Is the dinof lagellate Takayama xiamenensis a synonym of Takayama acrotrocha (Kareniaceae, Dinophyceae)?*
Songhui LÜ , Aimin CHAO , Qianyan LIANG , Jingyi CEN , Jianyan WANG ,Tao JIANG , Si LI
1 Research Center of Harmful Algae and Marine Biology, Jinan University, Guangzhou 510632, China
2 Key Laboratory of Eutrophication and Red Tide Prevention of Guangdong Higher Education Institutes, Jinan University,Guangzhou 510632, China
3 Southern Marine Science and Engineering Guangdong Laboratory, Zhuhai 519000, China
4 Zhejiang Ecological and Environmental Monitoring Center, Hangzhou 310012, China
5 Department of Science Research, Beijing Museum of Natural History, Beijing 100050, China
6 School of Ocean, Yantai University, Yantai 264005, China
Abstract The naked dinof lagellate Takayama acrotrocha was identif ied as responsible for a bloom in Shenzhen Bay, Guangdong, China, in early spring 2021. The identif ication was conf irmed by light, scanning,and transmission electron microscopy and molecular data based on the LSU ribosomal DNA (rDNA) and ITS rDNA sequences. This is the f irst record of T. acrotrocha bloom in the South China Sea. The sulcus of T. acrotrocha was wide and extended onto the epicone as a short intrusion in general, sometime the intrusion was not apparent and some were f inger-like. The apical groove was deeply sigmoid. The nucleus was large,ovoid to cup-shaped and occupied most of the epicone. A large, rounded pyrenoid surrounded by a starch sheath was located at the left side to the centre of the hypocone. Under epif luorescence illumination, a row of large vesicular knobs was observed on the upper border of the cingulum. The intraspecif ic morphological variabilities in the clonal cultures of T. acrotrocha were investigated carefully. Cells that share the same diagnostic characters used for the description of Takayama xiamenensis such as the f inger-like sulcus, a large nucleus located in the epicone and the similar pyrenoid type were observed. The LSU rDNA sequences of T. acrotrocha and T. xiamenensis only diff ered in 3 base pairs (bp) for a sequence length of 673 bp (with a similarity of 99.55%). For these reasons, we propose T. xiamenensis as a junior synonym of T. acrotrocha.
Keyword: Takayama spp.; harmful algal bloom; morphology; phylogeny; South China Sea
1 INTRODUCTION
The genusTakayamawas proposed by de Salas et al. (2003) and was later classif ied into the family Kareniaceae with two other genera,KareniaandKarlodinium(Bergholtz et al., 2006). Typical characteristics of this genus are a sigmoid apical groove on the epicone, and fucoxanthin and/or its derivatives as the main accessory pigments. Species in the genusTakayamawere formerly classif ied under the generaGymnodiniumandGyrodinium,for exampleTakayamaacrotrocha(syn.Gyrodiniumacrotrochum) (Larsen, 1996),Takayamacladochroma(syn.Gyrodiniumcladochroma) (Larsen, 1994) andTakayamapulchella(syn.Gymnodiniumpulchellum)(Larsen, 1994). Seven species have now been reported in the genusTakayama:T.acrotrocha,T.cladochroma,Takayamahelix,T.pulchella,Takayamatasmanica,Takayamatuberculata, andTakayamaxiamenensis(Larsen, 1994, 1996; de Salas et al., 2003, 2008; Gu et al., 2013);Takayamatasmanicais the type species.
Takayamaspecies have been detected in Australia(Larsen, 1996), China (Gu et al., 2013), Italy (Siano et al., 2009), Japan (Omura et al., 2012), Korea (Cho et al., 2021), Singapore (Tang et al., 2012; Leong et al.,2015), and Florida, USA (Steidinger, 1998). Blooms dominated byTakayamaspecies have been reported in China (Gu et al., 2013), Singapore (Tang et al.,2012; Leong et al., 2015), Japan (Omura et al., 2012),Italy, and the USA (Steidinger, 1998).Takayamaspecies have been suspected to be toxic to marine organisms. For example,T.pulchella(reported asGymnodiniumpulchellum) blooms in the Indian River(Florida, USA) in 1990 and 1996 caused the deaths of many species of fish (Centropomusundecimalis,Mugilcephalus,Ariusfelis,Sciaenopsocellatus,Archosargusprobatocephalus, andPogoniascromis)and invertebrates (CallinectessapidusandPenaeusspp.) (Steidinger, 1998).
In China, 4 species in the genusTakayamahave been identif ied:T.xiamenensis,T.pulchella,T.tuberculata,andT.tasmanica(Gu et al., 2013; Law and Lee, 2013;Cheng et al., 2020).Takayamaxiamenensis(identif ied asT.pulchellawhen it was f irst reported by Gu et al. (2006)) was isolated from Xiamen Bay during a bloom in 2003 (Gu et al., 2013).Takayamapulchellawas commonly detected with a low density in Hong Kong coastal waters (Law and Lee, 2013). In 2011,a bloom ofT.pulchellawas reported in Tolo Harbor,Hong Kong coastal waters, although no f ish kill was reported during the bloom (Law and Lee, 2013).Takayamatuberculatabloomed discontinuously from March to June in 2021 in Hong Kong coastal waters (https://www.afcd.gov.hk/english/publications/publications_press/pr2223.html) (Cheng et al., 2020).Takayamatasmanicawas reported in the East China Sea (Gu et al., 2013) and Hong Kong waters (Law and Lee, 2013), and its toxicity is uncertain.
Takayamaspecies are characterized by a sigmoid groove on the epicone, which is distinctly diff erent from the linear apical groove of the generaKareniaandKarlodinium(Daugbjerg et al., 2000). Species in the genusTakayamaare distinguished mainly by the details of cell nucleus, chloroplasts, pyrenoids,and sulcal intrusion onto the epicone (de Salas et al., 2003, 2008). However, the morphological interspecies diff erences betweenTakayamaspp. are minimal, and accurate identif ication to species-level is extremely diffi cult, even for a taxonomy specialist.It has been considered that large subunit (LSU)rDNA, the commonly used molecular marker for identifying marine dinof lagellates, is even insuffi cient to distinguishTakayamaspecies (de Salas et al., 2008;Gu et al., 2013), and thus they may be misidentif ied or omitted in traditional surveys based on morphological identif ication.
In this study, 5 isolates ofT.acrotrochawere established from the South China Sea (SCS), and two of these were isolated from seawater duringTakayamablooms. Morphological features of the isolates were carefully checked based on light microscopy(LM), scanning electron microscopy (SEM), and transmission electron microscopy (TEM), and analysis of their phylogenetic relationship with the related dinof lagellates was based on the LSU rDNA and the internal transcribed spacer (ITS) region.Examination of the lethality ofT.acrotrochaon brine shrimp (Artemiasalina) and marine medaka (Oryziasmelastigma) was also conducted.
2 MATERIAL AND METHOD
2.1 Algae isolation and cultivation
Seawater was sampled from the coastal waters of the SCS for kareniaceans collection. Specif ic isolation for the bloom-causing species was conducted during an unarmoured dinof lagellate bloom in Shenzhen Bay(22°28ʹ30″N, 113°57ʹ46″E), Guangdong Province,from March 19thto April 15th, 2021. The peak cell density of the bloom was 7.1×107cells/L. The bloom area was about 6.5 km2. The sea surface temperature during the bloom was 22.97–26.57 °C. The sea surface salinity was 23.62–29.02. Microalgae in the seawater were concentrated using a phytoplankton net with a pore size of 10 μm, and the samples were taken to the laboratory for cell isolation. The algal clone was established by isolating a single cell from the net sample using a capillary pipette under the microscope. The single cell was f irst cultured in a well of a 96-well cell plate, and then transferred to a test tube for mass cultivation. The isolates were cultivated in L1 medium (Guillard, 1975) with a salinity of 28±1 and incubated at 20±1 °C with a photoperiod of 12-h light꞉12-h dark under 60±10 μmol photons/(m2·s). The isolates were deposited at the Research Center for Harmful Algae and Marine Biology, Jinan University, Guangzhou, China.
