Kyoto classification of gastritis: Advances and future perspectives in endoscopic diagnosis of gastritis
Osamu Toyoshima, Toshihiro Nishizawa
Abstract This editorial provides an update of the recent evidence on the endoscopy-based Kyoto classification of gastritis, clarifying the shortcomings of the Kyoto classification, and providing prospects for future research, with particular focus on the histological subtypes of gastric cancer (GC) and Helicobacter pylori (H. pylori)infection status. The total Kyoto score is designed to express GC risk on a score ranging from 0 to 8, based on the following five endoscopic findings: Atrophy,intestinal metaplasia (IM), enlarged folds (EF), nodularity, and diffuse redness(DR). The total Kyoto score reflects H. pylori status as follows: 0, ≥ 2, and ≥ 4 indicate a normal stomach, H. pylori-infected gastritis, and gastritis at risk for GC,respectively. Regular arrangement of collecting venules (RAC) predicts noninfection; EF, nodularity, and DR predict current infection; map-like redness(MLR) predicts past infection; and atrophy and IM predict current or past infection. Atrophy, IM, and EF all increase the incidence of H. pylori-infected GC.MLR is a specific risk factor for H. pylori-eradicated GC, while RAC results in less GC. Diffuse-type GC can be induced by active inflammation, which presents as EF, nodularity, and atrophy on endoscopy, as well as neutrophil and mononuclear cell infiltration on histology. In contrast, intestinal-type GC develops via atrophy and IM, and is consistent between endoscopy and histology. However,this GC risk-scoring design needs to be improved.
Key Words: Kyoto classification; Gastritis; Endoscopy; Gastric cancer; Histology;Helicobacter pylori
INTRODUCTION
The Kyoto classification of gastritis aims to match the endoscopic and histopathological findings of gastritis. It further aims to evaluate gastric cancer (GC) risk andHelicobacter pylori(H. pylori) infection of gastritis. The Kyoto classification was first advocated by the Japan Gastroenterological Endoscopy Society in 2013 and is widely used in recent clinical practice worldwide[1]. Technological advances in endoscopy have significantly improved the accuracy of identifying premalignant mucosal changes[2].This editorial provides an update of the recent evidence on the Kyoto classification, clarifying the shortcomings of the Kyoto classification, and providing prospects for future research. This article is divided into the following four chapters: (1)H. pyloriinfection according to the Kyoto classification; (2)The histological consistency of the Kyoto classification; (3) Risk of GC according to the Kyoto classification; and (4) Future prospects in the Kyoto classification.
In the Kyoto classification, the total Kyoto score has been developed as a GC risk score. The total Kyoto score is calculated as the sum of the following 5 endoscopic findings: Atrophy, intestinal metaplasia (IM), enlarged folds (EF), nodularity, and diffuse redness (DR); and ranges from 0 to 8 (Table 1 and Figure 1)[1]. The Kyoto DR score includes the disappearance of the regular arrangement of collecting venules (RAC). Map-like redness (MLR) frequently appears afterH. pylorieradication, and is generally pathologically consistent with IM[3]. This article describes the total Kyoto score and its five individual findings along with RAC and MLR.
GCs consist of two distinct histological subtypes: Lauren’s diffuse and intestinal GC[4]. Diffuse-type GC develops directly from highly active inflammation, whereas intestinal-type GC develops through destruction and replacement of tissues, such as atrophy and IM, and is termed Correa’s cascade[5-7].GCs can also be described according to the different rates of incidence[8,9], lesion characteristics[10-12],and prognoses[13-16]as per the correspondingH. pyloriinfection status. In this editorial, we specifically describe the histological subtypes of GC andH. pyloriinfection status.
H. PYLORI INFECTION IN THE KYOTO CLASSIFICATION
H. pylori non-infection
Evidence of RAC as an indicator of non-infection has been reported in both in Japan[17,18]and several other countries[19-21], including in the west[22-24], as shown in Table 2. Two recent meta-analyses reported that the sensitivity and specificity of RAC for predicting non-infection were 78%-80% and 94%-97%, respectively[25,26]. The high reliability of RAC for non-infectious cases has also been verified.
