Biodiversity and distribution of benthic dinof lagellates in tropical Zhongsha Islands, South China Sea*
Hang XIE , Jian ZOU , Chengzhi ZHENG , Yuchen QU , Kaixuan HUANG ,**,Songhui LÜ
1 Research Center of Harmful Algae and Marine Biology, College of Life Science and Technology, Jinan University, Guangzhou 510362, China
2 Southern Marine Science and Engineering Guangdong Laboratory, Zhuhai 519000, China
3 Key Laboratory of Eutrophication and Red Tide Prevention of Guangdong Higher Education Institutes, Jinan University,Guangzhou 510632, China
Abstract Benthic dinof lagellates have attracted increasing attention in recent years because of their toxicity and ability to form extensive harmful algal blooms. Ostreopsis producing palytoxin and its analogs,Gambierdiscus producing ciguatera toxins, and Prorocentrum producing okadaic acid and dinophysis toxins,have been concerned as serious human poisonings. We explored the benthic dinof lagellate biodiversity and distribution characteristics of a series of tropical reefs in 20–40-m water depth in wet season in the Zhongsha Islands in South China Sea using morphological, phylogenetic, and cell counting methods. Results show that benthic dinof lagellates in the islands are rich in biodiversity and 15 species from genera Amphidinium,Coolia, Ostreopsis, and Prorocentrum were identif ied: Amphidinium carterae, A. magnum, A. massartii,A. operculatum, Coolia canariensis, C. malayensis, C. palmyrensis, C. tropicalis, Ostreopsis cf. ovata,Prorocentrum concavum, P. cf. sculptile, P. emarginatum, P. hoff mannianum, P. lima, and P. rhathymum.Among them, A. magnum is reported for the f irst time in Chinese waters. The abundance of benthic dinof lagellates was relatively low at 88–4 345 cells/100 cm 2 on sediment and 10–91 cells/g on macroalgae.Prorocentrum and Amphidinium were the dominant and subdominant genera, respectively. It is speculated that the low abundance of benthic dinof lagellates is closely related to the scarcity of macroalgae and stronger water motion at the depth >15 m in Zhongsha Islands. This study expanded the study in biodiversity of benthic dinof lagellates in Chinese waters, and revealed the distribution characteristics of harmful benthic microalgae in reef habitats.
Keyword: benthic dinof lagellates; Zhongsha Islands; South China Sea; biodiversity; distribution
1 INTRODUCTION
Benthic dinof lagellates, e.g., species ofOstreopsis,Gambierdiscus,Fukuyoa,Prorocentrum,Coolia, andAmphidinium, are group of microalgae that attach to substrates such as macroalgae, dead coral, sand, and rocks (Fukuyo, 1981; Boisnoir et al., 2019a). Harmful benthic algal blooms have aroused widespread concern due to benthic-dinof lagellate-produced toxins and bloom-induced anoxia, which endangers human health and marine ecosystem (Yasumoto et al., 1987; Litaker et al., 2010; Mangialajo et al.,2011; Kudela et al., 2017). Palytoxin and its analogs,the metabolites of someOstreopsisspecies, are the most toxic non-protein algal toxins (Amzil et al.,2012; Ben Gharbia et al., 2016). The ciguatoxins and maitotoxins generated by species ofGambierdiscusandFukuyoa, which are responsible for ciguatera f ish poisoning (CFP) in humans, are the most serious nonbacterial illness associated with seafood consumption(Litaker et al., 2010). Some species ofProrocentrumproduce okadaic acid and its analogs, which are the culprit of diarrheic shellf ish poisoning (DSP) in humans (Tripuraneni et al., 1997; Foden et al., 2005).Furthermore, someCooliaandAmphidiniumspecies can threaten marine benthic animals (Pagliara and Caroppo, 2012; Karafas et al., 2017).
In 1979, with the discovery ofGambierdiscustoxicus— a toxic species isolated from a ciguateraendemic area — the study of harmful benthic dinof lagellates was initiated (Yasumoto et al., 1977;Adachi and Fukuyo, 1979). An increase in number of CFP incidences andOstreopsisblooms reported from around the world has led to the increasing studies highlighting their ecological impacts (Litaker et al.,2010; Rhodes, 2011). In recent decades, scholars have paid more attention to the taxonomy, toxicity,and ecology of benthic dinof lagellates, as well as the geographical distribution of toxic and harmful species (Kudela et al., 2017). CFP producers, species ofGambierdiscusandFukuyoa, are mainly reported in tropical and subtropical waters (Bienfang et al.,2008; Chinain et al., 2020). A review summarized that the Caribbean Sea and the Western Pacif ic Ocean are the areas where these two genera are most requently reported (Chinain et al., 2021). This distribution pattern has also expanded to higher latitude temperate waters, including Korea (Jang et al., 2018) and the northeast Mediterranean Sea in the past decade (Aligizaki and Nikolaidis, 2008).Ostreopsisspecies are distribution globally. Blooms ofOstreopsishave frequently recorded in waters of Mediterranean Sea (Mangialajo et al., 2011; Rhodes,2011; Kang et al., 2013; Acaf et al., 2020; Nascimento et al., 2020). BesidesOstreopsis, other generaCoolia,Amphidinium,Prorocentrum, andSinophysisis with higher biodiversity of benthic dinof lagellates(Rhodes, 2011; Ben Gharbia et al., 2016, 2019; Reñé et al., 2020). Benthic dinof lagellates are abundantly distributed on coral reefs and island ecosystems.Fukuyo (1981) isolated eleven benthic dinof lagellate species belonging to f ive genera from coral reefs and systematically described the whole benthic dinof lagellate community. Some islands in the South Pacif ic, such as French Polynesia, the Cook Islands,and the Republic of Kiribati, are considered to be“hot spots” ofGambierdiscusbiodiversity (Chinain et al., 2016). So far studies of benthic dinof lagellates have been mostly focused on a certain genus or toxic species. There have been relatively few studies on entire benthic dinof lagellate communities (Nishimura et al., 2013; Boisnoir et al., 2018; Lim and Jeong,2021). Those studies of community composition and substrate preferences have only been carried out in a few areas (Yong et al., 2018; Mustapa et al., 2019;Accoroni et al., 2020).
To date, three species ofGambierdiscus(Zhang et al., 2016), one species ofFukuyoa(Leung et al.,2018), two species ofOstreopsis(Zhang et al., 2018),four species ofCoolia(Leung et al., 2017), eleven species ofAmphidinium(Luo et al., 2022), and more than tenProrocentrumspecies, in which some of the species were okadaic acid and/or DSP toxins producers (Luo et al., 2017; Zou et al., 2021), have been reported in the South China Sea. Recently,a harmfulProrocentrumconcavumbloom was reported at Hainan Island, which is the f irst benthic dinof lagellate bloom in China at present (Zou et al.,2020).
To date, most studies on benthic dinof lagellates have been concentrated in intertidal areas, bays,lagoons, and islands, especially in the Caribbean Sea and Mediterranean Sea (Mangialajo et al., 2011;Durán-Riveroll et al., 2019; Chinain et al., 2021). A study of how water movement, depth, and diff erent habitats inf luenced on the abundance and distribution of benthic dinof lagellate communities was carried out at Johnston Atoll, Pacif ic Ocean (Richlen and Lobel,2011). It was found that moderate hydrodynamic disturbance promoted the distribution of benthic dinof lagellate community. The sampling depth of this study was concentrated below 5 m and the maximum depth was only 13 m. Lee et al. (2020) studied the eff ects of substratum and depth on benthic harmful dinof lagellate assemblages at Perhentian Islands and Terengganu, Malaysia. Though the deepest sampling depth was 25 m, sampling sites were mostly on the coasts of the islands. In short, there are few studies on reefs of more than 20-m depth, especially the areas far away from the mainland. Compared with the coasts of continents or large islands, reef ecosystems with water depths of more than 20 m are subject to stronger hydrodynamic disturbances. The Zhongsha Islands are the typical tropical coral reef ecosystems composed of reef-building corals and the marine organisms that grow in and around the reefs.The main body of the Zhongsha Islands, Zhongsha Great Atoll, is fully submerged beneath water depth of 13–26 m. The seabed surface of Zhongsha Great Atoll is coral sand and shell debris. In this study,we investigated the biodiversity and distribution of benthic dinof lagellates in the Zhongsha Islands based on morphology, phylogeny, and cell counting.Samples of sediments and macroalgae were collected to analyze the epibenthic dinof lagellate community composition and abundance at diff erent shoals.
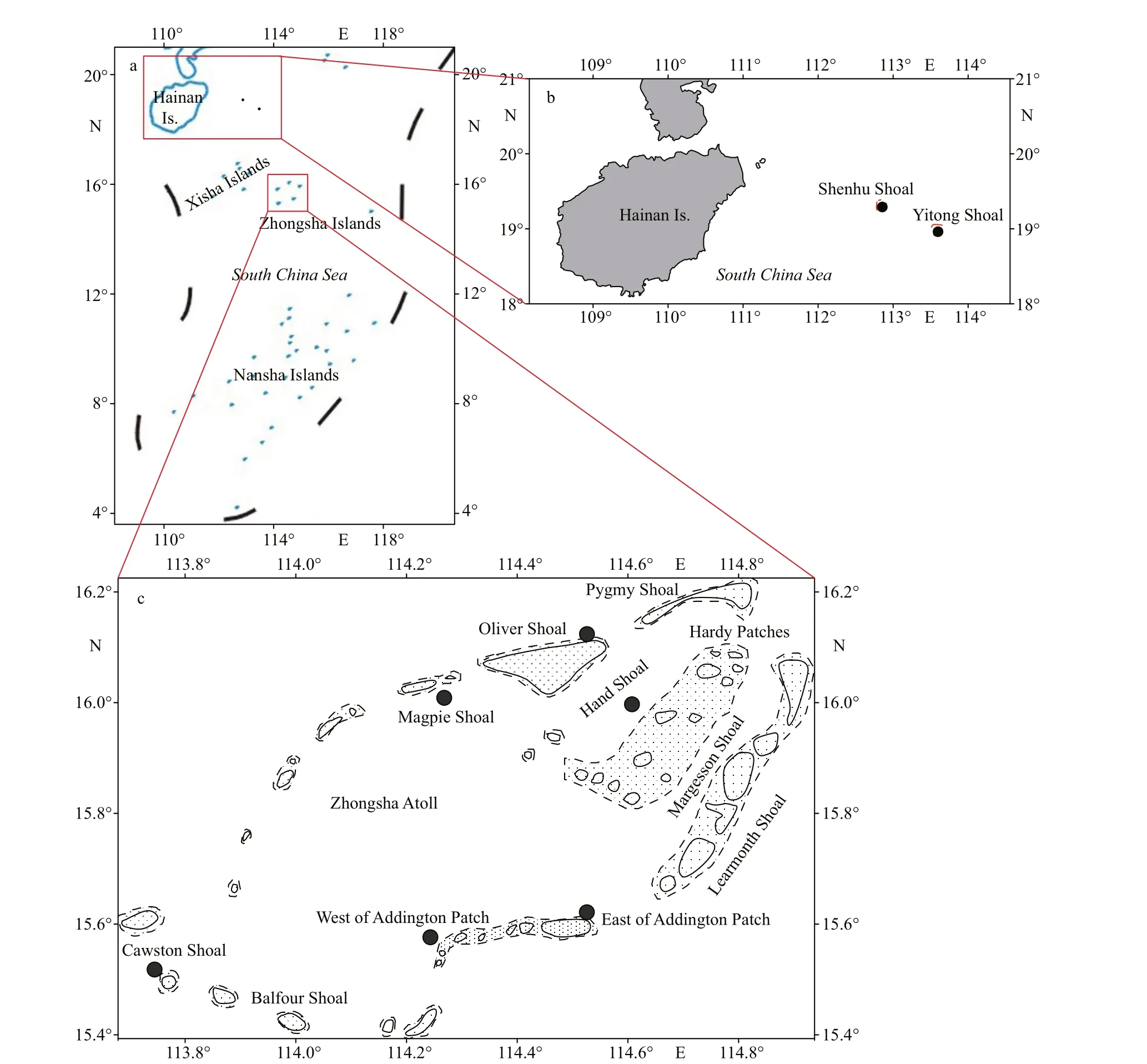
Fig.1 The location of South China Sea (a) and sampling sites in Zhongsha Islands (b), Yitong Shoal, and Shenhu Shoal (c)
2 MATERIAL AND METHOD
2.1 Study area and sampling strategy
The Zhongsha Islands (13°57ʹN–19°33ʹN,113°02ʹE–118°45ʹE), are composed of 26 named shoals on Zhongsha Great Atoll and Huangyan Island, and four scattered shoals: the Yitong, Xianfa,Shenhu, and Zhongnan Shoals. The Zhongsha Islands belong to a tropical monsoon climate zone, with the nature characteristics of strong sunshine exposure,low temperature variation, and high temperature and salinity. In addition, the Zhongsha Islands have distinct dry and wet seasons, and abundant precipitation that increases from north to south.