2.2 Light microscopy
Live cells were observed using an Olympus BX61 microscope (Olympus, Tokyo, Japan) with magnif ications of 400× and 1 000×. Micrographs of more than 45 randomly selected cells were taken using a QImaging Retiga 4000R digital camera (QImaging,Surrey, BC, Canada). Cell length and width were measured by Image-Pro Plus 6.0 image acquisition software (QImaging, Surrey, BC, Canada). For epif luorescence microscopy, 2 mL of algal culture at the exponential growth stage was stained with SYBR Green stain (Thermo Fisher, Waltham, MA, USA).The shape, size, and location of the cell nucleus and chloroplasts were identif ied under an Olympus BX61 epif luorescence microscope.
2.3 Scanning electron microscopy
Cells collected from theTakayamabloom and cells of the isolates were f ixed by OsO4with a f inal concentration of 2%. Fixed samples were f iltered,dehydrated, critical point dried, and sputter coated as described in Wang et al. (2018). The mounted samples were examined using a Zeiss Ultra 55 f ield emission scanning electron microscope (Zeiss, Jena,Germany).
2.4 Transmission electron microscopy
Vegetative cells in the exponential phase were double f ixed by OsO4(with a f inal concentration of 0.5%) and glutaraldehyde (with a f inal concentration of 2%) at room temperature for 30 min. The f ixed samples were dehydrated in a series of ethanol solutions (10%, 30%, 50%, 70%, 90%, and 95%),followed by three changes in 100% ethanol. The dehydrated cells were embedded in Spurr’s resin via propylene oxide. The embedded blocks were sectioned on an Ultracut E Ultramicrotome (Leica Microsystems, Wetzlar, Germany) using a diamond knife. The sections were transferred to a formvar f ilm and double-stained with uranyl acetate and lead citrate. The stained sections were examined in a JEOL JEM-1010 electron microscope (Jeol Ltd., Tokyo,Japan) operated at 30–50 kV.
2.5 Genomic DNA extraction and PCR amplif ication
A 2-mL aliquot of the isolates in the exponential growth phase was harvested by centrifugation. The genomic DNA was extracted using a Takara MiniBEST Universal Genomic DNA Extraction Kit (TaKaRa,Dalian, China) according to the protocol provided by the manufacturer. Partial LSU rDNA and the ITS sequences were amplif ied by PCR using primers D1R-F (5ʹ-ACCCGCTGAATTTAAGCATA-3ʹ)(Scholin et al., 1994), D3B-R (5ʹ-TCGGAGGGAACCAGCTACTA-3ʹ) (Nunn et al., 1996), ITS1F(5ʹ-TCGTAACAAGGTTTCCGTAGGTG-3ʹ) and ITS1R (5ʹ-ATATGCTTAAGTTCAGCGGG-3ʹ) (Pin et al., 2001). The PCR amplif ication protocols for LSU rRNA and ITS rDNA were followed as described in Cen et al. (2020). The LSU rDNA sequences of the causative species in theTakayamablooms on March 19th2021 were determined by single-cell PCR using the same PCR protocol mentioned above.
2.6 DNA sequence alignment and phylogenetic analysis
The partial LSU rDNA and ITS rDNA of the isolates were deposited in GenBank with accession numbers MZ948839–MZ948843 for the LSU rDNA sequences and MZ948833–MZ948837 for the ITS rDNA sequences. LSU rDNA and ITS rDNA sequences of the kareniacean species closely related to theTakayamaspp. were searched from GenBank using the online software BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Sequences were aligned using Clustal X software in MEGA 7.0 (Kumar et al.,2016). BioEdit 7.0.5 was used to edit and excise the redundant bases at the two ends of the sequences for the phylogenetic analysis (Hall, 1999). The aligned lengths used for phylogenetic tree construction were 673 base pairs (bp) for the LSU rDNA and 588 bp for the ITS rDNA. The genetic divergence (p-distance)betweenTakayamaspecies was calculated by MEGA 7.0. The phylogenetic trees were constructed by MEGA7.0 software using the maximum likelihood(ML) (model T3+G+I) in 1 000 bootstrap running.The optimal model of Bayesian inference (BI) was selected by MrModeltest 2.2 and phylogenetic analysis was conducted using MrBayes 3.2 (Ronquist and Huelsenbeck, 2003).
2.7 Pigment extraction and analysis
A 10-mL algal culture in the mid-exponential phase was f iltered onto a f iber membrane with a 0.45-μm pore size (Whatman, Maidstone, UK), and was stored in a refrigerator at -80 °C for later treatment. Pigment extraction and analysis were performed according to the methods described in Zapata et al. (2000) and Wang et al. (2018).
2.8 Cell acute toxicity analysis
Artemiasalinabioassay:A.salinawas incubated at 25 °C in a 500-mL glass beaker for 24 h.Seawater collected from theTakayamabloom with a concentration of 2.5×107cells/L was used for the lethality test. AProrocentrumobtusidens(3×107cells/L) culture was used as the blank control and non-bloom seawater was used as the negative control. Twenty specimens ofA.salinawere used in each test, and tests were performed in triplicate.Survival ofA.salinawere recorded every 6 h for 48 h.
Marine medaka (Oryziasmelastigma) bioassay:Brood stock ofO.melastigmawere kindly provided by the State Key Laboratory in Marine Pollution of the City University of Hong Kong. Ten healthy,adultO.melastigmawere transferred into a 5-L bucket containing 3-L bloom seawater at 25 °C. A photoperiod of 12-h light꞉12-h dark was used, and the f ish were fed twice daily. Acute toxicity was assessed by measuring the lethal eff ect of the seawater collected from theTakayamabloom (with a concentration of 2.5×107cells/L) on the marine medaka over 48 h.The non-bloom seawater andP.obtusidensculture(1×107cells/L) were used as the negative control and the blank control, respectively. The survival ratio ofO.melastigmawas observed at 0, 4, 8, 12, 24, and 48 h. Three replications were set for each test.
3 RESULT
Five strains ofTakayamaspp. were established in this study, and detailed information for the isolates is listed in Table 1. All strains have an apparent sigmoid groove around the cell apex, which is consistent with the typical apical groove of the genusTakayama.All the 5 strains and single cells from theTakayamabloom shared the same LSU rDNA and ITS rDNA sequences. After a careful and comprehensive analysis of the taxonomy and phylogeny, the 5 isolates were identif ied asT.acrotrocha. The causative species of the dinof lagellate bloom in Shenzhen Bay,Guangdong Province from March 19thto April 15th2021 was determined to beT.acrotrocha.
3.1 Morphological characteristics of Takayama acrotrocha
3.1.1 Morphology ofTakayamaacrotrochaunder LM
Cells ofT.acrotrochawere unarmoured, small and oval, with a length of 14–20 μm and a width of 11–17 μm (Supplementary Table S1). Cells were slightly f lattened dorso-ventrally, with a length towidth ratio of 1.02–1.64 (Supplementary Table S1).Some small cells less than 14 μm in length and no more than 10 μm in width were also observed. The epicone was hemispherical (Fig.1a–b). The hypocone was irregular in shape, and slightly longer than the epicone (Fig.1c–d). In the ventral view, the hypocone was bilobed, truncated, and incised antapically(Fig.1a–b). The cingulum was shallow, with a displacement of 1–1.5 times of the cingulum width(approximately 1/4 of the cell length) (Fig.1a–b).The sulcus was wide in the hypocone, becoming narrow in the inter-cingular region, and extended onto the epicone as a short intrusion (sometimes the intrusion was not apparent) (Fig.1a–b). The apical groove was sigmoid and curved around the cell apex,starting above the anterior of the sulcal intrusion and extending to the dorsal part of the epicone (Fig.1a &c). A large, rounded pyrenoid was located at the left side or in the middle of the hypocone from the ventral view (Fig.1d). The chloroplasts were numerous,irregularly shaped, and distributed peripherally in the cell, arranged in a helicoidal pattern on the epicone and more abundant in the hypocone (Fig.1a–c &e–f). The nucleus was large, ovoid to cup-shaped,and occupied the most part of the epicone (Fig.1d–i).Under epif luorescence illumination, a row of large vesicular knobs was clearly observed on the upper border of the cingulum (Fig.1e–f).
