as the temperature treatments, signif icant positive relationship betweenFv/Fm(R2=0.45), Yield (R2=0.43) and ETR (R2=0.44)and hemolytic activity were also found.
3.3 Eff ect of irradiance
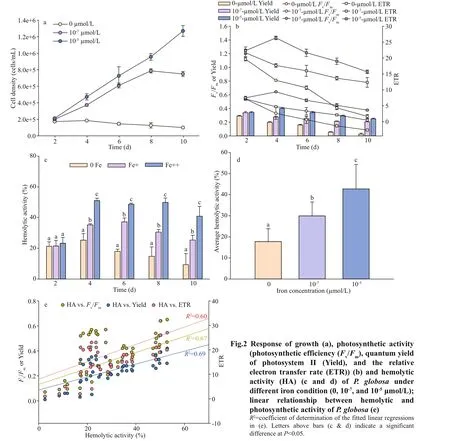
Light intensities of 30, 60, 100, 180, and 270 μmol/(m2·s) were used in the present study.In Fig.3a, the apparent growth was found in light treatments of over 60 μmol/(m2·s). The growth rates varied but not signif icant, with around 0.45/d on average. The highest growth rate, 0.68/d, was found at light of 100 μmol/(m2·s). In contrast,P.globosagrew much slower, ~0.13/d at the lowest irradiance(30 μmol/(m2·s)). Interestingly, the exponential growth last 9 days in the highest two light treatments(I180, I270), however, 11 days in the middle light treatments (I100and I60), resulting to the signif icant diff erence of maximum cell concentrations (180 000 vs. 200 000 cells/mL).
Photosynthetic activities ofP.globosa,Fv/Fm,Yield, and ETR are shown in Fig.3b. The two highest light treatments, I180and I270, stressed the photosynthetic activity ofP.globosaas the evidence of signif icant low values ofFv/Fm, Yield, and ETR(Fig.3b). Light of 100 μmol/(m2·s) was suffi cient forFv/Fm(no declined through the entire growth), but shrunk after day 5 in both Yield and ETR. Low light,I30and I60, did not stress the PSII ofP.globosa, but no signif icant growth was found in I30(Fig.3a).
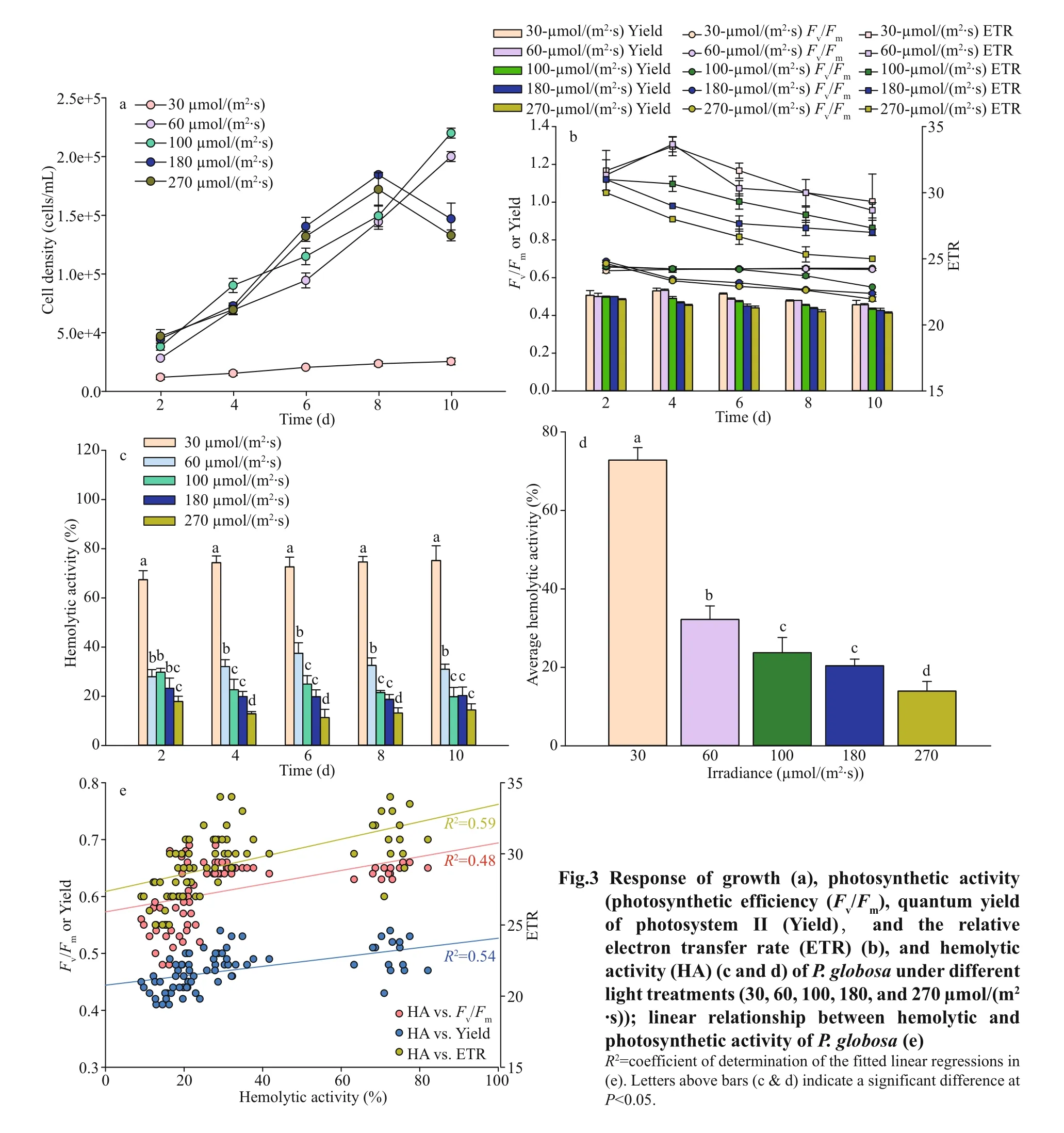