Fig.2 The method of sediment sampling
Sediments and macroalgal samples were collected by scuba diving between June 24 and July 5, 2020, at the Zhongsha Great Atoll (six stations), Yitong Shoal,and Shenhu Shoal, China (Fig.1 & Table 1). Sediment samples were collected in triplicate using a 10-cm×10-cm×3-cm mud sampler and were transferred with seawater into a 1-L sample bag (Fig.2). Sediment samples were placed for 10 min for sediment setting,and then seawater was slowly collected and the volume was recorded. Later, the seawater was f iltered with a 120-μm sieve to collect the epibenthiccells.Benthic dinof lagellates were retained by a sieve with 20-μm mesh, then immediately f ixed in 1.5% Lugo’s solution. Water samples were collected using a water sampler. Macroalgae were sampled carefully with the surrounding seawater and transferred into sampling bags. Macroalgal samples were shaken for 30 s to detach attached dinof lagellate cells and then rinsed twice with 0.22-μm f iltered seawater. The seawater containing epibenthic algal cells were f iltered using a 120-μm sieve and then epibenthic algal cells were retained by a 20-μm mesh, and immediately f ixed in 1.5% Lugol’s solution. The separated macroalgae was dried using absorbent paper before weighing(Boisnoir et al., 2019b).
2.2 Dinof lagellate cell abundance
Cells were counted by 1-mL Sedgewick Rafter Counting Cell using the light microscope (Olympus BX 61, Tokyo, Japan). The cell abundance of the sediment was described as cells per 100 cm2.Abundance on macroalgae was measured as cells per gram of fresh macroalgal weight.
2.3 Isolation and culture
Live cells during the exponential period were isolated with micropipette under the light microscope.Isolated benthic dinof lagellates cells were cultured in a 96-well plate with L1 medium (Guillard and Hargraves, 1993). The culture plate was under a dark꞉light cycle of 12 h꞉12 h with 100-μmol/(m2·s)irradiance, 25 °C, and salinity of 30. Strains that divided a number of cells were ported to a 25-mL culture f lask with a same culture conditions.
2.4 Morphological observation
Cell during the exponential period was observedunder a microscope. Morphological data (cell shape,length, and width) were obtained using Image-Pro Plus 6.0 Image.
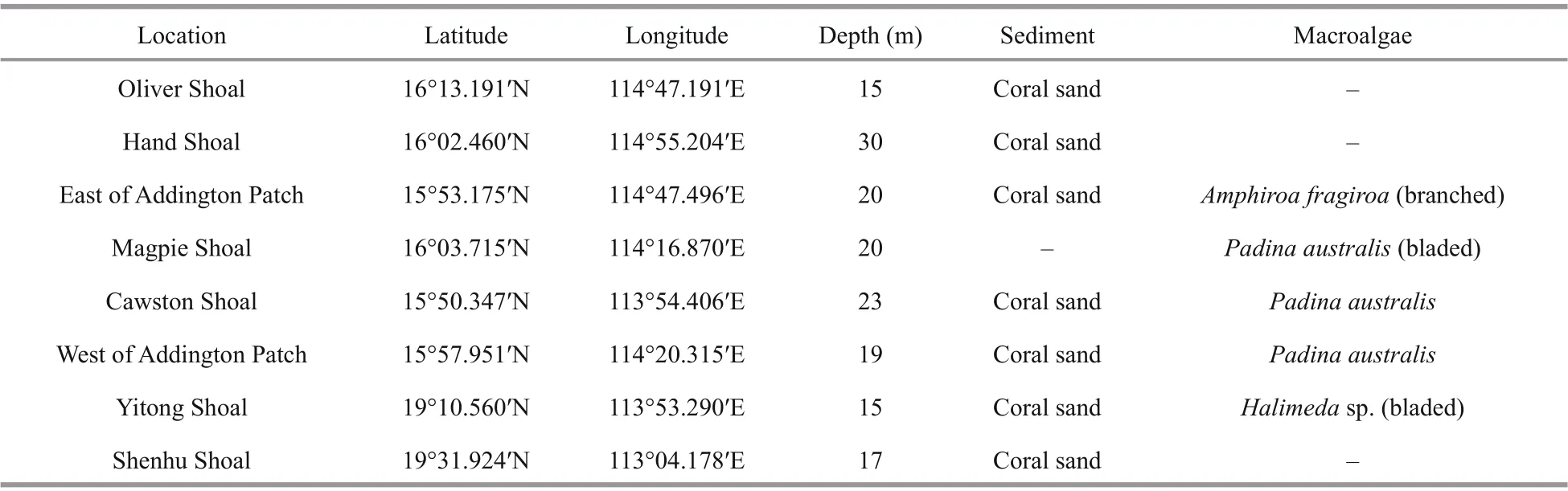
Table 1 Locations of sampling sites, sampling depth, and sample types
The morphology and distribution of chloroplasts and nuclei were observed using a f luorescence microscope, then image acquisition was conducted using the QImaging Retiga 400R digital camera(QImaging, Surrey, BC, Canada) at 400 magnif ications.
Sample preparation for the scanning electron microscope (SEM) was performed according to Lim et al. (2019). Cells grown in the exponential phase were f ixed in glutaraldehyde (f inal concentration:2.5%) for 12 h at 4 °C. One milliliter of algal cells was pipetted onto a round glass slide and soaked in 10%polylysine for more than 2 h. The slide was observed under a microscope to ensure that a large number of cells were adsorbed on the surface. The cells were soaked using a gradient of 100%, 80%, 60%, 40%,and 20% of the seawater and Milli-Q water for 5 min.Dehydration was carried out through a series of ethanol gradients (10%, 30%, 50%, 70%, and 90% and three times at 100%, for 5 min each). The dehydrated cells were dried by a CO2critical point dryer (Leica Microsystems, Mannheim, Germany) and puttercoated with gold (Leica, Germany). Finally, the morphology and structure of cell was presented and photographed under a scanning electron microscope(Carl Zeiss Inc., Germany).
2.5 DNA isolation and gene amplif ication
The clonal cells of all benthic dinof lagellate strains were harvested at 12 000×gfor 1 min at 4 °C. Total genomic DNA of exponential cells were extracted by MiniBEST Universal Genomic
DNA Extraction Kit (TaKaRa, Japan) according to the instructions. A 50-μL PCR reaction system includes: 2-μL DNA, 25-μL Accurate TaqMaster Mix (2X) (Zoman Biotechnology, China), each 1 μL of the forward primer and reverse primer, and 21-μL ultrapure water. The D1–D3 regions of large subunit (LSU) was amplif ied using general primers(D1R: 5ʹ-ACCCGCTGAATTTAAGCATA-3ʹ, D3B:5ʹ-TCGGAGGGAACCAGCTACTA-3ʹ) described in Scholin et al. (1994). The PCR cycle procedure:pre-denaturation at 94 °C, 4 min and followed by 36 cycles, each cycle included denaturation at 94 °C for 20 s, annealing at 56 °C for 30 s, and extension at 72 °C for 1.5 min, and a f inal extension step at 72 °C for 10 min (Zou et al., 2020). All sequences were uploaded to GenBank and the accession numbers were listed in Supplementary Table S4.
2.6 Phylogenetic analysis
34 sequences of the LSU (D1–D3) regions obtained by sequencing was aligned and edited by ContigExpress software. The obtained sequences from our study and related species sequences from GenBank, which consist of 41Amphidiniumsequences, 43Cooliasequences, 61Ostreopsissequences, and 64Prorocentrumsequences, were analyzed for multiple alignments at the website (https://www.ebi.ac.uk/Tools/msa/muscle/). Sequence group of diff erent genera were aligned separately. Then the aligned results were break off both ends using BioEdit (Version 7.2.9) and then were analyzed using the maximum likelihood (ML) analysis in an online program (https://www.phylo.org/) with RAxMLHPC2 and XSEDE V. 8.2.10. The best models were determined by the program MrModeltest2.3 under the Akaike Information Criterion. In addition, Bayesian inference (BI) was conducted at Mr. Bayes 3.2.7 with four Markov Chain Monte Carlo (MCMC) chains and each chain was run for 1 000 000 cycles and sampling every 100 cycles using the best f it model(GTR). The uncorrected P-distances among all species and its closely related species were used by the software Mega X and the results are displayed in the Supplementary Table S5.
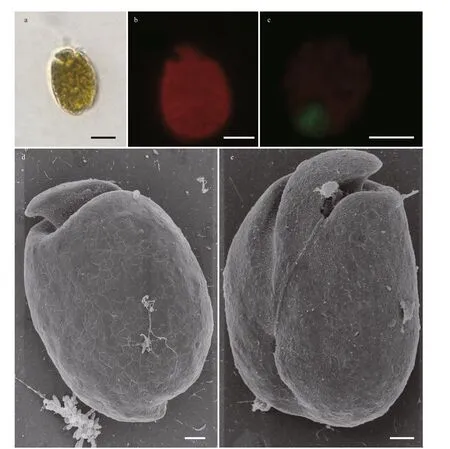
Fig.3 Light microscopy (LM) and scanning electron microscopy (SEM) photographs of Amphidinium magnum
3 RESULT
3.1 Biodiversity of benthic dinof lagellates species
Fifteen species of benthic dinof lagellates belonging to four genera were identif ied in morphology:Amphidinium carterae,A. magnum,A. massartii,A. operculatum,Coolia canariensis,C. malayensis,C. palmyrensis,C. tropicalis,Ostreopsiscf.ovata,Prorocentrumconcavum,P. cf.sculptile,P.emarginatum,P.hoff mannianum,P.lima, andP.rhathymum. We focus the morphology of a species f irst discovered in the waters of China (A. magnum),and potentially widespread toxic species (O. cf.ovata,P.lima, andP.hoff mannianum). The morphological micrographs ofP. cf.sculptileandA. carteraewere not obtained because of cultivation failure.
3.1.1 Morphology of benthic dinof lagellates
3.1.1.1Amphidiniummagnum(Fig.3)
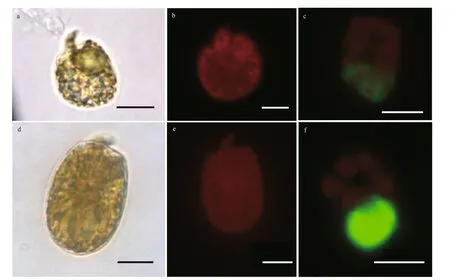
Fig.4 Light microscopy (LM) photographs of Amphidinium massartii (a–c) and Amphidinium operculatum (d–f)
Cells are 26.94–38.10 μm long (mean 34.05±2.31 μm,n=30) and 20.04–29.70 μm wide(mean 24.04±1.93 μm,n=30). The length/width(L/W) ratio is from 1.03 to 1.67 (mean 1.42±0.10,n=30). The cells are generally ovoid, and the epicone extends to the right with an approximate triangular structure. The antapex of the hypocone is rounder,while its right is almost vertical (Fig.3a). No pyrenoid is observed but numerous chloroplasts f ill the whole cell with a block distribution (Fig.3b). The nucleus is posterior (Fig.3c). The longitudinal f lagellum is located approximately a third of the way up the cell (Fig.3e). Irregular scales are scattered across the hypocone surface. The ventral ridge is long and narrow, which is about half the length of the cell,connecting the two f lagella insertion points (Fig.3e).