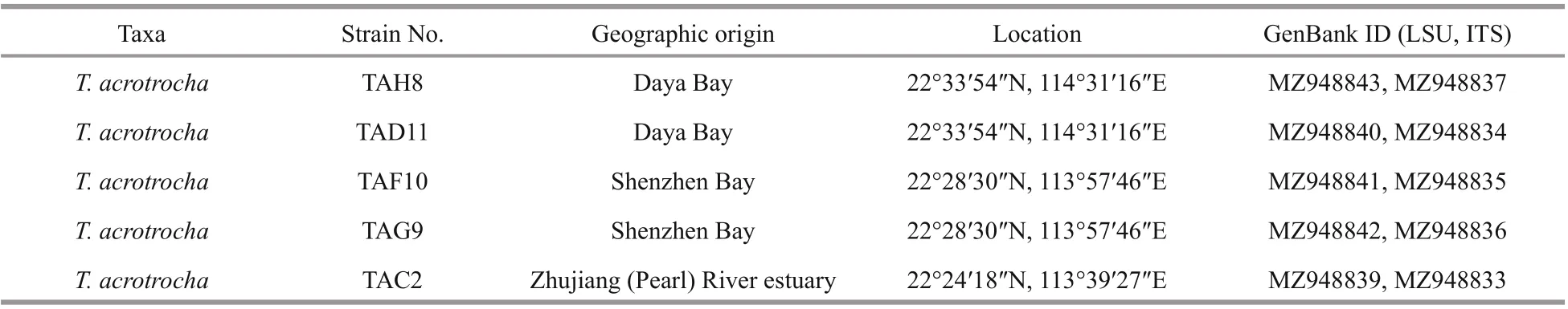
Table 1 Information for the Takayama acrotrocha isolates from the South China Sea
Generally, there was no signif icant morphological variance among the diff erent strains, except for the minimal variability in the length and width of the sulcus intrusion onto the epicone. For cells sampled from the bloom seawater, the sulcal intrusion was short but could be observed under LM (Fig.2a–b).The sulcal intrusion in strain TAG9 was of a similar length and shape as in the f ield cells (Fig.2c–d). Most cells of strain TAH8 had a relatively long and f ingerlike sulcal intrusion (Fig.2e–f), and some cells with a short intrusion were also observed (Fig.2g–h). The sulcal intrusion was not obvious in strain TAF10(Fig.2i–j), while a short intrusion in some cells was visible (Fig.2k–l).
3.1.2 Morphology ofTakayamaacrotrochaunder SEM
Cells were oval (Fig.3a–d). The epicone was hemispherical and the hypocone was slightly irregular in shape (Fig.3a–e). The posterior of the hypocone was truncated and incised by the sulcus (Fig.3c–d).The cingulum displaced approximately 1/4–1/3 of the cell length (Fig.3b). The sulcus extended brief ly onto the epicone (Fig.3a–d), and the sulcus intrusion was not obvious in some cells (Fig.3e–f). A tubular structure was observed in the sulcus (Fig.3a–b & e–f).The apical groove was coiled along the apex of the cell in a ‘S’ shape (Fig.3a–b, e, & g–h). The apical groove started from the right of the sulcal intrusion,ran around the cell apex and extended approximately 1/3 of the dorsal epicone (Fig.3b–c). Both rims of the apical groove were slightly ridged (Fig.3b–c). The ventral pore was small, sometimes slit-like, situated on the left side of the epicone and well above the anterior of the cingulum (Fig.3a–b & e–f). A tube-like structure was positioned along the sulcus in the intercingular region (Fig.3a–b & e–f). The epicone surface on the right lobe, which lies between the ventral end of the apical groove and the sulcus was swollen. A slightly slit-like ventral pore was observed on the swollen structure (Fig.3e–f). In some well-preserved cells, a row of amphiesmal knobs was neatly arranged along the upper rim of the cingulum (a structure that has not been reported in the genusTakayamabefore)(Fig.3b–c & g). A dorsal pore was observed in the posterior end of the apical groove of some (but not all) cells (Fig.3h–i).
The cell surface was covered by a thick layer of large and wart-like amphiesmal vesicles, especially on the hypocone (Fig.4a–h). The amphiesmal vesicles on the epicone were arranged in approximately 6 rows,running in a direction that followed the sigmoid apical groove (Fig.4c). The amphiesmal vesicles on the hypocone were much bigger, pentagonal to hexagonal in shape, and were also in approximately 6 rows(Fig.4h). The SEM photos showed that the sulcus intrusion onto the epicone ofT.acrotrochawas short in general (Figs.3–5); however, some f inger-like sulcus intrusions were also observed (Figs.4d–e, 5h, & 5k).