Hemolytic activities ofP.globosaunder variable light treatments were shown in Fig.3c & d. Signif icant high amount (One Way ANOVA,P<0.05) of hemolytic toxins were produced under the low light treatment, 30 μmol/(m2·s), with an average of 72%through the entire growth, followed by 60, 100, 180,and 270 μmol/(m2·s) (Fig.3d). Hemolytic activities reached 32%, 24%, 20%, and 13% under the light of 60, 100, 180, and 270 μmol/(m2·s), respectively.The hemolytic activity decreased with the increasing irradiance during the whole exponential period(Fig.3c). Linear relationship between hemolytic and photosynthetic activity showed the signif icant positive response ofFv/Fm(R2=0.48), Yield (R2=0.54),and ETR (R2=0.59) (Fig.3e).
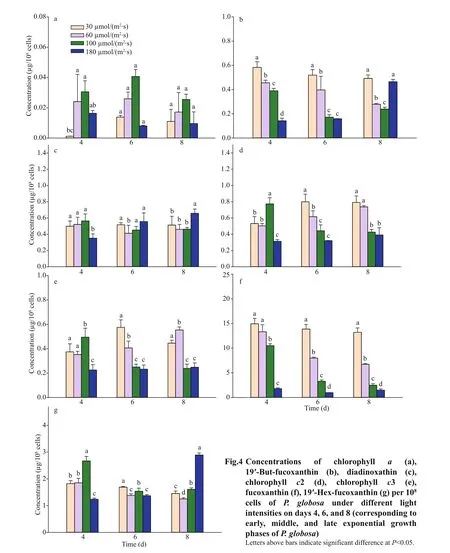
Pigment contents varied greatly with light and growth phase (Fig.4). Pigments of chlorophyllc3 (Chlc3), chlorophyllc2 (Chlc2), 19ʹ-But-fucoxanthin(But-fuco), fucoxanthin (Fuco), 19-Hex-fucoxanthin(Hex-fuco), diadinoxanthin (Diad), and chlorophylla(Chla) were detected inP.globosa. Chlc3, Chlc2,and Fuco were the dominant pigments inP.globosa.Cellular Chlc3 and Fuco ofP.globosa, ranging from 78% to 31%, decreased with the increasing light intensity. Photopigments were further investigated with the relationship of hemolytic activity by principal component analysis (PCA) (Fig.5). The scores of the f irst two principal components (PC1 and PC2) reached 49.9% and 24.5% forP.globosa. The hemolytic activity was apparent in highly positive PC1 space and appeared quite correlated with the pigments of Chlc2, Chlc3, and Fuco.