H. pylori current and past infection
All five Kyoto scores, atrophy (61.1%-85.8% and 58.5%-85.3%)[19,23,27], IM (95.6% and 86.0%)[27], EF(96.6%-99.1% and 85.0%-85.3%)[17,27], nodularity (98.3%-100% and 76.5%-89.1%)[17,20,27,28], and DR(73.6%-97.6% and 65.0%-89.7%)[17,19,20,27], commonly offer high specificity and accuracy for categorizing current infections (Table 2).
Three studies have previously compared patients with non-infectious, current, and past infections, all of which reported that RAC was strongly correlated with non-infection [odds ratios (ORs) = 4.6-55.0];MLR was a highly specific finding indicative of past infection (ORs = 7.8-12.9), and DR, EF, and nodularity provided high ORs of 10.5-26.4, 6.0-8.6, and 4.0-22.5, respectively, for current infection.Atrophy and IM were associated with both current (ORs = 1.9-21.6 and 4.3) and past infections (ORs =1.9-22.8 and 4.4), respectively[17,19,25]. A previous study reported an algorithm with an accuracy of 80.0% for defining the presence of RAC as non-infection, DR and mucosal edema as current infection,and MLR as post-eradication[24].H. pylorieradication decreases the Kyoto EF, nodularity, and DR scores, but does not improve the Kyoto atrophy and IM scores[29]. These results indicate that the presence of RAC predicts non-infection; EF, nodularity, and DR predict current infection; MLR predicts past infection; and atrophy and IM predict current or past infection.
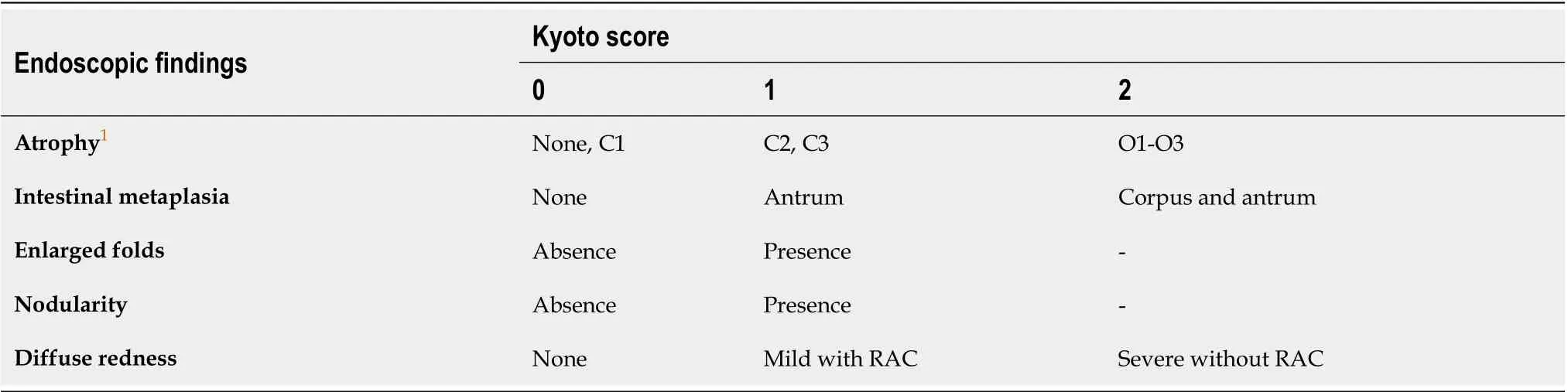
Table 1 Kyoto classification score
Total Kyoto score
Several studies have previously focused on the association between the total Kyoto score andH. pyloriinfection. The sensitivity and specificity of the total Kyoto score for current infection were good at 78.3%-98.7% and 92.0%-98.4%, respectively (Table 2)[27,30]. The area under the curve (AUC) of the total Kyoto score for predicting current infection was 0.85, with a cutoff value of 2[27]. Current infection rates increased stepwise, with total Kyoto scores of 0-1, 2-3, and ≥ 4 (8.6%, 61.4%, and 85.7%, respectively)[31]. The mean total Kyoto scores differed among patients with current, past, and non-infection (3.4, 1.1,and 0.0, respectively)[32]. A combination of the total Kyoto score and serumH. pyloriantibody titer allows for the accurate diagnosis of current infection[33]. The total Kyoto score decreases from 3.9 to 2.8 followingH. pylorieradication[29]. In summary, total Kyoto scores of 0 and ≥ 2 express non-infection and current infection, respectively.