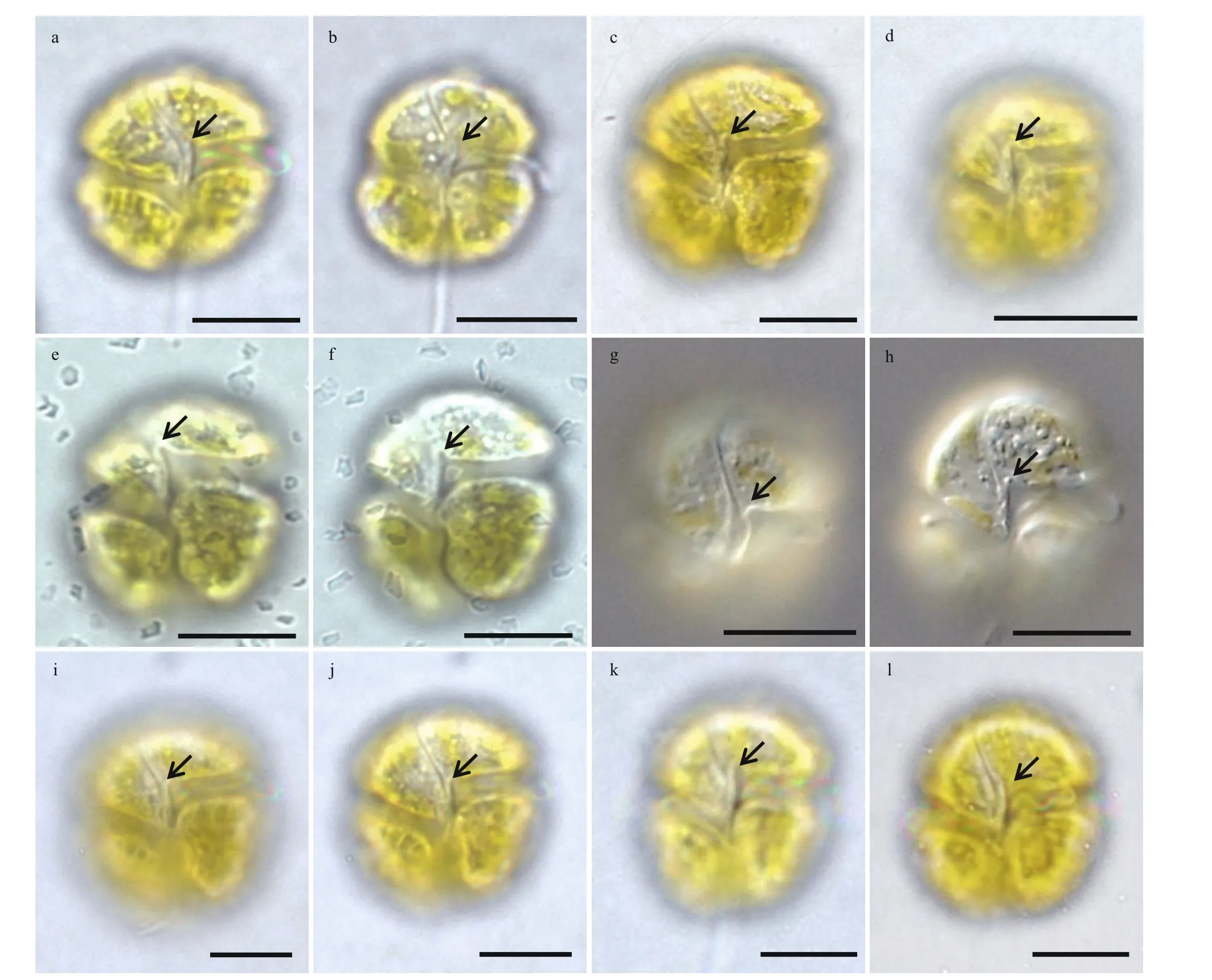
Fig.2 Light microscopy images of vegetative cells of Takayama acrotrocha in ventral view, focused on the sulcal intrusion(arrows)
3.1.3 Ultrastructure ofTakayamaacrotrochaunder TEM
The longitudinal section of the cell from the ventral side revealed the general ultrastructure ofT.acrotrocha(TAH8): a dinokaryotic nucleus with condensed chromosomes, chloroplasts, a pyrenoid and trichocysts (Fig.6a–c). The nucleus was oval,situated in the epicone and extending to the cell centre(Fig.6a). The trichocysts were scattered sparsely in the cell (Fig.6a & d). Numerous chloroplasts were distributed peripherally in the cell, and were more abundant in the hypocone (Fig.6a–c). Stacks of three thylakoids were arranged parallel to each other along the longitudinal axis of the chloroplasts (Fig.6a–e).A large, rounded pyrenoid surrounded by a starch sheath was observed in the hypocone, situated at the anterior of the chloroplast and sometimes separated from it (Figs.6e–f & 7a–b). The pusular system consisted of a central collecting chamber and a number of pusular vesicles which opened directly into the collecting chamber and connected with the canal of the f lagellum (Fig.7c). The amphiesmal vesicles were regularly distributed on the surface,and were larger on the hypocone than on the epicone(Figs.6a–e & 7d–e). Sections through the apical groove showed that the apical groove was composed of 4 rows of amphiesmal vesicles (Fig.7d–e). Several small vacuoles of electron-opaque material were gathered beneath the apical groove, and some of these were fused with the cytoplasm membrane (Fig.7d–e).Rows of cortical microtubules were arranged beneath the inner membrane, sometimes in groups of f ive(Fig.7e).

Fig.3 Scanning electron microscopy of the cells of Takayama acrotrocha collected from Shenzhen Bay, Guangdong during a bloom
3.2 Phylogenetic analysis
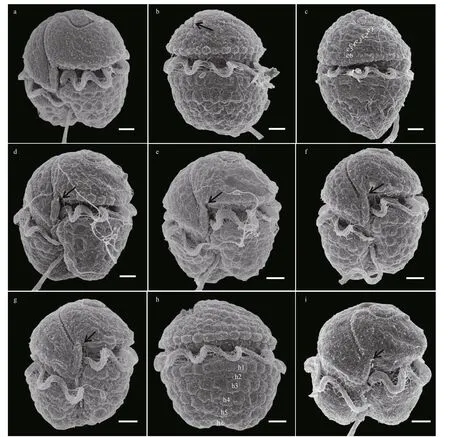
Fig.4 Scanning electron microscopy of the vegetative cells of Takayama acrotrocha (Strain Nos: a–f, TAG9; g–i, TAF10)
The 5 isolates ofT. acrotrochafrom the SCS shared the identical LSU rDNA and ITS rDNA sequences. The divergence based on the partial LSU rDNA sequence (673 bp) betweenT.acrotrochafrom the SCS and the other 17 strains ofTakayamaspecies (Supplementary Table S2)ranged from 0.52% to 2.88%; for example, 0.36%fromT.xiamenensis, 2.14% fromT.helix, and 2.88% fromT.tuberculata(Table 2). The 5 strains ofT.acrotrochafrom the SCS diff erentiated fromT.xiamenensis(which was isolated from the East China Sea, GenBank ID: AY764178) in only 3 bp.Based on the ITS rDNA (total 589 bp), the diff erence between theT.acrotrochafrom the SCS andT.acrotrochafrom Italy (strain No. MC728-D5 and GenBank ID: HM067011) was 0.50%, and fromT.xiamenensis(strain No. TPXM and GenBank ID:AY764179) was 0.30%.