3.4 Daily cycle variation
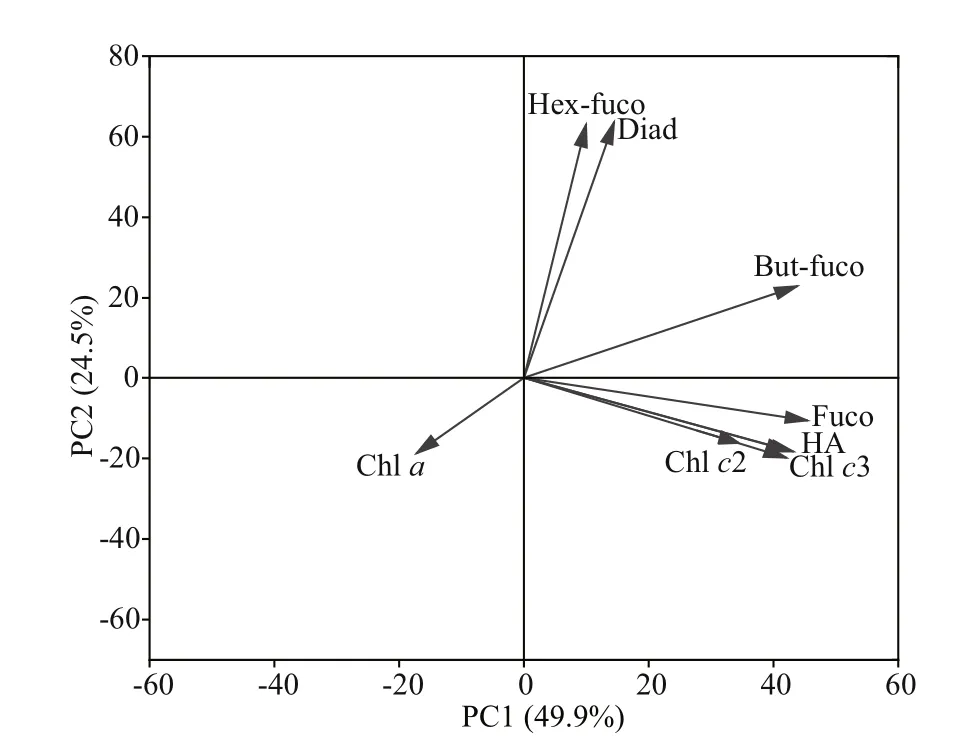
Fig.5 Results of principal component analysis (PCA) of hemolytic activity (HA) and the concentrations of photosynthetic pigments of P. globose

Fig.6 Variation of photosynthetic effi ciency ( F v/ F m),quantum yield of photosystem II (Yield), the relative electron transfer rate (ETR), and hemolytic activity(HA) of P. globosa over 24 h, 12-h꞉12-h light꞉dark cycle
Photosynthetic and hemolytic activity ofP.globosawere investigated in a daily cycle(Fig.6). The variations ofFv/Fm, Yield, and ETR ofP.globosaare S-shaped. After entering the light time, the photosynthetic transfer effi ciency gradually increased, and reached the highest value at 7-h light exposure, then gradually decreased. The lowestFv/Fmwere found after 3-h dark cycle. Similarly, hemolytic activity was higher during the day time, and lower during the night time. The maximum hemolytic activity of 74%±0.48% was obtained from 16꞉00 pm to 20꞉00 pm and the minimum of 64%±3.5% from 8꞉00 am to 16꞉00 pm and from 0꞉00 pm to 8꞉00 am.