3.1.1.2AmphidiniummassartiiandAmphidiniumoperculatum(Fig.4; Supplementary Fig.S1 and Table S1)
Amphidiniummassartii(Fig.4a–c; Supplementary Fig.S1a–b): Cells are 16.44–21.92 μm long (mean 19.52±1.57 μm,n=30) and 10.31–18.08 μm wide(mean 15.34±1.72 μm,n=30). The ratio of L/W is from 1.21 to 1.59 (mean 1.29±0.20,n=30). Cell shape varies greatly from round to elliptical and the epicone extends to the left with a crescent structure (Fig.4a).No pyrenoid is observed. The nucleus is oval and locates at posterior (Fig.4c). The antapex of hypocone is ovoid generally while its right is almost vertical(Fig.4a; Supplementary Fig.S1a–b).
Amphidiniumoperculatum(Fig.4d–f;Supplementary Fig.S1c–d): Cells are 27.57–37.95 μm long (mean 31.89±2.66,n=30) and 20.04–29.70 μm wide (mean 18.73–26.39 μm,n=30). The ratio of L/W is from 1.24 to 1.63 (mean 1.43±0.09,n=30).Cell shape varies greatly from round to elliptical and the epicone extends to the right with an approximate triangular structure and the hypocone is relatively round and broad (Fig.4d; Supplementary Fig.S1c–d).Numerous chloroplasts are f ill the whole cell with a block distribution (Fig.4e). The nucleus is oval and located at posterior (Fig.4f).
3.1.1.3Cooliacanariensis,Cooliamalayensis,Cooliapalmyrensis, andCooliatropicalis(Fig.5;Supplementary Fig.S2 and Table S2)
Cooliacanariensis(Fig.5a–c; Supplementary Fig.S2a): Cells are round or oval in ventral view (Fig.5a;Supplementary Fig.S2a). They are 25.28–36.34 μm long (dorsal to ventral, mean 30.93±2.46 μm,n=30), 23.53–33.53 μm wide (transdiameter, mean 25.51±2.93 μm,n=30). The ratio of L/W is from 1.02 to 1.24 (mean 1.11±0.06;n=30). The chloroplast is granular and f ills the entire cell (Fig.5b). The elongated nucleus locates at the posterior (Fig.5c).
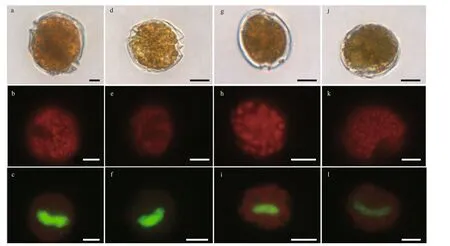
Fig.5 Light microscopy (LM) photographs of Coolia canariensis, Coolia malayensis, Coolia palmyrensis, and Coolia tropicalis
Cooliamalayensis(Fig.5d–f; Supplementary Fig.S2b): Cells are round or oval in ventral view (Fig.5d;Supplementary Fig.S2b). They are 20.21–33.79 μm long (dorsal to ventral, mean 25.56±2.87 μm,n=30), 17.47–26.90 μm wide (transdiameter, mean 22.38±2.36 μm,n=30). The ratio of L/W is from 1.01 to 1.30 (mean 1.14±0.08;n=30). The chloroplast is granular and f ills the entire cell (Fig.5e). A beanshaped nucleus locates at the right posterior end of cell. (Fig.5f).
Cooliapalmyrensis(Fig.5g–i; Supplementary Fig.S2c): Shape of cells is nearly spherical (Fig.5g;Supplementary Fig.S2c). They are 21.01–25.97 μm long (dorsal to ventral, mean 23.89±1.41 μm,n=30), 17.43–24.04 μm wide (transdiameter, mean 21.46±1.67 μm,n=30). The ratio of L/W is from 1.01 to 1.22 (mean 1.12±0.07;n=30). The granular chloroplast was f illed the entire cell (Fig.5h). A beanshaped nucleus locates at the right posterior end of cell (Fig.5i).
Cooliatropicalis(Fig.5j–l; Supplementary Fig.S2d): Shape of cells is nearly spherical (Fig.5j;Supplementary Fig.S2d). They are 28.74–40.84 μm long (dorsal to ventral, mean 34.82±3.08 μm,n=30), 25.34–35.71 μm wide (transdiameter, mean 32.07±3.33 μm,n=30). The ratio of L/W is from 1.02 to 1.19 (mean 1.09±0.05;n=30). The granular chloroplast are f ill the entire cell (Fig.5k). A U-shaped nucleus locates at the middle posterior end of cell(Fig.5l).
3.1.1.4Ostreopsiscf.ovata(Fig.6)
The cells of this species are compressed and tear-shaped in general (Fig.6a). In the ventral view,they are 32.55–42.87 μm long (dorsal to ventral,mean 36.04±2.72 μm,n=30), 22.30–32.95 μm wide(transdiameter, mean 25.51±2.93 μm,n=30). The L/W ratio ranges from 1.24 to 1.60 (1.42±0.09;n=30).An oval nucleus located at the posterior end of the cell (Fig.6c). Thecal plate pattern: Po, 3ʹ, 7ʹʹ, pyrenoid(arrowhead), 5ʹʹʹ, 2ʹʹʹʹ. The plate surface is smooth with scattered pores (Fig.6d–f). The pore plate (Po)is narrow and long in shape with a few pores and generally looks like a slit (Fig.6f). In the epitheca, it is hard to observe apical plate 2ʹ, which is very close to Po in the ventral view. Compared with apical plate 2ʹ, the other two apical plates are much larger. Apical plate 1ʹ is a broad hexagon. The f irst apical plate (1ʹ)is much longer and broader than the other two apical plates (Fig.6d). Additionally, apical plate 1ʹ occupies the middle in the ventral view. The hypotheca is made up of eight plates. Antapical plates 2ʹʹʹʹ are hexagonal and smaller than postcingular plates 2ʹʹʹ, 3ʹʹʹ, and 4ʹʹʹ(Fig.6e). All postcingular plates are about the same size, except postcingular plate 1ʹʹʹ. The ventral pore is not observed.
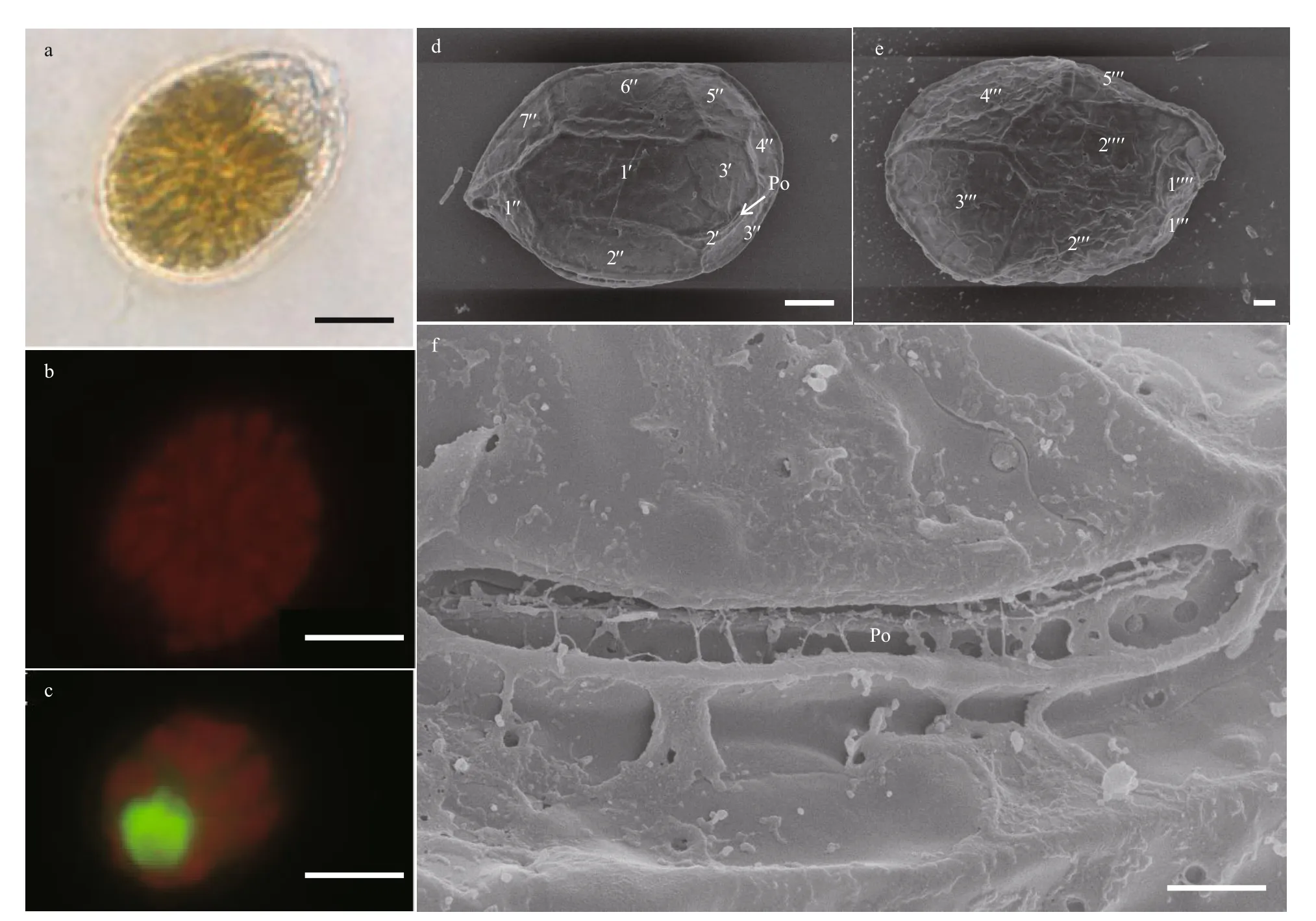
Fig.6 Light microscopy (LM) and scanning electron microscopy (SEM) photographs of Ostreopsis cf. ovata
3.1.1.5Prorocentrumhoff mannianum(Fig.7)
Cells ofP.hoff mannianumare oval and symmetrical (Fig.7a). They are 46.81–53.91 μm long(mean 50.52±1.60 μm,n=30) and 40.05–51.60 μm wide (mean 47.32±2.66 μm,n=30). The L/W ratio varies from 0.98 to 1.27 (mean 1.13±0.05,n=30). The pyrenoid is locates at the cell center, with abundant chloroplasts scattered all around the cell (Fig.7b).The nucleus is located at the posterior (Fig.7c).Kidney-shaped pores are scattered across the thecal surface, with no pore in the central area (Fig.7d–e).The number of thecal pores varies from 139 to 159 and 0.52–0.96 μm length, 0.23–0.42 μm. Numerous small and shallow depressions are observed in the thecal plate and the intercalary band is surrounded by marginal pores varies from 82 to 93 (Fig.7d).Additionally, there is a big depression in the plate center. Small round to ovoid pores were found within some depressions. The left thecal margin exhibited a f lared and f lattened curved apical collar that bordered the perif lagellar area (Fig.7e). An apical collar is just above the f lagellar region. The perif lagellar area is wide and V-shaped, with a large f lagellar pore (fp)and an accessory pore (ap) (Fig.7f). The plate patten is 1, 2, 3, 4, 5, 6, 7, and 8.
3.1.1.6Prorocentrumlima(Fig.8)
Cells ofP.limaare oval, slightly thin, and symmetrical (Fig.8a). They are 34.39–41.17 μm long(mean 38.27±1.68 μm,n=50) and 21.36–29.65 μm wide (mean 30.29±1.69 μm,n=50) with a L/W ratio varies from 1.34 to 1.67 (mean 1.45±0.07,n=50).The pyrenoid is observed in cell center with a starch ring (Fig.8a). Numerous chloroplasts f ill the whole cell (Fig.8b). The thecal plate is smooth, and round or kidney-shaped pores are dispersed across the surface (Fig.8d–f). Approximately 56–71 pores are observed, but no pore is observed in the center of the plate (Fig.8d). The nucleus is oval and locates at the posterior (Fig.8c). The intercalary bands are surrounded by a series of 44–56 neatly arranged pores(Fig.8d–e). The perif lagellar area is wide V-shaped.Eight platelets and two large pores are observed(Fig.8f).