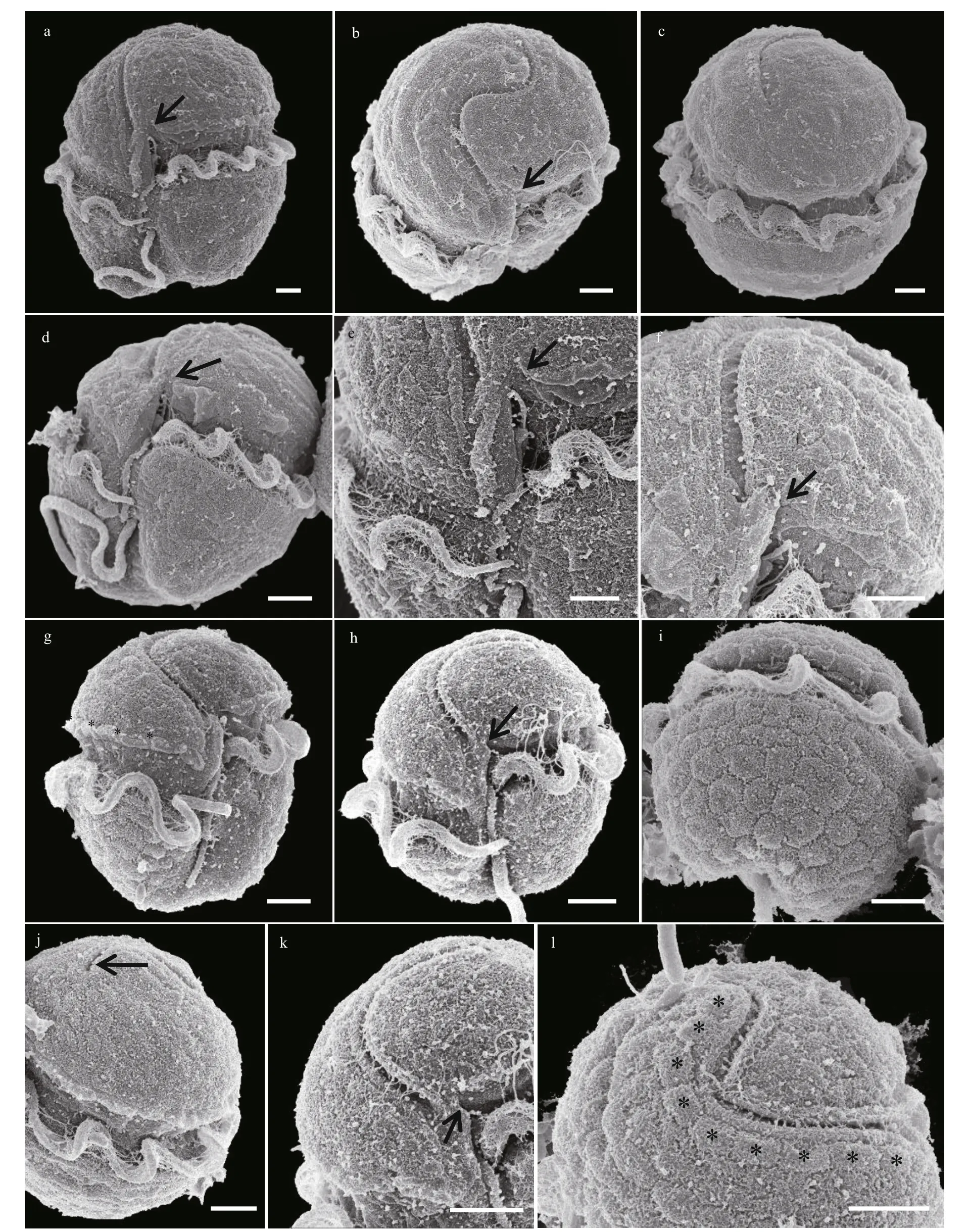
Fig.5 Scanning electron microscopy of vegetative cells of Takayama acrotrocha (strain Nos: a–f, TAH8; g–l, TAD11)
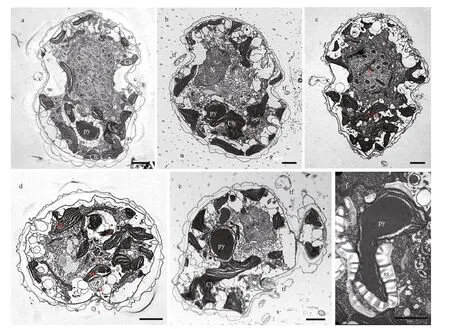
Fig.6 Transmission electron microscopy images of Takayama acrotrocha (TAH8)
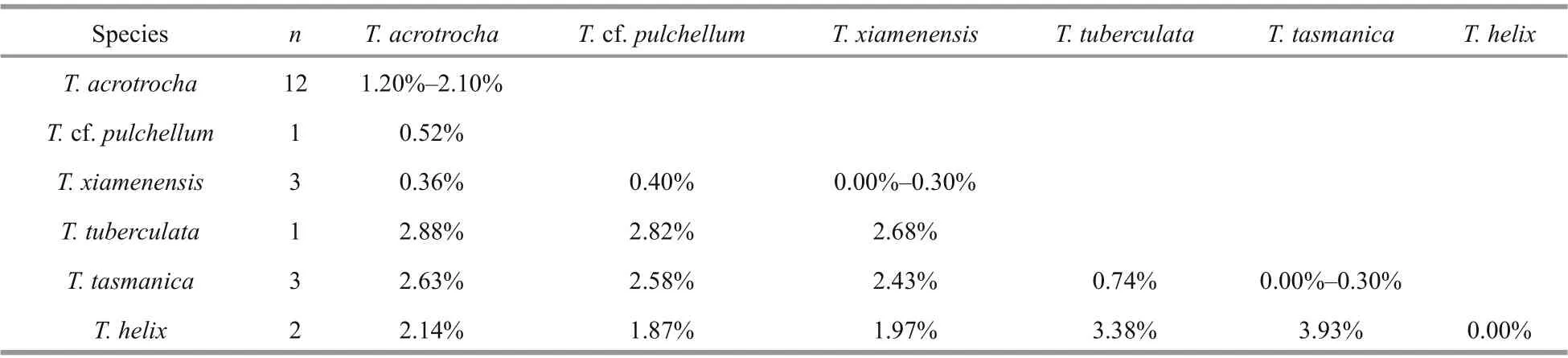
Table 2 The genetic divergence ( p-distance) between Takayama species based on the partial large subunit ribosomal DNA(LSU rDNA) (673 bp)
Phylogenetic tree inferred from the LSU rDNA showed that the 5 isolates ofT.acrotrochafrom the SCS clustered into the genusTakayamawith the otherTakayamaspecies (Fig.8), and formed a single branch withT.acrotrochafrom Italy (strain No. MC728-D5 and GenBank ID: FJ024703), Korea (strain No.TaLomme01 and GenBank ID: MZ358881),Singapore (strain No. GT15 and GenBank ID:DQ656116) andT.xiamenensisfrom the East China Sea (strain No. TPXM and GenBank ID: AY764178)with a support value of 80/0.98 (Bootstrap values/posterior probabilities). Based on the LSU rDNA phylogeny, species in the genusTakayamaclustered into two sister clades: clade 1 comprisedT.acrotrocha,T.xiamenensis,T. cf.pulchellum, andT.helix(99/1.00), and clade 2 comprisedT.tasmanicaandT.tuberculata(99/1.00). In the genusTakayama,the ITS rDNA sequences were only available forT.acrotrochaandT.xiamenensis, and the ITS rDNA sequences of the two species formed a single clade with a support value of 67/0.91 (Fig.9).
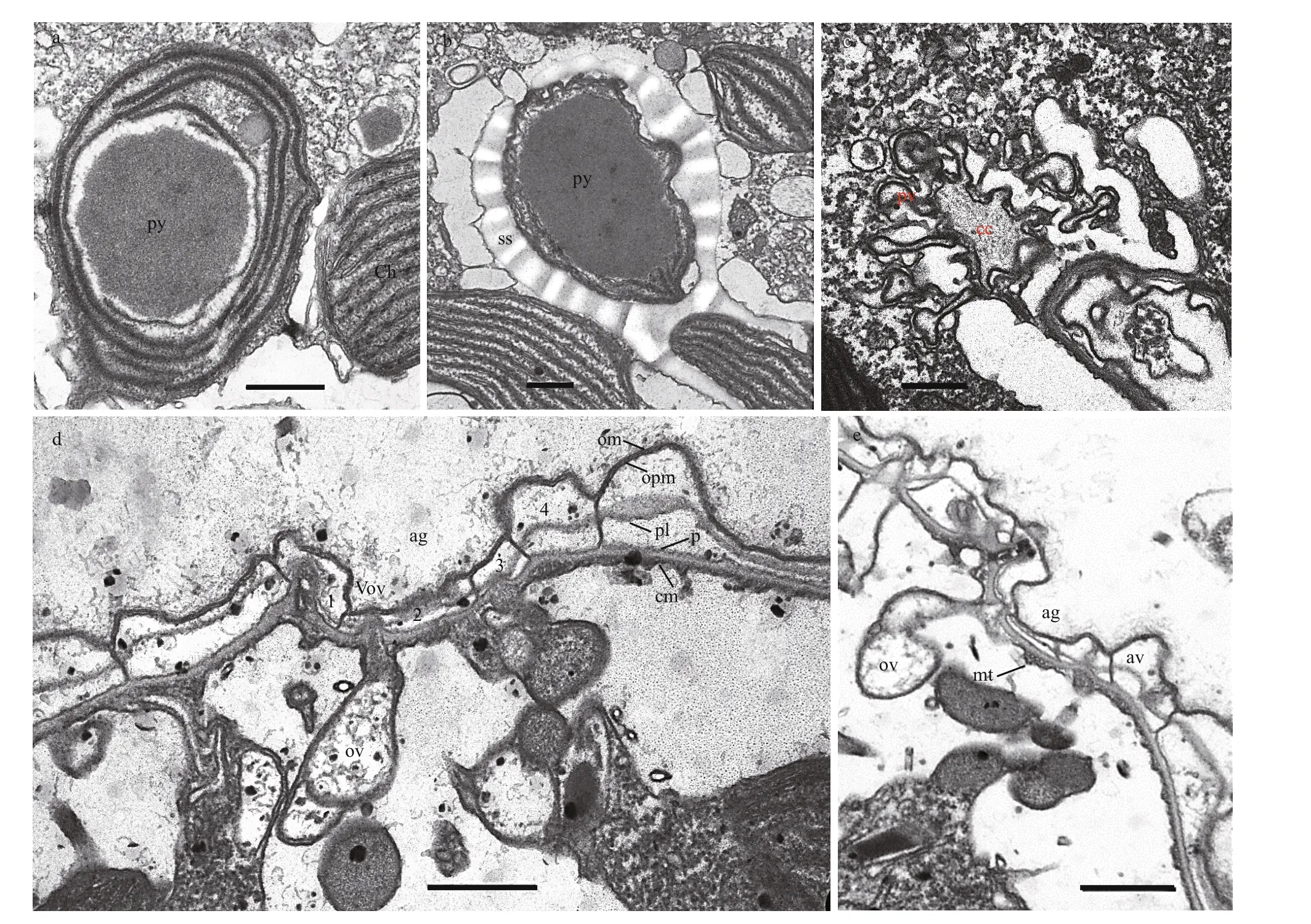
Fig.7 Ultrastructure of Takayama acrotrocha (TAH8)
3.3 Pigment composition
The pigment composition ofT.acrotrocha(strain No. TAH8) is similar to that of the fucoxanthincontaining dinof lagellates within the genusTakayama(Table 3). A total of 11 pigments: chlorophyll-c2(chlc2), chlorophyll-c3 (chlc3), chlorophylla(chla), Mg-2,4-divinylpheoporphyrin (MgDVP),19ʹ-butanoyloxy-fucoxanthin (but-fuco), fucoxanthin(fuco), prasinoxanthin (pras), 19ʹ-hexanoyloxyfucoxanthin (hex-fuco), diadinoxanthin (diad),canthaxanthin (cantha), and gyroxanthin-diester(gyro) were detected using high performance liquid chromatography (HPLC) based on the absorption spectra and retention time according to the commercial standard pigments (Fig.10). The main pigment is chla, and fucoxanthin is the main accessory pigment.The carotenoid pigments were quantif ied as ratios relative to chlaand were hex-fuco (0.29%), butfuco (4.92%), fuco (338.30%), diad (16.88%), cantha(5.66%), and gyro (1.83%). Seven unidentif ied pigments were detected at retention times of 10.20,22.70, 24.00, 26.30, 28.60, 29.00, and 32.60 min.