3.5 Eff ect of photosynthetic electron inhibitors
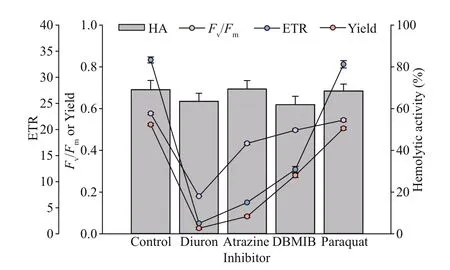
Fig.7 Eff ect of four photosynthesis blockers on percent hemolytic toxicity, photosynthetic effi ciency ( F v/ F m),quantum yield of photosystem II (Yield), and the relative electron transfer rate (ETR) of P. globosa after one hour of exposure, relative to the control
Four inhibitors of diuron, atrazine, DBMIB, and paraquat inhibited the photosynthetic activity ofP.globosa, but had no eff ect on hemolytic activity(Fig.7).Fv/Fmdecreased from 0.58 to 0.18 (diuron),0.43 (atrazine), 0.5 (DBMIB), and 0.55 (paraquat),respectively. Similar trends were observed in Yield and ETR ofP.globosa. Diuron was the most eff ective inhibitors on PSII ofP.globosa(Oneway ANOVA;P<0.05).
4 DISCUSSION
Phaeocystisglobosahas attracted much attention and research due to the fundamental role and harmful algal bloom eff ects in many regions of the world (Blauw et al., 2010; Buchan et al., 2014). The temperature was one of the triggers that can aff ect the hemolytic activity ofP.globosain many aquatic system (Cao et al., 2015; Kang et al., 2020). Cells ofP.globosain an optimum temperature condition, i.e.,24 and 27 °C (Xu et al., 2017) or 27–30 °C (Guo et al., 2007), produce more hemolytic toxins into the water system (Guo et al., 2007). Similar results were also found in our study (Fig.1). A higher hemolytic activity was observed in the faster growing rate ofP.globosaand the healthier cells (higher values of PSII activity).
Iron is an important nutrient in marine phytoplankton (Lill, 2009), and would be a limiting factor in the metabolism of phytoplankton during algal blooms (Larson et al., 2018). Furthermore, metal oxides are photocatalysts that facilitate photocatalysis by absorbing solar photons over a broad spectral range, ultimately enhancing the absorption of visible light (Zhou et al., 2018; Wan et al., 2019). As forP.globosa, trace amount of iron, i.e., 10-7μmol/L,can trigger the growth, as well as the active PSII and hemolytic activity (Fig.2). Iron suffi cient would contributed the growth ofP.globosa(Slagter et al.,2016) and enhanced the hemolytic activity (Liu et al., 2006; Jiang et al., 2012). The stress of iron did not inhibit the growth, PSII and toxic activity inP.globosa, indicating the production of hemolytic compounds may associate with the growth and PSII activity.
Light was reported as the trigger of hemolytic compounds in many harmful algal species (Cao et al.,2015; de Q Mendes et al., 2017; Wang et al., 2019).The saturation light for one isolate ofP.globosawas determined as 60 μmol/(m2·s) and the photoinhibition point was 230 μmol/(m2·s) (Xu et al., 2017). Lower light, i.e., 80 μmol/(m2·s), inhibited the growth rate ofP.globosaisolated from North Sea, but had no eff ect on photosynthetic activity (Hoogstraten et al.,2012). Light intensities from 40 to 150 μmol/(m2·s)promotedP.globosaisolated from the East China Sea to growth faster (Liu et al., 2011). In our study, the growth rate ofP.globosadid not change dramatically within the light of 60 to 270 μmol/(m2·s). However,the exponential duration under higher light intensities(I180and I270) were inhibited to 9 days compared to 11 days under the relatively low light condition.Another evidence ofP.globosapreferred low light is that the value ofFv/Fmin low light (25 μmol/(m2·s))was 10%–20% higher than that in high-light intensity(Maat et al., 2016). As shown in Fig.3b, light of 60–100 μmol/(m2·s) was the most PSII active condition forP.globosain the present study. The continuous light at daytime may inhibit the growth ofP.globosa,which may due to the circadian rhythm pattern of DNA synthesis and cell division (Wang et al., 2014).
The hemolytic activity ofP.globosavaried signif icantly under high and low light conditions(Cao et al., 2015). This f inding can be seen in the light and daily cycle experiments in the present study.The signif icant high amount of hemolytic activity under light of 30 μmol/(m2·s) (Fig.3c–d), and down regulated hemolytic activity at dark cycle (Fig.6)indicated that light was essential forP.globosato produce hemolytic toxins and a lower light promoted the production of the toxins.