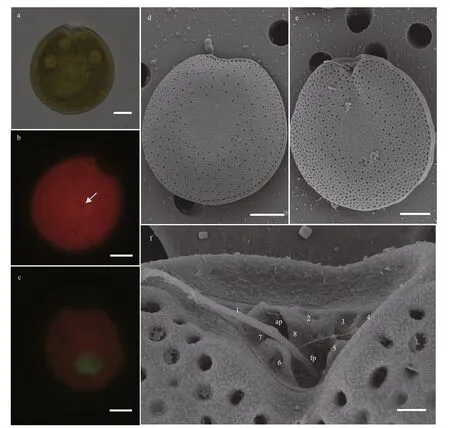
Fig.7 Light microscopy (LM) and scanning electron microscopy (SEM) photographs of Prorocentrum hoff mannianum
3.1.1.7Prorocentrumconcavum,Prorocentrumemarginatum, andProrocentrumrhathymum(Fig.9;Supplementary Fig.S3 and Table S3)
Prorocentrumconcavum(Fig.9a–c; Supplementary Fig.S3a): cells ofProrocentrumconcavumare oval and symmetric (Fig.9a; Supplementary Fig.S3a). They are 43.77–49.29 μm long (mean 46.7±1.67 μm,n=30)and 35.80–44.70 μm wide (mean 41.52±2.17 μm,n=30). The ratio of L/W is from 1.04 to 1.27 (mean 1.13±0.05,n=30). A pyrenoid could be brightly observed in the center of cell, which be rounded by a starch ring (Fig.9a). Numerous chloroplasts f ill the whole cell (Fig.9b). Nucleus locates at the posterior of the cell (Fig.9c).
Prorocentrumemarginatum(Fig.9d–f;Supplementary Fig.S3b): cells ofProrocentrumemarginatumare broad, oval and asymmetric (Fig.9d;Supplementary Fig.S3b). They are 28.56–40.56 μm long (mean 35.36±2.63 μm,n=30) and 20.67–33.77 μm wide (mean 27.63±3.22 μm,n=30). The ratio of L/W is from 1.07 to 1.73 (mean 1.29±0.16,n=30). No pyrenoid is observed, while numerous chloroplasts f ill the whole cell (Fig.9e). The nucleus is oval and locates at the posterior of the cell (Fig.9f).
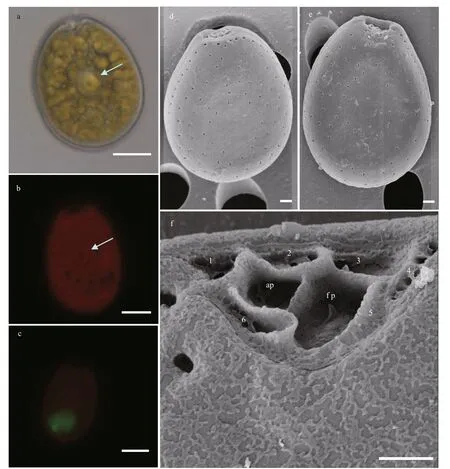
Fig.8 Light microscopy (LM) and scanning electron microscopy (SEM) photographs of Prorocentrum lima
Prorocentrumrhathymum(Fig.9g–i;Supplementary Fig.S3c): cells of this species are oval and asymmetric (Fig.9g; Supplementary Fig.S3c). They are 27.79–36.86 μm long (mean 31.73±2.23 μm,n=30) and 19.22–26.43 μm wide(mean 22.55±1.98 μm,n=30). The ratio of L/W is from 1.25 to1.73 (mean 1.41±0.11,n=30). A pyrenoid could be observed in the center of cell, which be rounded by a starch ring (Fig.9g). Numerous chloroplasts f ill the whole cell (Fig.9h). The nucleus is oval and located at the posterior of the cell (Fig.9i).
3.1.2 Phylogenetic analysis
3.1.2.1Amphidinium
In the present study seven strains, comprising four species, were used for phylogenetic analysis of theAmphidiniumgenus with related species sequences(Fig.10). ThreeA.massartiistrains isolated from this study were grouped together and they formed a sister clade to the Brazilian strain Am_Cub_1. TheA.carteraestrain ZS130 diff ered from the Chinese strain 3WZD8, Australian strain CS740, and New Zealand strain CAWD57.Amphidiniummagnumwas clustered with three other Bahamian strains with maximal support. TwoA.operculatumstrains were used for phylogenetic analysis. Strain ZS701 diff ered from strain WZD225, all of which were isolated from the South China Sea. Strain ZS716 was clustered with the Brazilian strain Ao-Rec-1 and Australian strain K_0663.
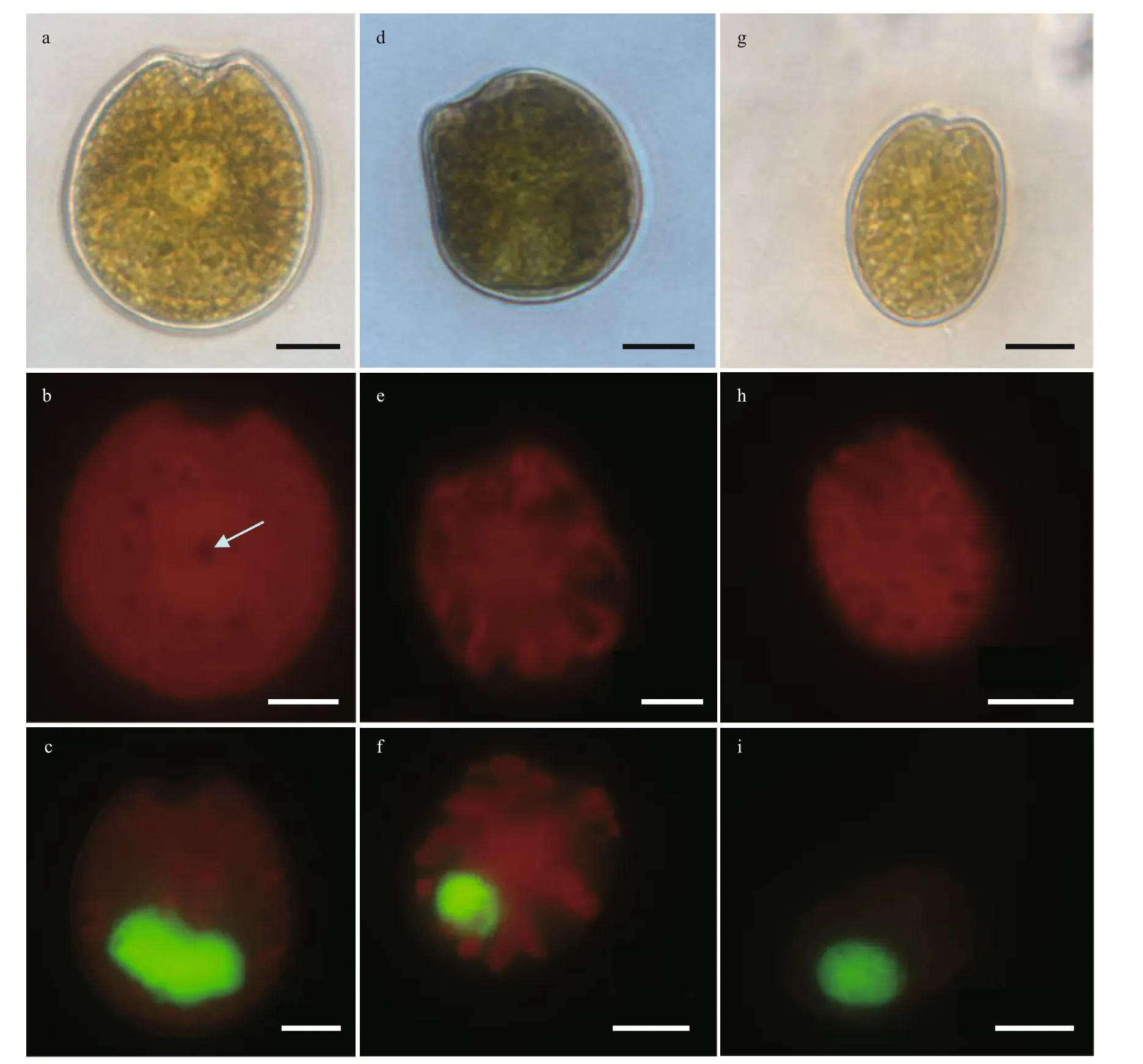
Fig.9 Light microscopy (LM) photographs of Prorocentrum concavum, Prorocentrum emarginatum, and Prorocentrum rhathymum
3.1.2.2Coolia
In the present study, a total of eight strains,consisting of four species, were used for phylogenetic analysis of theCooliagenus with related species sequences (Fig.11). TheC.malayensisstrains ZS719 and ZS733 were grouped with two Hong Kong strains SKLMP_W059 and SKLMP_W072, and the USA strain CCMP1345 with a 53 Bootstrap value.In addition,C.palmyensisstrain ZS601 clustered together with the northeast Atlantic strain Cp1412_1 and two Hong Kong strains, SKLMP_S017 and SKLMP_W085. ThreeC.canariensisstrains acquired in the present study clustered together and shared identical sequences with the Hainan Island strain D1C2. TwoC.tropicalstrains, ZS602 and ZS619,grouped together with strain XS554 isolated from the Xisha Islands, South China Sea.
3.1.2.3Ostreopsis
FiveOstreopsissequences belonging to this study were aligned with related species sequences from GenBank. The phylogenetic relationship is shown in Fig.12. TheO. cf.ovataclade contained three groups, one consisted of some strains isolated from the western Pacif ic (South China Sea and Gulf of Thailand) and Indonesia. A Brazil and two Portugal(East Atlantic) strains were formed the Atlantic group. The remaining strains isolated from the Mediterranean Sea (Spain, Greece, Italy, Tunisia, and Manoca) and West Atlantic area (Brazil) clustered together as the Mediterranean/West Atlantic group.AllO. cf.ovatastrains acquired in the present study grouped together with other strains from the western Pacif ic.