HISTOLOGICAL CONSISTENCY OF KYOTO CLASSIFICATION
The purpose of the Kyoto classification is to match endoscopic and histological findings of gastritis.Regarding atrophy and IM, considerable evidence exists to indicate the consistency between endoscopy and histology. In recent studies, a high Kyoto atrophy score and severe endoscopic IM are associated with histologically advanced stages of operative link for gastritis assessment and operative link for gastric IM assessment, respectively[34,35].
Consistency between the endoscopic findings of the Kyoto scores and histological grading of the updated Sydney system (USS) scores has been examined individually. All five Kyoto scores were associated with histological inflammation, namely the USS score for neutrophil and mononuclear cell infiltration, which is an indicator ofH. pyloriinfection. The Kyoto atrophy and IM scores correlated with both histological atrophy and IM in the corpus[34,36]. AmongH. pylori-infected patients, the Kyoto EF,nodularity, and DR scores indicated histologically high inflammation in the corpus[36-38]. In summary,the Kyoto atrophy and IM scores were concordant with histological corpus atrophy and IM scores. The Kyoto EF and nodularity scores were associated with the histological corpus inflammation.
GC RISK OF KYOTO CLASSIFICATION
Significant evidence to indicate endoscopic atrophy as a risk factor for GC has been accumulated. The incidence of GC based on atrophy is summarized in Table 3. GC incidences for mild, moderate, and severe atrophy are 0.06%-0.15%, 0.12%-0.34%, 0.31%-1.60%, respectively, indicating the severity of atrophy as a risk factor for GC development, even afterH. pylorieradication[39-41]. A recent study from Western countries also showed that a Kyoto atrophy score of 2 was associated with GC development with a hazard ratio of 6.4 in patients with baseline IM[42].
The ORs for the histological subtypes of GC based on the Kyoto classification are summarized in Table 4. The Kyoto atrophy score is a predictor of GC with ORs of 2.5-7.4[43-45]. Two recent metaanalyses showed that a Kyoto atrophy score of 2 had high risk ratios (2.8-8.0 for developing GC)[46,47].In an examination based on histological subtypes, a high Kyoto atrophy score was found to be associated with both diffuse-type and intestinal-type GCs with ORs of 2.3 and 6.2, respectively[44].
A high Kyoto IM score indicates a high risk for GC (OR = 1.6), especially intestinal-type GC (OR =1.7), but a low risk for diffuse-type GC (OR = 0.2)[44,45,48,49]. In a direct comparison of diffuse-type and intestinal-type GCs, a high Kyoto IM score was associated with intestinal-type GC (ORs = 1.7-2.1)[44,49]. Furthermore, a high Kyoto IM score was associated with multiple GCs[50].
In a study on asymptomaticH. pylori-infected patients, the hazard ratio of patients with EF for GC development during the 5 years was high at 43.3[51]. In contrast, EF was associated with a low risk of intestinal-type GC (OR = 0.5)[44]. Furthermore, a direct comparison between diffuse-type and intestinaltype GCs indicated EF as a risk factor for diffuse-type GC (OR = 1.3)[44]. EF is reported to be an indicator of submucosal invasion in patients with GC (OR = 3.4; submucosal invasionvsintramucosal depth)[52].