3.4 Cell toxicology on brine shrimp and marine medaka
Acute toxicity ofT.acrotrochaonA.salinaand adultO.melastigmawas checked by exposing the two organisms to seawater samples containing the bloomingT.acrotrochawith a concentration of 3.05×107cells/L for 48 h. No lethality ofT.acrotrochaonA.salinaand adultO.melastigmawas observed over 48 h.
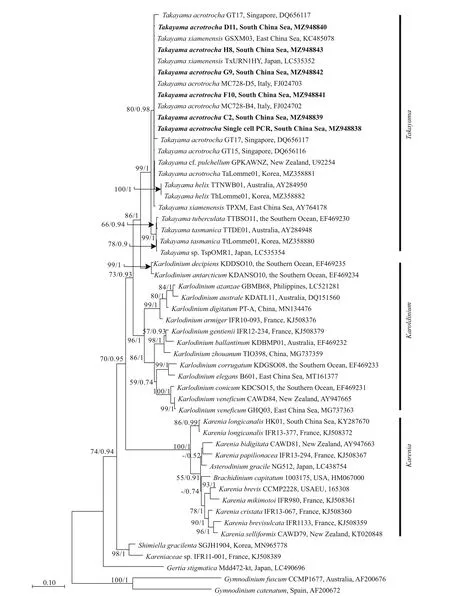
Fig.8 Molecular phylogeny of Takayama species and closely related species based on partial LSU rDNA
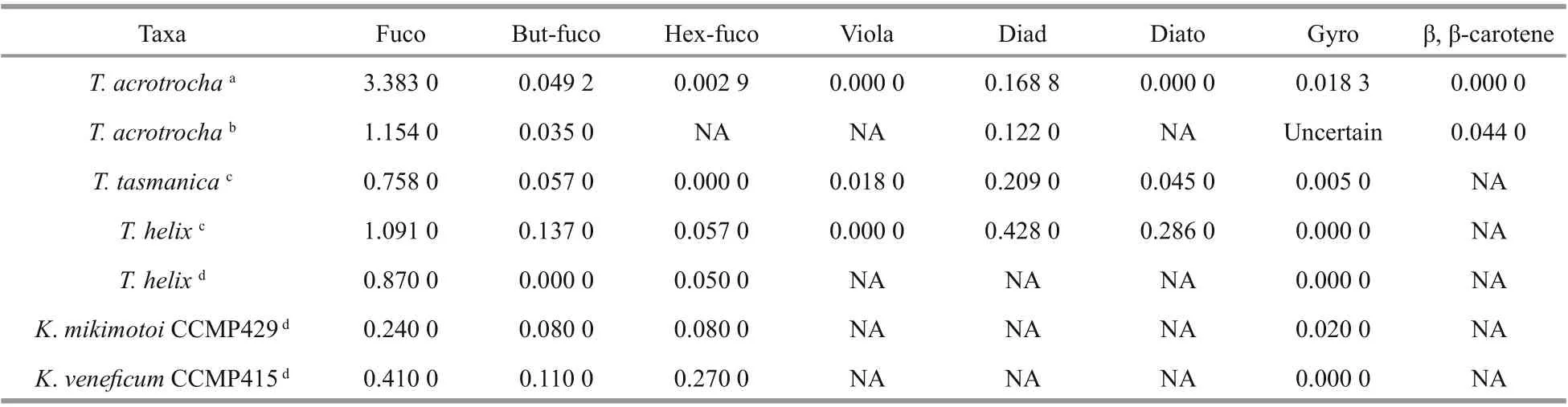
Table 3 Mass pigment ratios (w꞉w, carotenoids꞉Chl a) of Takayama kareniacean species
4 DISCUSSION
4.1 Morphology
Takayamaacrotrochawas f irst described by Larsen(1996) asGyrodiniumacrotrochum, an unarmoured dinof lagellate with a sigmoid apical groove, discshaped chloroplasts with individual pyrenoids and no sulcal intrusion onto the epicone. The original description ofT.acrotrochawas based on eight cells from f ield samples from Hobsons Bay, Australia(referred to in Larsen (1996): ‘At the laboratory the water samples were kept in culture cabinets at 15 °C until concentrated for microscopy by continuous centrifugation’). The description in Larsen (1996) was based on light microscopy, and no SEM details or DNA sequences were given. Siano et al. (2009) redescribedT.acrotrocha(based on clonal cultures from the Gulf of Naples, Italy) with more morphological features such as the arrangement of the apical groove, the presence of a pore on the ventral epicone, the slit in the sulcal zone, the small sulcal intrusion into the epicone and the tube-shaped structure along the sulcus in the inter-cingular region, as well as LSU rDNA sequences.Four cultures ofT.acrotrochafrom tropical waters in Singapore were established for a bloom monitoring project by Tang et al. (2012). The Singapore isolates ofT.acrotrochahad morphological characteristics which were consistent with the descriptions by Larsen(1996) and Siano et al. (2009). Numerous multilateral plate-like surface vesicles, and possibly a peduncle between the two emerging points of f lagellates, were additionally observed in the Singapore isolates ofT.acrotrochaunder SEM.
Takayamaacrotrochacan be distinguished under LM by the presence of a large, oval nucleus in the epicone, a sigmoid apical groove and chloroplasts which are located mostly in the hypocone (Larsen,1996; Siano et al., 2009). Under SEM,T.acrotrochawas characterized by a sigmoid apical groove starting from the ventral epicone and extending to less than halfway along the dorsal epicone, passing mostly on the left side instead of the middle of the cell apex.A small and deep sulcal intrusion onto the epicone ofT.acrotrochawas clearly observed by Siano et al. (2009, their f igure 27) and Tang et al. (2012, their f igure 2C).