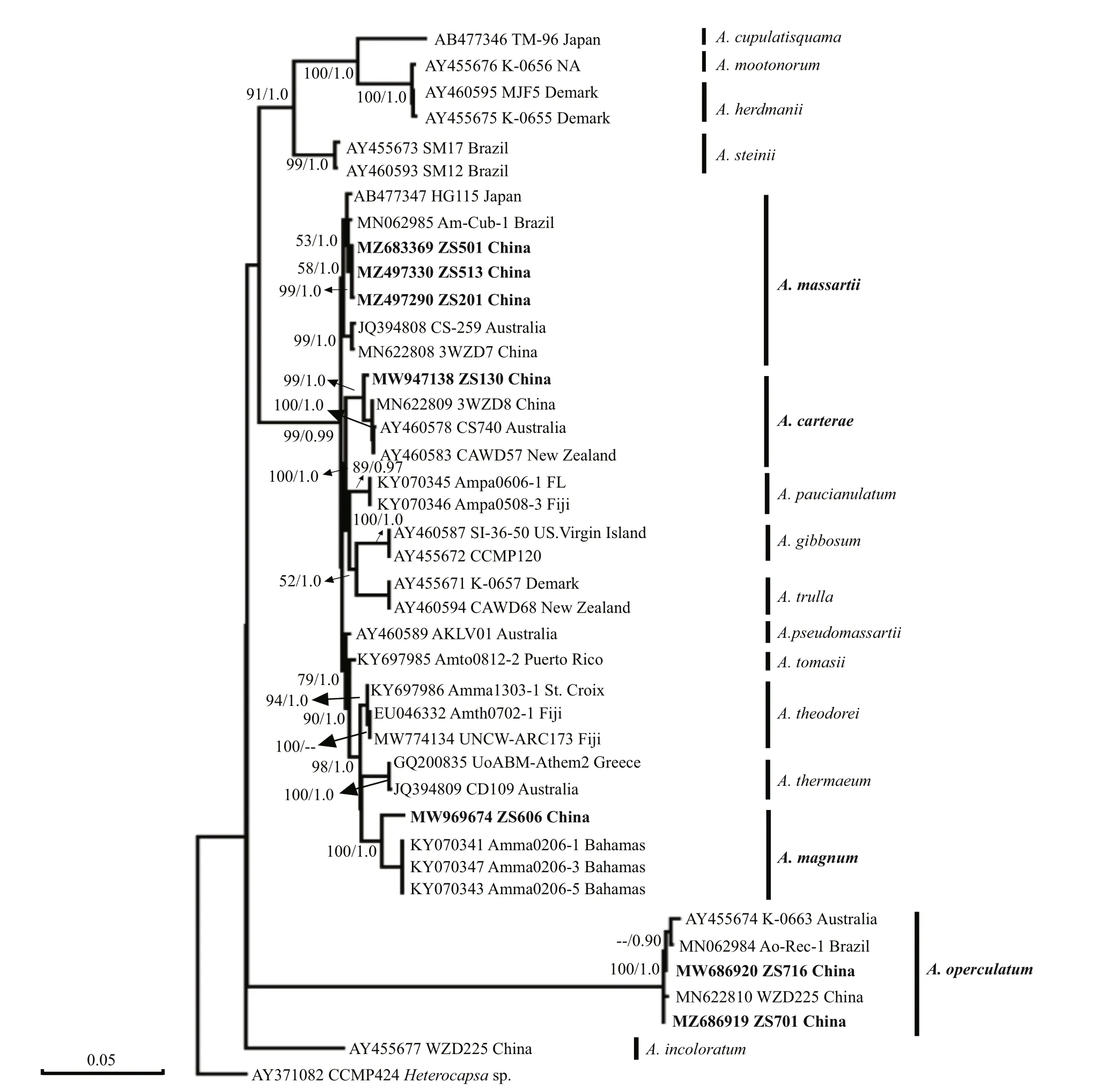
Fig.10 Phylogenetic tree of partial LSU rDNA (D1–D3) sequences of Amphidinium based on the maximum likelihood (ML)and Bayesian inference (BI)
3.1.2.4Prorocentrum
In this study, 14 strains belonging to fourProrocentrumspecies, were used for phylogenetic analysis of theProrocentrumgenus with related species sequences (Fig.13). There was one point for which the D1–D3 region sequence ofP.concavumwas not obtained. Based on the phylogenetic tree, theP.limacomplex separated into four subclades, which roughly showed diff erent geographical distributions.Nine strains belonging toP.limawere isolated from this study.Prorocentrumlimastrain ZS102 was grouped together with other Chinese strains,andP.limastrain ZS516 was grouped together with strain TIO115a and strain S4, isolated from Guangxi,China, and Australia, respectively. Another sevenP.limastrains were clustered with strain NMN07,and they were a sister clade of a Martinique strain.TwoP.hoff mannianumstrains isolated from this study were the sister clade of the La Réunion strain PBMA_01.Prorocentrumrhathymumstrain ZS405 clustered with the Xisha Islands strain XS501.TheP. cf.sculptilestrain ZS615 formed a clade and clustered with two Martinique strains.P. cf.emarginatumstrain ZS251 was the sister clade of La Réunion strain PERN06. Besides, strain ZS251 grouped with three other strains, two Xisha Islands strains, and one was isolated from the USA.
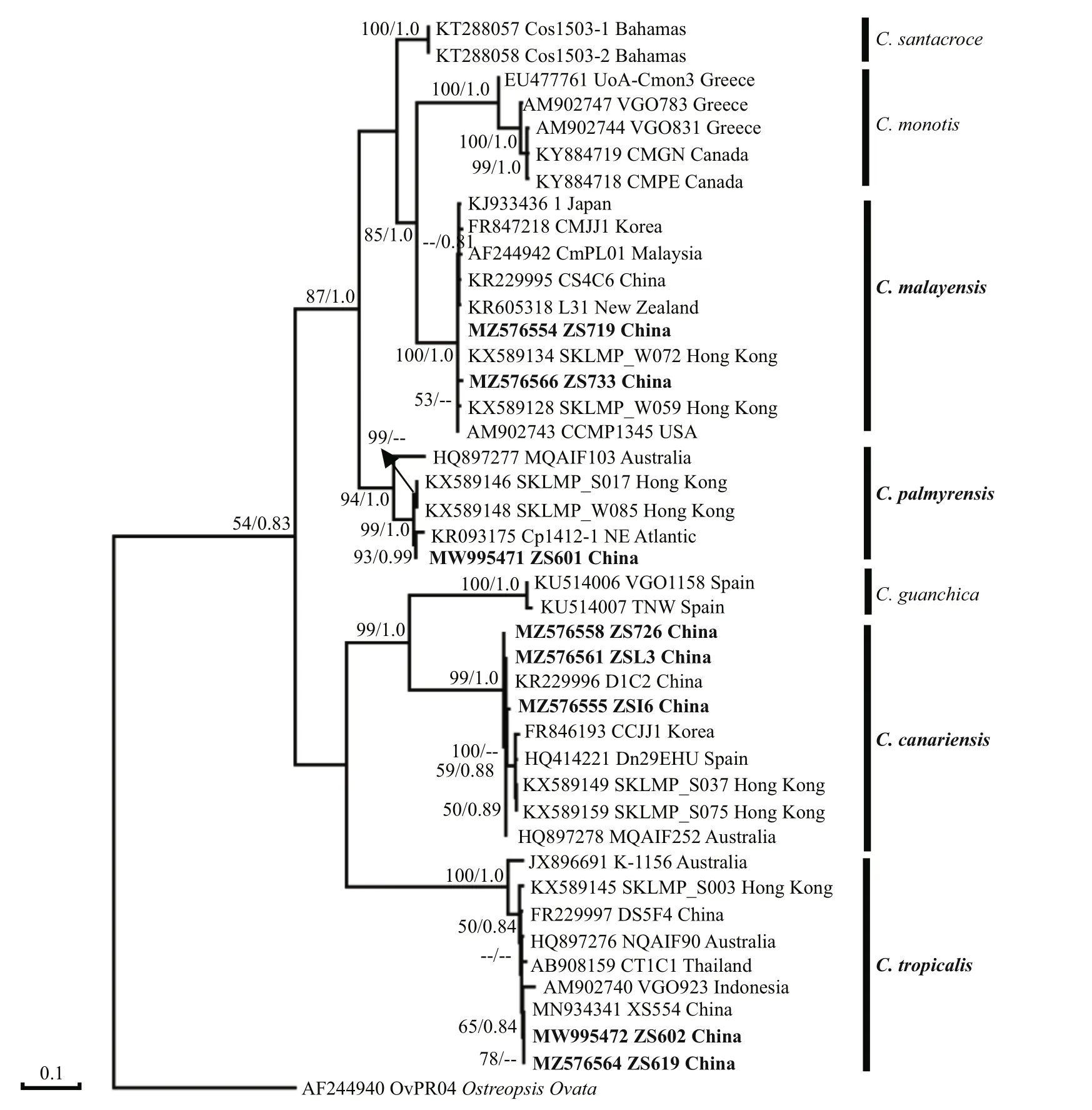
Fig.11 Phylogenetic tree of partial LSU rDNA (D1–D3) sequences of Coolia based on the maximum likelihood (ML) and Bayesian inference (BI)
3.2 Distribution of benthic dinof lagellates
3.2.1 Spatial distribution of benthic dinof lagellates
At Oliver Shoal (15 m), Hand Shoal (30 m), and Shenhu Shoal (17 m), no macroalgal sample was collected, so all species at those sites were isolated from coral sand (Table 1). Hand Shoal was located at the middle of Zhongsha Great Atoll, which had a deepest sampled depth of 30 m and only 3 species were isolated from coral sand at this Shoal. Additionally, 8 species were only identif ied fromPadinaaustralisat Magpie Shoal (20 m) (Table 2). At south Zhongsha Great Atoll, 10 benthic dinof lagellates species were identif ied from all types of samples at Addington Patch,which had a medium sampled depth of 20 m. Also,7 species were identif ied from coral sand orPadinaaustralisat Cawston Shoal with a middle sampled depth of 23 m (Table 2).AmphidiniumcarteraeandA.magnumwere only isolated from macroalgaePadinaaustralisat Magpie Shoal and coral sand at Oliver Shoal, respectively, andA.massartiiappeared at half of the Shoals, whatever sediment or all macroalgae (Table 2). Except Oliver Shoal and Hand Shoal,A.operculatumwas found at all shoals from coral sand,Padinaaustralis, andHalimedasp. In Shenhu Shoal,C.canariensisandC.malayensiswere present.C.palmyrensisandC.tropicalisonly isolated from coral sand andPadinaaustralisat Addington Patch (Table 2). Additionally, except Oliver Shoal,O. cf.ovataappeared at all Shoal.Prorocentrumcf.sculptileappeared at half of the shoals, including Oliver Shoal, east of Addington Patch, Magpie Shoal,and Yitong Shoal. This species was found in all types of samples (sediment or macroalgae). In addition,P.concavumwas only identif ied from coral sand at Hand Shoal with a 30-m sampled depth. At all shoals,Prorocentrumlimawas found in coral sand,Amphiroafragiroa,Padinaaustralis, andHalimedasp. (Table 2). However,P.hoff mannianumonly appeared in coral sand andPadinaaustralisat Oliver Shoal(Table 2). Only identif ied from coral sand,Padina australis, andHalimedasp.,P.rhathymumwere found at Magpie Shoal, Cawston Shoal, and Yitong Shoal (Table 2). Furthermore, although classif ication data forGambierdiscuswere not obtained in this study,Gambierdiscusspp. appeared at all shoals, no matter coral sand or other macroalgae except Oliver and Cawston Shoal (Table 2). In short, Benthic dinof lagellates are most diversely represented on south of Zhongsha Great Atoll and Yitong Shoal, as well as macroalgae. Additionally, more species were identif ied from macroalgae samplesPadinaaustralis.