The risk of nodularity is controversial. Previous studies found that nodularity was associated with a high risk for diffuse-type GC (OR = 10.0)[53], notably in youngH. pylori-infected patients (OR = 64.2)[54]. In contrast, nodularity was described as a low risk factor for GC (OR = 0.5), especially intestinaltype GC (OR = 0.3)[44]. Nodularity decreases with age and the risk of intestinal-type GC increases with age[28]. Therefore, the risk of nodularity in GC should be stratified according to age.
Previously, RAC has been revealed as a predictor of non-GC[45]. Collectively, the Kyoto atrophy, EF,and nodularity scores were associated with diffuse-type GC, whereas the Kyoto atrophy and IM scores were related to intestinal-type GC, as shown in Figure 2.
GC risk after H. pylori eradication
Recently, the risk of GC afterH. pylorieradication has been intensively investigated. Table 5 shows the risk of GC followingH. pylorieradication. A Kyoto atrophy score of 2 and MLR are both indicators of GC after eradication, with ORs of 8.1 and 1.8-5.3, respectively[55-57]. Additionally, RAC was inversely associated with eradicated GC (ORs = 0.3-0.4)[56,58]. Studies have further revealed that the hazard ratios of Kyoto atrophy 2 and MLR for GC development were 4.9 and 3.6, respectively[55,59]. Takeet al[41]previously reported the long-term incidence of GC after eradication based on endoscopic atrophy.The incidence of diffuse-type GC was higher in the second decade of follow-up than in the first decade.This increase was only observed in patients with mild-to-moderate gastric atrophy, indicating that even if atrophy is not severe, the risk of GC can persist long after eradication.
Total Kyoto score
The total Kyoto scores of patients with GC,H. pylori-infected GC, andH. pylori-eradicated GC were 4.0-4.6, 4.8-5.6, and 4.2, respectively[44,50,58,60]. A high total Kyoto score was associated not only with GC(ORs = 1.5-1.6), but also with both diffuse-type and intestinal-type GCs (ORs = 1.3 and 1.7, respectively,Table 4)[44,61]. Additionally, some investigators showed that the incidence of GC increased stepwise with the total Kyoto scores of 0-1, 2-3, ≥ 4, and that the AUC of the nomogram to predict GC using the total Kyoto score was 0.79[31,61]. Taken together, a total Kyoto score of 4 or more is useful for determining GC risks, including histological subtypes, even afterH. pylorieradication.
FUTURE PERSPECTIVES IN KYOTO CASSIFICATION
The total Kyoto score was developed to evaluate GC risk, with a score ≥ 4 indicating risk. However,designing a method to simply add each component of the Kyoto score is problematic. First, the GC risksof the diffuse and intestinal types were distinctly different. For example, IM is associated with a high risk of intestinal-type GC but a low risk of diffuse-type GC. Conversely, EF and nodularity are high risk factors for diffuse-type GC, but indicate a low risk of intestinal-type GC (Table 4 and Figure 2). The majority of GC cases are classified as intestinal type, which indicates that the intestinal-type GC riskmay be overestimated, whereas the diffuse-type GC risk may be underestimated. Second, two points were assigned to Kyoto atrophy, IM, and DR scores in the total Kyoto score. The verification of the weighting of the total Kyoto score is a future task. Therefore, this scoring method should be revised in the future. A modified Kyoto score has been suggested as the sum of the following points: 2 points for invisible RAC, and 1 point each for Kyoto atrophy score 2, Kyoto IM score 2, and corpus MLR.Compared with the scores of 0-1, the ORs of the GC morbidity for the modified Kyoto scores of 2-3 and 4-5 were higher, at 8.6 and 28.0, respectively. Although statistical significance was not reached, the AUC of the modified Kyoto score had a higher predictive ability than that of the original total Kyoto score(0.75vs0.71, respectively)[45]. Furthermore, a scoring system specific to histological GC subtypes is needed. Third, MLR has been shown to predict GC afterH. pylorieradication. Since IM manifests as MLR afterH. pylorieradication[3], MLR may be more suitable than IM to assess the risk of eradicated GC.