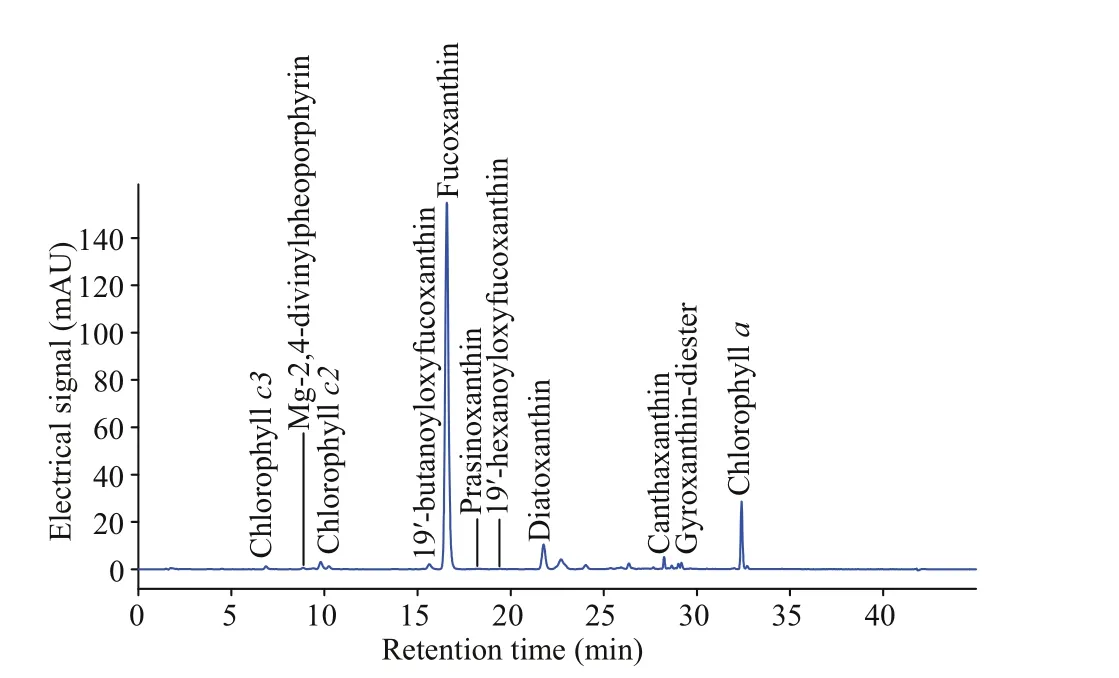
Fig.10 Pigment chromatogram of Takayama acrotrocha(strain No. TAH8)
The isolates ofT. acrotrochafrom the SCS shared the typical features described by Larsen (1996), Siano et al. (2009) and Tang et al. (2012) as the presence of a large oval nucleus in the epicone, the sigmoid apical groove that encircles the cell epicone and the chloroplasts located mostly in the hypocone, allowing the identif ication of our isolates asT.acrotrocha.According to the original description, the sulcus ofT.acrotrochadoes not intrude onto the cell epicone,and this is a key feature diff erentiatingT.acrotrochafrom the otherTakayamaspecies. However, with the high resolution of SEM, sulcus intrusion was found inT.acrotrochaby Siano et al. (2009) and Tang et al. (2012). The sulcus intrusion was apparent in our study both under LM and SEM, though cells with a less obvious sulcus intrusion were observed in strain TAF10 (see Fig.2i–l). The original micrographs in Larsen (1996, their f igures 2–4) were focused on the anterior and posterior of the apical groove and nucleus, not on the sulcus. An extension of the sulcus onto the epicone inT. acrotrochawas clearly observed in our strains (Fig.2). Our f inding was conf irmed inT.acrotrochafrom Korean coastal waters that the sulcus was not apparent in f igure 2A but was visible in f igure 2B in Cho et al. (2021), despite it was not marked by the author.
The shape and position of the nucleus is another feature for the identif ication ofTakayamaspecies.As illustrated in de Salas et al. (2008), the nucleus inTakayamaspecies can be classif ied into two shapes,oval or cup-shaped, located mostly in the epicone or on the left side of the cell from epicone to hypocone,see f igure 10 in de Salas et al. (2008).T.acrotrochadiff ered fromT.tasmanicaandT.tuberculatain having a nucleus that is oval, not cup-shaped, and diff ered fromT.pulchellaandT.cladochromain having a nucleus located in the epicone rather than on the left side of the cell.Takayamaacrotrochawas similar toT.helixin having elongated chloroplasts arranged in spiraling bands in the epicone, and with spiraling surface impressions that parallel the chloroplasts on the epicone. However, the very shallow sigmoid groove ofT.helixdistinguished it from the other members in the genusTakayama.
Chloroplasts of diff erent shapes and with/without individual pyrenoids were the most apparent diff erence among theTakayamaspecies. Chloroplasts in the genusTakayamacan be grouped into 2 types:chloroplasts radiating from a central pyrenoid (inT.tasmanicaandT.tuberculata) and chloroplasts containing individual pyrenoids (inT.cladochroma,T.helix, andT.pulchella). As described by Larsen(1996), chloroplasts ofT.acrotrochahad individual pyrenoids: ‘Several irregular, disc-shaped chloroplasts are present, mostly located in the hyposome, each with a pyrenoid’. However, the individual pyrenoids in chloroplasts ofT.acrotrochawere not conf irmed in the later re-examinations by Siano et al. (2009)and Tang et al. (2012). In the present study, an oval pyrenoid surrounded by a starch sheath under the nucleus was observed using both LM (Fig.1d) and TEM (Fig.6a), and such an oval pyrenoid was also observed in theT.acrotrochafrom Korea, see f igure 2C in Cho et al. (2021).
The amphiesma vesicles ofT.acrotrochawere clearly present on the surface of some well-preserved cells in Tang et al. (2012) and also in our isolates.The vesicles on the epicone were distributed in approximately 6 rows, extending along the ‘S’-shaped apical groove. The amphiesma vesicles on the hypocone were large and wart-like, and were also arranged in 6 layers. A line of amphiesma vesicles lying on the upper layer of the cingulum and forming a pearl necklace-like structure in the well-f ixed cells were observed in our isolates, this feature has not been reported inTakayamaspp. before. Wart-like amphiesma vesicles on the cell surface were also recorded inT.tuberculata, after which the species was named. We suggest that the wart-like amphiesmal vesicle might not be a species-specif ic characteristic only forT.tuberculata. Irregularly shaped vesicles could exist on the surface of otherTakayamaspecies,and may have been lost in the preparations for SEM, and this has led to a lack of useful features to diff erentiateT.tasmanicafromT.tuberculata.
In the f irst report ofT.acrotrochaby Larsen(1996), the common ventral pore on the left side of the epicone was not found, and this pore was also not mentioned by Tang (2012). A very small ventral pore was present in the Italian strain ofT.acrotrochaunder SEM (Siano et al., 2009). In this paper, a ventral pore above the sulcal extension on the left side of the epicone was observed both in f ield cells and clonal cultures, although sometimes it was not obvious in cells covered by the amphiesmal vesicles or cell external mucus. In addition to the ventral pore, a slitlike ventral pore well below the anterior of the apical groove was observed both in the Italian strains and in our cells. Another narrow, shallow slit, above and parallel to the entire anterior edge of the cingulum observed in the Singaporean strains (see f igure 2C in Tang et al. (2012)), was not detected in all our strains;however, an unapparent narrow and shallow furrow(rather than a slit) was found in our cultures (see Fig.3e–f), at the same position mentioned by Tang et al. (2012).
4.2 Phylogeny
Gu et al. (2013) considered that the LSU rDNA was too conservative to set boundaries forTakayamaspecies. No DNA sequence was available for the holotypes ofT.acrotrocha,T.cladochroma, andT.pulchella, and DNA sequences are only available forT.acrotrocha,T.helix,T.xiamenensis,T.tasmanica,andT.tuberculatain GenBank. Both ML and the BI phylogenetic tree allow us to infer that species in the genusTakayamacluster into 3 subclades: the f iveT.acrotrochastrains established in this study formed a branch with the Singapore strain ofT.acrotrocha(DQ656117) andT.xiamenensis(Tang et al., 2012),T.tasmanicaandT.tuberculataformed the second branch andT.helixwas the third branch. The 3 branches ofTakayamain the tree were consistent with the morphological diff erences among theTakayamaspecies: the f irst clade, which includedT.acrotrochaandT.xiamenensis, has a deep ‘S’-shaped apical groove, an oval nucleus occupies most of the epicone and some chloroplasts have bulging pyrenoids. The second branch, which consists ofT.tasmanicaandT.tuberculata, has a deep S-shaped apical groove, a cup-shaped nucleus on the epicone and chloroplasts radiating from a central pyrenoid agglomeration. The third branch,T.helix, has a very shallow, sigmoid,apical groove. Additionally, the wart-like amphiesma vesicle in our strains ofT.acrotrochameant that there were no useful features left to distinguishT.tasmanicafromT.tuberculata, and the two species might be conspecif ic; thus, we concluded that the LSU rDNA is still a valid marker for separatingTakayamamorphotypes.