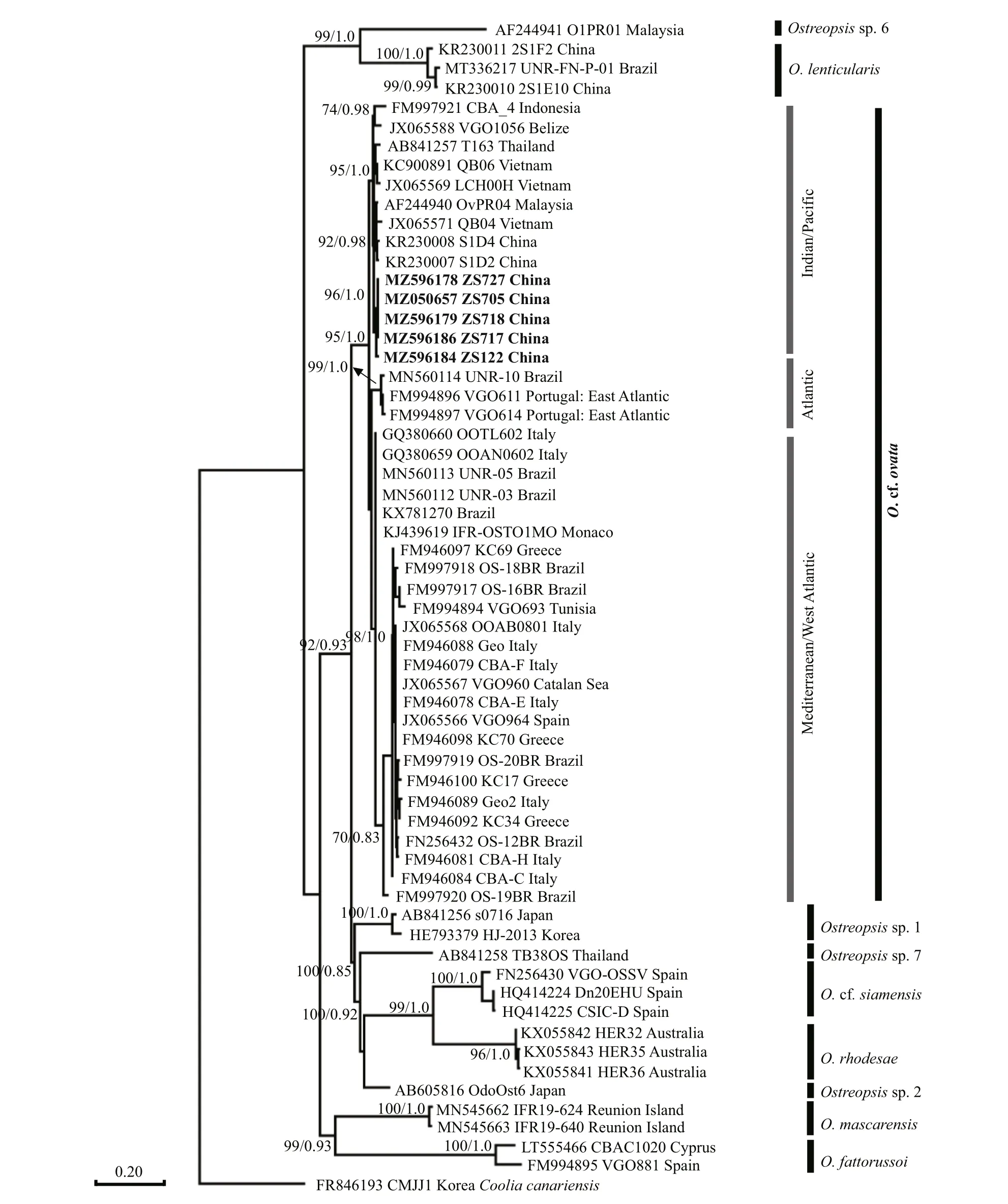
Fig.12 Phylogenetic tree of partial LSU rDNA (D1–D3) sequences of Ostreopsis based on the maximum likelihood (ML) and Bayesian inference (BI)
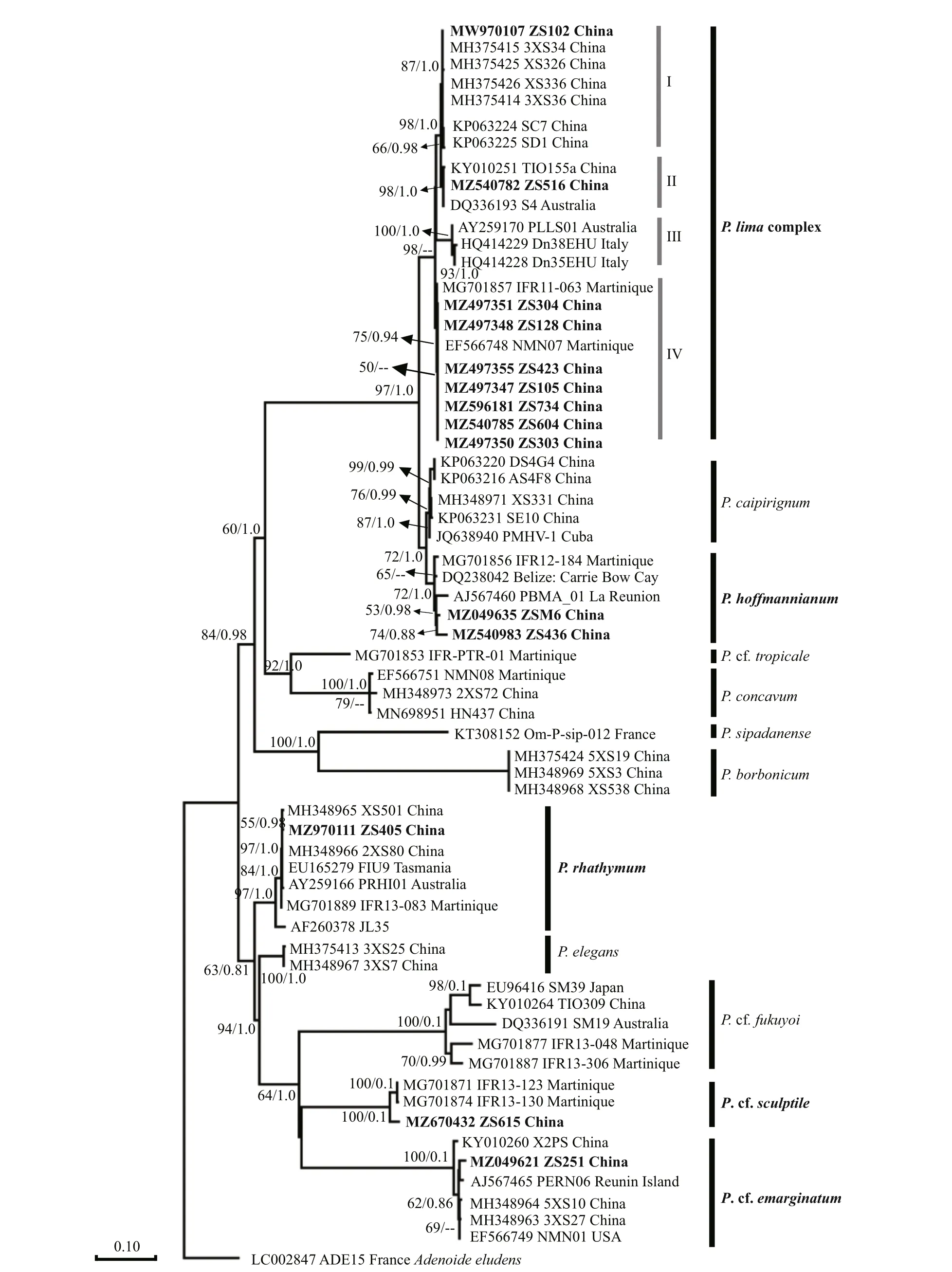
Fig.13 Phylogenetic tree of partial LSU rDNA (D1–D3) sequences of Prorocentrum based on the maximum likelihood (ML)and Bayesian inference (BI)
3.2.2 Quantitative distribution of benthic dinof lagellates
The abundance of benthic dinof lagellates in the sediment was between 88 and 4 345 cells/100 cm2(Fig.14). Except for Shenhu Shoal and west of Addington Patch, the genusProrocentrumwas dominant at all sites. The lowest cell number averaged 88 cells/100 cm2was at Oliver Shoal, which has the lowest sampled depth of 15 m, while the maximum cell abundance averaged 88 cells/100 cm2was found at Cawston Shoal with a 23-m sampled depth (Fig.14).The two sites were located at the northernmost and southernmost part of the Zhongsha Great Atoll(Fig.14a). OnlyProrocentrumandOstreopsisspecieswere found at Oliver Shoal, with an average of 82 cells/100 cm2and 5 cells/100 cm2, respectively.At Hand Shoal with the deepest sampled depth, a cell abundance of averaged 334 cells/100 cm2was collected andCooliaspecies were absent in samples collected (Fig.14a). At southern Zhongsha Great Atoll,Amphidiniumwas dominant, with cell abundance averaging 54 cells/100 cm2at west of Addington Patch. However, at east of Addington Patch, cell abundance was as high as 1 815 cells/100 cm2, which was more than 10 times that west of Addington Patch (142 cells/100 cm2), although their sampling depths are very close (20 m and 19 m, respectively).Yitong Shoal (15 m) and Shenhu Shoal (17 m) had a lower sampled depth and there were similar levels of cell abundance of benthic dinof lagellates were recorded, respectively averaging 642 cells/100 cm2and 534 cells/100 cm2(Fig.14b). Compared with that at other shoals, the maximum cell abundance ofCooliawas found at Shenhu Shoal, with an average of 408 cells/100 cm2, representing approximately 76.4%of all benthic dinof lagellates (Fig.14b).Gambierdiscusspecies were not observed in samples from Cawston Shoal, or Shenhu Shoal (Fig.14a–b). As the dominant genus,Prorocentrumoccupied more than 50% of the total abundance of benthic dinof lagellates at all shoals, except west of Addington Patch, where they comprised only 6.8%. Diff erent distribution patterns in the abundance of benthic dinof lagellates were observed on the east and west sides of Addington Patch. The benthic dinof lagellates abundance near the outside of Zhongsha Great Atoll was higher,while that of the inside was much lower. At the same time, the abundance in the south of Zhongsha Great Atoll was higher than that in the northern (Fig.14a).In addition, the relationship between depth and abundance was not very close, but the abundance at a medium depth of 20 m was much higher (averaged 1 815 cells/100 cm2at East of Addington Patch and averaged 4 345 cells/100 cm2at Cawston Shoal).