Table 3 Gastric cancer incidence based on endoscopic atrophy

Table 4 Odds ratio for histological subtype of gastric cancer based on the Kyoto classification

Table 5 Odds ratio of gastric cancer after Helicobacter pylori eradication based on the Kyoto classification
In Western countries, RAC and endoscopic IM have been extensively studied; however, other endoscopic findings, such as atrophy, EF, nodularity, DR, and MLR, have been less extensively explored, and further studies in more varied populations are required. This article does not mention a variety of important endoscopic findings, including spotty redness as a predictor ofH. pyloriinfection[62]; xanthoma[57,63,64], foveolar hyperplastic polyp[65], refluxed bile[66], and a lack of fundic gland polyp[64]to predict GC; and depressive erosion and fundic gland polyp as indicators of functional dyspepsia[32,67]. Further research is required to confirm these findings. RAC provides high kappa values of intra-observer and inter-observer agreements of 0.88-0.91 and 0.74-0.79, respectively[21,68];however, agreement between the other endoscopic findings needs to be clarified.
Autoimmune gastritis (AIG) is gaining attention as an important factor owing to the decrease inH.pyloriinfection[69]. Both severe endoscopic atrophy and a Kyoto IM score of 2 have been reported as AIG features[70,71]. Further steps should be taken to elucidate the differential diagnosis between AIG andH. pylori-associated gastritis using the Kyoto classification.
Recently, image-enhanced endoscopy (IEE) has been widely used in clinical practice. Two metaanalyses previously reported the utility of narrow-band imaging (NBI) for the diagnosis of IM[72,73].Additionally, an improved diagnostic accuracy on using NBI, blue laser imaging, and linked color imaging have been reported[74-77]. In the future, research on endoscopic assessment using IEE,including texture and color enhancement imaging[78,79]will be required.
CONCLUSION
In conclusion, the total Kyoto score and individual Kyoto score, including atrophy, IM, EF, nodularity,and DR, can predict GC risk andH. pyloriinfection. Total Kyoto scores of 0, ≥ 2, and ≥ 4 indicate a normal stomach,H. pylori-infected gastritis, and gastritis at risk for GC, respectively; RAC predicts noninfection; EF, nodularity, and DR predict current infection; MLR predicts past infection; and atrophy and IM predict current or past infection. Atrophy, IM, and EF all increase inH. pylori-infected GC, MLR is a specific risk factor forH. pylori-eradicated GC, while RAC indicates a lesser GC risk. Diffuse-type GC can be induced by active inflammation, which presents as EF, nodularity, and atrophy on endoscopy, and neutrophil and mononuclear cell infiltration on histological examination. In contrast,intestinal-type GC developsviaatrophy and IM, and is consistent on endoscopy and histology.However, the GC risk-scoring design still needs to be improved.
ACKNOWLEDGEMENTS
We would like to thank Dr. Hidenobu Watanabe to provide histological gastric cancer images.
FOOTNOTES
Author contributions:Toyoshima O and Nishizawa T contributed to this paper; Toyoshima O contributed to the design, writing of the manuscript, illustrations, and review of literature; Nishizawa T contributed to the design,discussion, and editing the manuscript.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Japan
ORCID number:Osamu Toyoshima 0000-0002-6953-6079; Toshihiro Nishizawa 0000-0003-4876-3384.
S-Editor:Wang JJ
L-Editor:A
P-Editor:Wang JJ
 World Journal of Gastroenterology2022年43期
World Journal of Gastroenterology2022年43期
- World Journal of Gastroenterology的其它文章
- Management of non-alcoholic fatty liver disease patients with sleep apnea syndrome
- Differential analysis of intestinal microbiota and metabolites in mice with dextran sulfate sodium-induced colitis
- Correction to “MicroRNA-596 acts as a tumor suppressor in gastric cancer and is upregulated by promotor demethylation”
- Salvia miltiorrhiza extract may exert an anti-obesity effect in rats with high-fat diet-induced obesity by modulating gut microbiome and lipid metabolism
- Current status of minimally invasive liver surgery for cancers
- Upper gastrointestinal endoscopic findings in celiac disease at diagnosis: A multicenter international retrospective study