The generaTakayamaandKarlodiniumformed a monophyletic group in the phylogenetic trees, whileBrachidiniumcapitatum,Asterodiniumgracile, andKareniaspecies clustered in a single clade (Fig.8).The main character ofBrachidiniumandAsterodiniumis an unarmored pelliculate cell with four or f ive radiating elongated extensions. The extensions of brachidiniaceans are quite variable, with some intermediate specimens resembling cells ofKareniapapilionaceaandKareniabidigitata(Gómez et al.,2005), which was further validated by DNA evidence demonstrating thatBrachidiniumis phylogenetically related toKareniaspecies (Henrichs et al., 2011;Benico et al., 2019). In 2012, Gómez (2012) proposed that the generaTakayama,Karlodinium, andKareniashould be included in the family Brachidiniaceae,alongside the generaAsterodinium,Brachidinium,Gynogonadinium,Microceratium,Pseliodinium, andTorodinium. However, no cell with long extensions has been found in kareniaceans, implying that more life cycle and morphology studies are needed for members of these two families. Recently, new taxaShimiellaandGertiahave been placed to the family Kareniaceae (Takahashi et al., 2019; Ok et al., 2021).As a result, we will revisit the def initions for members of the two families Brachidiniaceae and Kareniaceae in the future, and we will continue to include the genusTakayamain the Kareniaceae family in this publication.
4.3 Pigment composition
The pigment prof ile ofT.acrotrochawas categorized as Type-3 following the pigment classif ication of dinof lagellates by Zapata et al. (2012). The Type-3 prof ile was characterized with fucoxanthin/19ʹ-acyloxyfucoxanthins/gyroxanthin diesters/chlc2 and chlc3. Gyroxanthin diester, which is usually a marker pigment forKareniaspp. andKarlodiniumspp.(Bergholtz et al., 2006; Johnsen et al., 2011; Chang and Gall, 2013), was also detected inT.acrotrocha,but not inT.helix.Takayamaacrotrochafrom the SCS contained fucoxanthin as the most abundant carotenoid among the 5Takayamaspp., with a ratio to chlaas high as 3.383 0. Usually, species in the family Kareniaceae are known for containing fucoxanthin and/or its derivatives as major carotenoids, and lack peridinin; however,Gertiastigmatica, a species with the peridinin-type chloroplast has been reported in the family Kareniaceae recently (Takahashi et al., 2019),suggesting a more-diverse plastid origin in this family.
4.4 Toxicity
The genusTakayamais affi liated to the toxigenic family Kareniaceae, and some species have been associated with toxic bloom events. However, no ichthyotoxicity forT.pulchellumwas determined in vivo (Steidinger, 1998). No toxicity or stress was observed in the bioassay by all SCST.acrotrochacultures and the bloom seawater in the laboratory;and no f ish kill was associated with theT.acrotrochabloom in the Zhujiang (Pearl) River estuary, nor with the Singaporean isolates ofT.acrotrocha, suggesting the toxicity protocol ofTakayamaspecies need more investigation. Multi-dinof lagellate blooms dominated byT.xiamenensis,Karlodiniumvenef icum, andK.australehave been suspected of being associated with massive f ish kills in Singapore (Leong, 2015).Future research into accurate species-specif ic identif ication of these naked and harmful algal bloom species should be carried out.
4.5 Comparison between T. acrotrocha and T. xiamenensis
In 2013, Gu et al. (2013) identif ied a new speciesT.xiamenensisfrom Xiamen Harbour in the East China Sea. Partial LSU rDNA sequences of the two strains ofT.xiamenensiswere identical to theT.acrotrochastrains from Italy, and had a high similarity (99.72%)to the Singapore strain GT15.Takayamaxiamenensiswas characterized by a distinctive f inger-like intrusion onto the epicone; a large, cup-shaped nucleus on the epicone; and some chloroplasts with large, bulging pyrenoids. As described by Gu et al. (2013), the main diff erence betweenT.xiamenensisandT.acrotrochawas the f inger-like sulcus in the former, while no such intrusion was found in the latter. However, in our study, cells with or without a sulcus intrusion were both observed in the diff erent strains ofT.acrotrocha,and some sulcus intrusions were f inger-like,suggesting thatT.acrotrochaalso had a f inger-like sulcus intrusion. Although the author described the nucleus ofT.xiamenensisas cup-shaped, an ovoid nucleus was also recognized inT.xiamenensis(Gu et al., 2013, f igure 4). Moreover, diff erent from the cupshaped nucleus inT.tasmanicaandT.tuberculata(which surrounds the central pyrenoid agglomeration dorsally, laterally, and apically to form a cup shape),the cup-shaped nucleus ofT.xiamenensiswas seemed as a shading by the large, round pyrenoid in the hypocone (see f igure 4 in Gu et al. (2013)). Figure 13 in Gu et al. (2013) also suggests that the nucleus did not surround the pyrenoid. A bulging pyrenoids surrounded by a sheath of starch or starch granules inT.xiamenensiswere also clearly recognized in ourT.acrotrochastrains (Fig.6a–b & e–f). Additionally,the partial LSU rDNA sequences ofT.xiamenensiswere identical to theT.acrotrochastrains from Italy,and had a high similarity (99.7%) to the Singapore strain GT15 and our strains. All the data suggested thatT.xiamenensisandT.acrotrochaare conspecif ic species.
5 CONCLUSION
In this study, the morphology and phylogeny of a bloom species from the SCS,T.acrotrochais studied. The inter- and intra-species morphological and phylogenetic diff erences forT.acrotrochawere analyzed and compared.Takayamaacrotrochais characterized by a deeply sigmoid apical groove, a sulcus extending onto the epicone as a short intrusion,an ovoid to cup-shaped nucleus occupying most of the epicone, and a rounded pyrenoid located between the left side and the center of the hypocone. The main accessory pigment ofT.acrotrochais fucoxanthin. In the phylogenetic trees inferred from the LSU rDNA and ITS rDNA, theT.acrotrochafrom the SCS formed a subclade with theT.acrotrochafrom Singapore andT.xiamenensis.Takayamaacrotrochafrom the SCS has the typical morphological characteristics ofT.xiamenensis, and the two species shared the identical LSU rDNA and ITS rDNA sequences,suggesting thatT.acrotrochaandT.xiamenensisare the same species, with the priority forT.acrotrocha.
6 DATA AVAILABILITY STATEMENT
The data that support the f indings of this study are available from the corresponding author upon request.
7 ACKNOWLEDGMENT
Special appreciation is expressed to Dr. Zhaohe LUO from the Third Institute of Oceanography, the State Oceanic Administration for helpful discussions regarding the TEM photos. We would also like to thank Dr. Li LI from the Shenzhen Marine Monitoring and Forecasting Center for her help in the seawater sampling.
 Journal of Oceanology and Limnology2022年6期
Journal of Oceanology and Limnology2022年6期
- Journal of Oceanology and Limnology的其它文章
- Overview of harmful algal blooms (red tides) in Hong Kong during 1975–2021
- Information standardization for typical toxic and harmful algae in China’s coastal waters—a case study of Karenia mikimotoi*
- Biochemical composition of the brown tide causative species Aureococcus anophageff erens cultivated in diff erent nitrogen sources*
- Identif ication of paralytic shellf ish toxin-producing microalgae using machine learning and deep learning methods*
- Screening for lipophilic marine toxins and their potential producers in coastal waters of Weihai in autumn, 2020*
- First observation of domoic acid and its isomers in shellf ish samples from Shandong Province, China*