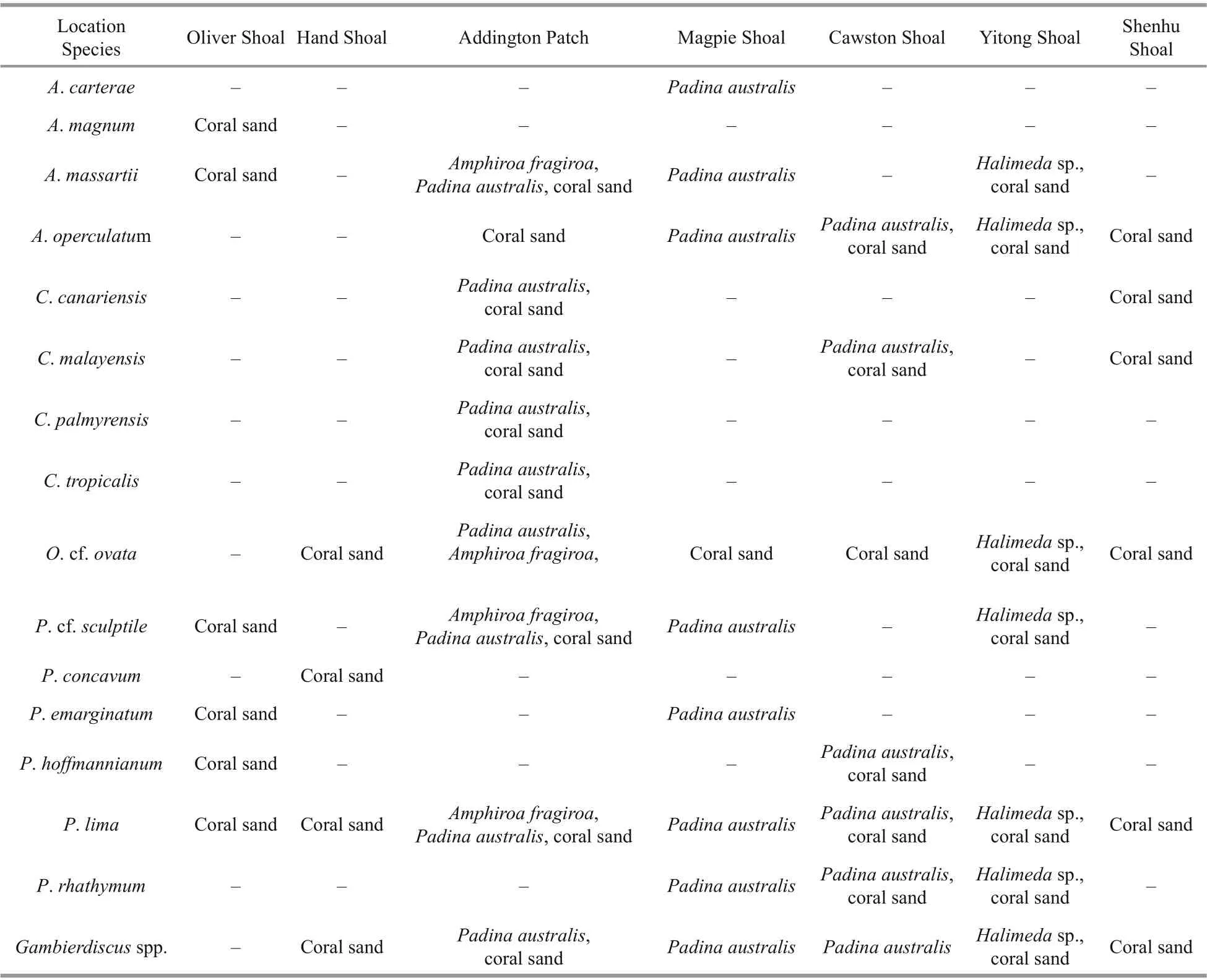
Table 2 Distribution of benthic dinof lagellate species in diff erent shoals
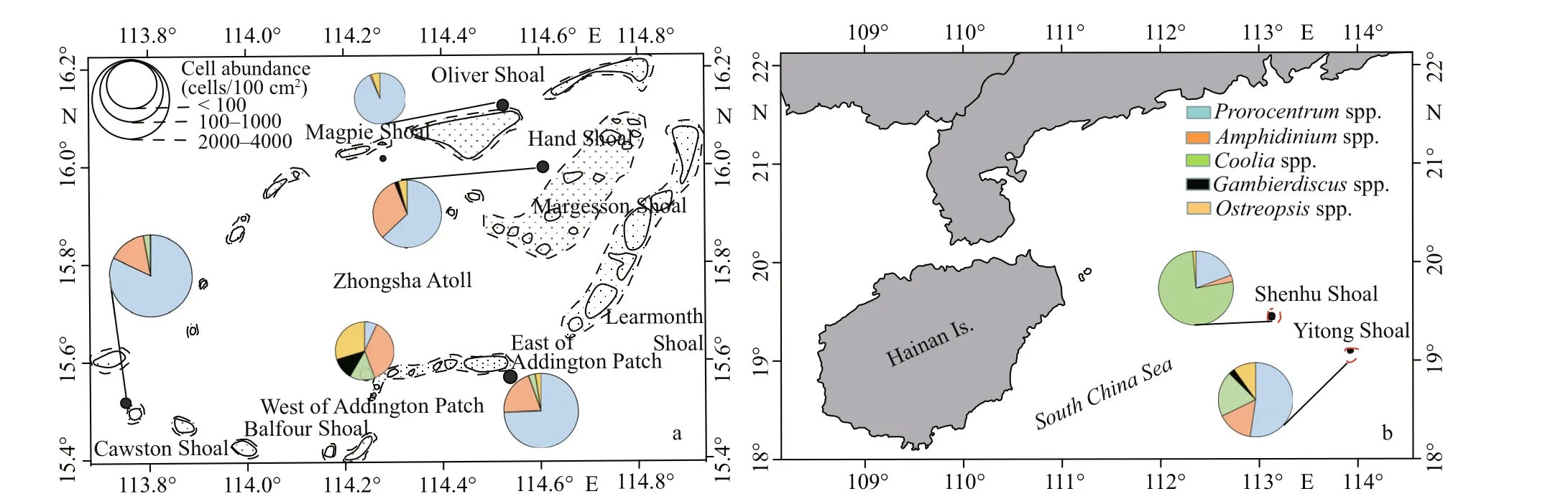
Fig.14 The cell abundances (cells/100 cm 2) of f ive benthic dinof lagellates on sediment in Zhongsha Great Atoll (a), Yitong Shoal, and Shenhu Shoal (b)
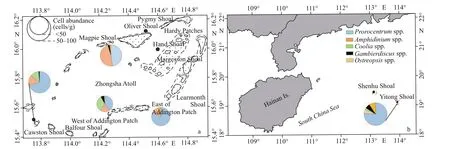
Fig.15 Cell abundances (cells/g) of f ive benthic dinof lagellates on macroalgae in Zhongsha Great Atoll (a), Yitong Shoal,and Shenhu Shoal (b)
Since only three kinds of macroalgae (Padinaaustralis,Halimedasp., andAmphiroafragiroa)were collected from all sites, macroalgal preference is not discussed in this study. The abundance of benthic dinof lagellates on macroalgae was between 10 and 91 cells/g andProrocentrumwas dominant on macroalgae at all sites (Fig.15). The northernmost site of Zhongsha Great Atoll, Magpie Shoal, had a total abundance of benthic dinof lagellates averaged 57 cells/g onPadinaaustraliswith a sampled depth of 20 m. However, the abundance ofGambierdiscusandOstreopsiswas as low as 1 cell/g (Fig.15a).At west of Addington Patch, which had a sampled depth of 19 m, benthic dinof lagellates cell abundance averaged 49 cells/g onPadinaaustralis, the diff erence was thatCooliacomprised 33.1% of total abundance.At east of Addington Patch, which had the same depth as Magpie Shoal, the cell abundance of benthic dinof lagellates was lowest onAmphiroafragiroa,averaging 10 cells/g, which was signif icantly diff erent from that at other sampling sites (Fig.15a).Additionally, noGambierdiscusspp. orCooliaspp.were observed onAmphiroafragiroa. At Yitong Shoal, with the lowest sampled depth of 15 m, the highest abundance of benthic dinof lagellates was found onHalimedasp., with an average of 91 cells/g(Fig.15a). In addition, all f ive benthic dinof lagellate genera were recorded from macroalgal samples.The benthic dinof lagellates abundance of Yitong Shoal was higher than that of Zhongsha Great Atoll because of its low water depth (Fig.15a–b).At north Zhongsha Great Atoll, Addington Patch had the lowest abundance. On macroalgae samples,the highest abundance was observed atHalimedasp. (91 cells/g at Yitong Shoal), while the lowest abundance appeared atAmphiroafragiroa(10 cells/g at east of Addington Patch).
4 DISCUSSION
4.1 Biodiversity of benthic dinof lagellates in Zhongsha Islands
4.1.1Amphidinum
Amphidiniummagnumidentif ied in Zhongsha Islands is the f irst record in Chinese waters. It is also the f irst report in addition to type species locality, Grand Bahama Beach, Bahamas (Karafas et al., 2017). Its morphological characteristics were consistent with those originally described. A long and narrow ventral ridge was clearly observable, but the pusule was absent in our strain. Scales were visible on the hypocone surface, which were absent in the strain from the type locality.
The phylogenetic tree based on the LSU region showed that the species isolated from Zhongsha Islands can be divided into two clades, one clade consisting ofA.massartiiandA.carterae, both of which are smaller thanA.operculatum, which formed a clade on its own, with 100% bootstrap support in the phylogeny.Amphidiniummagnumgrouped together with the three type locality strains, with maximum support. Additionally, the position ofA.magnumwas close to that ofA. cf.thermaeumandA.theodori,which is consistent with the results of Karafas et al.(2017).
4.1.2Coolia
Morphological characteristics (size, plate arrangement, and shape) of all fourCooliaspecies, includingC.canariensis,C.malayensis,C.palmyrensis, andC.tropicalis, identif ied from Zhongsha Islands, were generally similar to that of the type species descriptions. The four species were reported previously from Hong Kong and Hainan Island in China (Leung et al., 2017; Zhang et al.,2021).
The phylogenetic tree ofCooliashowed that theC.malayensisclosely related to the Hong Kong strains and New Zealand strain, which was consistent with that conducted by Zhang et al. (2021) in Hainan Island. The results reveal that allC.malayensisstrains isolated from Chinese waters have a common ancestor. Strains ofC.palmyensisfrom Zhongsha Islands and from NE Atlantic were a sister clade of Hong Kong strain, which suggested that there were few diff erences between Zhongsha Islands strain and Hong Kong strain in phylogeny (Leung et al., 2017).Two strains ofC.tropicalisolated from Zhongsha Islands grouped together with Xisha Islands, which also showed that there are diff erences among strains even they are geographically adjacent.
4.1.3Ostreopsis
Morphologically, it is diffi cult to distinguish the various species ofOstreopsis, which are similar to the type speciesO.siamensis. The cell size ofO. cf.ovatastrain from the Zhongsha Islands was 32.55–42.87 μm long (dorsal to ventral,mean 36.04±2.72 μm), 22.30–32.95 μm wide(transdiameter, mean 25.51±2.93 μm), which is signif icantly smaller than that ofO.siamensis(dorsoventral 60–100 μm; transdiameter 45–90 μm). In addition, the morphological characteristics of the Zhongsha Islands strain (ZS718) in this article were generally consistent with those of strains isolated by Fukuyo (1981), which were generally tear-shaped with a L/W ratio of 1.5–2.0 (Fig.6). In original description, a large antapical plate ofO. cf.ovatawas designated as postcingular intercalary plate (1p) (Fukuyo, 1981), now it is antapical plate(2ʹʹʹʹ). Compared with the original description, our plate pattern (Po, 3ʹ, 7ʹʹ, ?C, ?S, 5ʹʹʹ, 2ʹʹʹʹ) has been reported in recent literatures (Chomérat et al., 2020;Nascimento et al., 2020).
Penna et al. (2010) revealed thatO. cf.ovatacould phylogenetically divide into three clades, including Atlantic/Mediterranean/Pacif ic clade, Indian/Pacif ic clade, and Atlantic/Indian/Pacif ic clade. AllO. cf.ovatastrains from this study were clustered together to form an Indian/Pacif ic clade. The Indian/Pacif ic clade included all strains collected from the South China Sea (including the newly discovered Zhongsha Islands strain, Vietnamese strain, and Malaysian strain) and adjacent waters (Thai strain and Indonesian strain). The consistency in genetic characteristics and geographic distribution may be because of the similarity in ecosystems and the inf luence of ocean circulation. The islands and land surrounding the South China Sea make it a marginal sea, and the unique genetic characteristics ofO. cf.ovatasuggest that it is genetically separated from the Mediterranean/Indian OceanO. cf.ovatastrain. Thus,geographical isolation has had a strong inf luence on phylogenetic divergence.
4.1.4Prorocentrum
A number of studies have conf irmed thatP.limashows genetic diversity and phenotypic plasticity(Hoppenrath et al., 2013; Zhang et al., 2015). Similar toO. cf.ovata,P.limais also a cosmopolitan distributed species. Based on morphology and phylogeny constructed with LSU and internal transcribed spacer (ITS) regions, of strains ofP.limafrom Hainan Island, China were classif ied as f ive morphotypes (Zhang et al., 2015). Then Nascimento et al. (2017) def ined morphotype 4 ofP.limaas a new species,P.caipirignum. A study also discovered two morphological types (morphotypes 1 and 2) ofP.limain the Caribbean (Chomérat et al., 2019).The results showed that these two types had obvious morphological diff erences. Morphotype 2 was oblong oval, the cell length and width were 37.1–38.2 μm and 27.8–32.3 μm, respectively (L/W ratio was 1.18–1.36). The morphology and cell measurements of aP.limastrain (ZS102) from Zhongsha Islands was in accordance with the morphotype 2 in the Caribbean.The cell ofP.hoff mannianumstrain from Zhongsha Islands was broader than that of the type species,but it was similar to that ofP.belizeanum(Faust,1990). Herrera-Sepúlveda et al. (2015) summarized thatP.hoff mannianumandP.belizeanumhave some micromorphological similarities (cell size, surface ornamentation, and perif lagellar area architecture) and molecular data lack suffi cient evidence to distinguish these two species. Therefore, they are considered to be conspecif ics.
On the base of phylogenetic tree of LSU rDNA(D1–D3 region), we also identif ied our strains(ZS102 and ZS516) from Zhongsha Islands belonged toP.limamorphotype 2. Furthermore, theP.limacomplex could be clearly distinguished from the similar speciesP.caipirignumand had high genetic diversity, based on phylogenetic analysis of D1–D3 region. This same result is observed in previous studies (Zhang et al., 2015; Chomérat et al., 2019).Normally, the same geographical origin have similar phylogenies. However, theP.limacomplex in this study is divided into three groups. The f irst group has a neighboring geographical origin, the second is distributed in the northern and southern hemispheres, and the third strains are geographically separated from each other. This result suggests thatP.limain the same area also has diff erent genetic characteristics.