Therefore, how could the hemolytic toxin production associate with the PSII activity ofP.globosa? The signif icantly positive correlation between hemolytic and photosynthetic activity ofP.globosaunder temperature, light, iron, and daily cycle treatments made us to further consider whether the hemolytic compounds would be photopigments associated compounds or related with the electron transfer chain? Thereafter, pigments as well as electron inhibitors experiments were conducted.
The main pigments ofP.globosainclude Chlc3,magnesium-divinyl-pheo-porphyrin a5 monomethyl ester (Mg DVP), Chlc2, But-Fuco, Fuco, Hex-Fuco, Diad, Chla, and Caroteniods (Caro) (Zapata et al., 2004). Chlc2, andc3 also are light-harvesting pigments (Bollivar, 2006), and part of the Fuco-chla/cprotein complex. In addition, Chlc3, Fuco, and Chlc2 are particularly sensitive to low light conditions.But-fuco is often described a light-harvesting role(Van Leeuwe et al., 2014). As we know, pigment composition ofP.antarcticavaried with light and iron conditions (Seoane et al., 2009; Van Leeuwe et al., 2014). Here the increased Fuco were conf irmed at low light (Fig.4f). Under the high light,P.globosacells responded by reducing their content of light harvesting pigments, i.e., Chlc2, Chlc3, Fuco(Fig.4d, e, f) and by increasing the presence of photoprotective xanthophylls diadinoxanthin (Fig.4c). The results of PCA on hemolytic activity and pigments(Fig.5) suggested that the production of hemolytic compounds might associate with the light-harvesting pigments.
However, the inhibitors limited the PSII activity ofP.globosa, but no eff ect on hemolytic activity.DBMIB can block the PSII to PSI by the cytochrome b6f complex in activation of the kinase (Mao et al.,2002; Roberts et al., 2004; Trebst, 2007). Atrazine may cause the controversial response to diff erent organisms (Brain et al., 2012). Trace amount of DBMIB resulted to signif icant inhibition of PSII photochemistry (Belatik et al., 2013); however, no response of hemolytic activity may indicate not the electron transfer chains in PSII, but other biosynthetic pathway of hemolytic activity may involve inP.globosa.
Stress, such as low temperature, high light,iron deplete, dark, and electron inhibitors, showed signif icant impact on the growth and photosynthetic activity ofP.globosa, while, the external stresses do not correspond to the hemolytic activity ofP.globosa.The heathierP.globoseis, the higher hemolytic activity is. Stress could not be the driver of hemolytic activity ofP.globosa.
5 CONCLUSION
The current study focuses on regulation of external stressors, such as light, temperature, iron, and photosynthetic electron inhibitors, on photosynthetic system ofP.globosaand their hemolytic activity.Light was essential forP.globosato produce hemolytic toxins. However, high light intensity (i.e.,>100 μmol/(m2·s)) inhibited the hemolytic activity,together with the lower temperature (16 °C) and ironlimited stress. Meanwhile, photosynthetic activity(Fv/Fm, Yield, and ETR) and hemolytic activity ofP.globosawere positively correlated. However, the hemolytic activity ofP.globosawas not aff ected by photosynthetic electron inhibitors, which blocked the photosynthetic activity signif icantly. Therefore, it could be concluded that hemolytic toxin production ofP.globosawere associated with photosynthetic process but not with the electron transfer chain.The present study provides important information for understanding the formation mechanisms of hemolytic compounds ofP.globosa.
6 DATA AVAILABILITY STATEMENT
All data generated and/or analyzed during this study are contained within this article.
 Journal of Oceanology and Limnology2022年6期
Journal of Oceanology and Limnology2022年6期
- Journal of Oceanology and Limnology的其它文章
- Overview of harmful algal blooms (red tides) in Hong Kong during 1975–2021
- Information standardization for typical toxic and harmful algae in China’s coastal waters—a case study of Karenia mikimotoi*
- Biochemical composition of the brown tide causative species Aureococcus anophageff erens cultivated in diff erent nitrogen sources*
- Identif ication of paralytic shellf ish toxin-producing microalgae using machine learning and deep learning methods*
- Screening for lipophilic marine toxins and their potential producers in coastal waters of Weihai in autumn, 2020*
- First observation of domoic acid and its isomers in shellf ish samples from Shandong Province, China*