Hoppenrath et al. (2013) summarized thatP.belizeanum,P.hoff mannianum, andP.maculosumwere highly similar in morphology with small genetic diff erences. But their toxin-producing characteristics were diff erent, which blurred the boundaries between these species. However, with the application of more precise molecular techniques, these species are all attributed to theP.hoff mannianumspecies complex.In this study, the Zhongsha Islands strains ofP.hoff mannianumwere a sister clade of a La Réunion strain, which was once def ined asP.belizeanum.Therefore, we further demonstrate that ourP.hoff mannianumstrains are closer toP.belizeanum,which is consistent with the morphological results. However, these were our subclades ofP.hoff mannianumand these were directly related to geographic locations, indicating that geographical isolation has had a considerable inf luence on phylogenetic divergence. Therefore, another marker is needed to identifyP.hoff mannianumspecies more accurately.
4.2 Distribution of benthic dinof lagellates from Zhongsha Islands
4.2.1 Geographical distribution of benthic dinof lagellates There are approximately 200 species ofAmphidinium, both freshwater and seawater (Guiry and Guiry, 2022). Many species were heterotrophic(Taylor, 1971; Calado et al., 1998). Most ofAmphidiniumspecies identif ied in Zhongsha Islands were widespread and commonly reported in tropical and subtropical waters, as well as in temperate higher latitude regions (Murray and Patterson, 2002; Lee et al., 2013; Jiang, 2019; Luo et al., 2022).
Leaw et al. (2016) provided a new scenario for the phylogeographic history ofCooliaand it was speculated that the divergence of the two species,C.malayensisandC.monotis, may be due to geographic segmentation caused by vicariant events.FourCooliaspecies,C.canariensis,C.malayensis,C.palmyrensis, andC.tropicalisidentif ied from the Zhongsha Islands, were widely distributed in the western Pacif ic (C.tropicalisandC.canariensisin Vietnam,C.canariensisandC.malayensisin the Korean Sea,C.malayensis,C.palmyrensis, andC.tropicalisin Australia) (Jeong et al., 2012; Ho and Nguyen, 2014; Larsson et al., 2019). Aside from the western Pacif ic,C.malayensiswas also found in Florida, Belize, and the Cook Islands (Leaw et al., 2016).Cooliapalmyrensishas been found in the Caribbean Sea (Karafas et al., 2015) and western Atlantic Ocean (De Azevedo Tibiriçá et al., 2020).Cooliatropicaliswas f irst isolated from an Atlantic barrier reef ecosystem (Faust, 1995) and has then been expanded to Brazil (Nascimento et al., 2019)and many areas of the western Pacif ic (Larsson et al.,2019; Zhang et al., 2021).
The globally distributed speciesO. cf.ovatahas been reported in the Caribbean Sea, Mediterranean Sea, Atlantic, Indian, and Pacif ic Oceans (Monti et al., 2007; Mangialajo et al., 2008; Rhodes, 2011;Kang et al., 2013; Zhang et al., 2018). Parsons et al. (2012) summarized the global distribution ofOstreopsisand found thatOstreospsisspecies were also widely present in the western and southern Pacif ic, as well as western and northern Atlantic,except for the Mediterranean and Caribbean regions where the species was widespread. The genetic analyses indicated thatO. cf.ovatawere divided into three geographical areas: Atlantic/Mediterranean/Pacif ic, Atlantic/Indian/Pacif ic, and Pacif ic (Penna et al., 2010; Mangialajo et al., 2011; Sato et al., 2011).Ostreopsisspecies were commonly observed in Caribbean and Western Atlantic region such as Florida Keys (Accoroni et al., 2020), Brazil (Nascimento et al., 2020), and Bezile (Faust, 1995). Zhang et al.(2018) reportedO. cf.ovataandO.lenticularisfrom Hainan Island, South China Sea. Zheng et al. (2017)found that theO. cf.ovataisolated in Beibu Bay,southern China, were contain higher concentrations of ovatoxins-c (OVTX-c) and OVTX-d/e, as well as small amounts of OVTX-g and OVTX-f. So far there were only twoOstreopsisspecies have been reported in tropical waters of the southern China.
Prorocentrumas the dominant genus is a consistent feature of tropical waters (Mohammad-Noor et al.,2005; Parsons and Preskitt, 2007).Prorocentrumlimais a widely distributedProrocentrumspecies, which is often observed in water column, on macroalgal surfaces and sediments (Fukuyo, 1981; Faust, 1990;Aligizaki et al., 2008).Prorocentrumhoff mannianumwas f irst isolated in Belize (Faust, 1990). Subsequently,P.hoff mannianum,P.belizeanum, andP.maculosumwere revised as aP.hoff mannianumspecies complex(Herrera-Sepúlveda et al., 2015; Rodríguez et al.,2018).P.hoff mannianumspecies complex were mainly tropical and subtropical distribution, such as the Mediterranean (Gómez, 2003), Australia,New Zealand (Rhodes and Smith, 2019), and North America (Torres-Ariño et al., 2019).Prorocentrumconcavumwas widely distributed in the water column and reef ecosystems from tropical to high latitude temperate zones (Faust, 1994; Luo et al., 2017).
4.2.2 Quantitative distribution of benthic dinof lagellates
Although the Zhongsha Islands are called an archipelago, its main body, Zhongsha Great Atoll, is actually a series of reefs located below sea surface, far from human activities in water depths of approximately 20–40 m. The habitat is mainly coral reef ecosystems,which are greatly aff ected by ocean water dynamics such as turbulence. In this study, the environment in which benthic dinof lagellates grow is characterized by weak light intensity, low temperature relative to the intertidal zone, and low nutrient concentrations.
Benthic dinof lagellates are generally epiphyte on the seaf loor matrix (sand, silt, or debris shell) or on the surface of macrophytes, and are less abundant in water column unless bloom occurs (Fukuyo, 1981;Boisnoir et al., 2019a).Prorocentrumwas dominant on coral sand and three macroalgaes (Padinaaustralis,Halimedasp., andAmphiroafragiroa) except for those to the west of Addington Patch and Shenhu Shoal. A similar phenomenon thatProrocentrumwas the f irst dominant genus has also observed in previous researches (Richlen and Lobel, 2011;Widiarti and Anggrain, 2012; Accoroni et al., 2020).Interestingly, our results indicate thatOstreopsiswas the least abundant group in the Zhongsha Islands.The maximum density ofOstreopsiswas appeared at Yitong Shoal with averaged 64 cells/100 cm2on coral sand. Lee et al. (2020) studied the eff ect of substratum and depth on benthic dinof lagellates assemblages and found thatOstreopsiswere the second most dominant genera of benthic dinof lagellate assemblages, both in inorganic matrix and macrophytes. The maximum abundance ofOstreopsiswere observed in hard coral and turf algae. A similar phenomenon was observed at Johnston Atoll in the Pacif ic, which has a similar geographical characteristics and environmental conditions with Zhongsha Islands (Richlen and Lobel, 2011).Ostreopsisspp. from Johnston Atoll accounted for the largest proportion at several of hard coral reef sites, an opposite result to our study.Boisnoir et al. (2019a) indicated thatOstreopsispreferred to attach to blade-ramif ied macrophytes,particularly Florideophyceae. However, the minimum abundance was presented onPadinaaustraliswith averaged 8 cells/g. We speculate that other factors may have contributed to this phenomenon, such as the sampling depth. The maximum sampling depth at Johnston Atoll was 13 m, which was lower than the least depth in our study. AlthoughOstreopsishad generally low abundance on the three macroalgae and coral sand, they were signif icantly more abundant on blades with larger surface areas, e.g.Halimedasp. (8 cells/g). Whereas the abundance on branchedAmphiroafragiroaand foliatedPadinaaustraliswere as low as 1 cell/g.
LikeProrocentrum,Amphidiniumwas widely distributed in various substrates with a high abundance.Amphidiniumwas the genus with the second highest abundance in the benthic dinof lagellate community of Zhongsha Islands, both on coral sands and macroalgae. This was inconsistent with those of other studies that the abundance ofAmphidiniumwas generally the lowest (Richlen and Lobel, 2011; Lee et al., 2020).Cooliais present in low abundance. The same results are consistent with previous studies in the Gulf of Mexico (Okolodkov et al., 2007; Hachani et al., 2018; Ben Gharbia et al., 2019).
A study on the depth distribution of benthic dinof lagellates in the Caribbean demonstrated that their distribution was signif icantly correlated with light intensity. The cell density decreased signif icantly with increasing depth (Boisnoir et al., 2018). The benthic dinof lagellate density was <100 cells/g in both wet and dry seasons at a depth of 20 m. It was found that noOstreopsiscells were observed when the water depth exceeds 10 m. Interestingly, in this study,Ostreopsisspp. were observed at all depths on coral sands and macroalgae except that onPadinaaustralisat Cawston Shoal. A diff erent trend was observed by Lee et al. (2020) that at depths greater than 12 m,onlyProrocentrumandCooliawere presented. In our study, on coral sand samples,Gambierdiscuswere only observed at 15 m (Yitong Shoal) and 19 m (West of Addington Patch). AlthoughGambierdiscuswas also present in the samples of the macroalgaePadinaaustralisat Cawston Shoal (20 m) and Magpie Shoal(23 m), Their abundance was as low as 1 cell/g. On macroalgae samples, the maximum abundance ofProrocentrumwere observed at the minimum depth of 15 m (Yitong Shoal). At a maximum depth of 30 m, a second minimumProrocentrumabundance of 334 cells/100 cm2on coral sand were presented.However, noCooliaspp. was observed. Macroalgae generally grow in abundance in the intertidal zone with water depth less than 3 m. The Zhongsha Islands have a water depth of more than 15 m, and the bottom is a coral reef ecosystem. Increased depth means less macroalgae. Compared to the coasts of continents or large islands, reef ecosystems with water depths of more than 20 m are subject to stronger hydrodynamic disturbances. Benthic dinof lagellates were signif icantly more abundant in calm lagoons than on reefs heavily aff ected by waves (Boisnoir et al., 2018). Hydrodynamic conditions may be the most important factor aff ecting the growth of benthicepiphytic dinof lagellates. The abundance in sheltered areas is signif icantly higher than that in unsheltered areas (Shears and Ross, 2009; Mangialajo et al.,2011). It is speculated that the low abundance of benthic dinof lagellates is closely related to the scarcity of macroalgae and stronger water motion at the depth>15 m in Zhongsha Islands.
5 CONCLUSION
The biodiversity and distribution of benthic dinof lagellates in the Zhongsha Islands have been revealed. In general, 15 species of benthic dinof lagellates belonging to four genera were identif ied:A.carterae,A.magnum,A.massartii,A.operculatum,C.canariensis,C.malayensis,C.palmyrensis,C.tropicalis,O. cf.ovata,P. cf.sculptile,P.concavum,P.emarginatum,P.hoff mannianum,P.lima, andP.rhathymum.Benthic dinof lagellates were distributed broadly on coral sands and three species of macroalgae(Padinaaustralis,Halimedasp., andAmphiroafragiroa). However, the total abundance of benthic dinof lagellates in the Zhongsha Islands, which was 88–4 345 cells/100 cm2on sediment and 10–91 cells/g on macroalgae, is much lower than that in other areas.ProrocentrumandAmphidiniumwere the dominant and subdominant genera, respectively.It is speculated that the low abundance of benthic dinof lagellates is closely related to the scarcity of macroalgae and stronger water motion at the depth>15 m in Zhongsha Islands. This study has provided a deeper understanding of benthic dinof lagellate assemblages in the Zhongsha Islands.
6 DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
 Journal of Oceanology and Limnology2022年6期
Journal of Oceanology and Limnology2022年6期
- Journal of Oceanology and Limnology的其它文章
- Overview of harmful algal blooms (red tides) in Hong Kong during 1975–2021
- Information standardization for typical toxic and harmful algae in China’s coastal waters—a case study of Karenia mikimotoi*
- Biochemical composition of the brown tide causative species Aureococcus anophageff erens cultivated in diff erent nitrogen sources*
- Identif ication of paralytic shellf ish toxin-producing microalgae using machine learning and deep learning methods*
- Screening for lipophilic marine toxins and their potential producers in coastal waters of Weihai in autumn, 2020*
- First observation of domoic acid and its isomers in shellf ish samples from Shandong Province, China*
