肝脏分泌因子与代谢性疾病
梁佳琦,刘畅,张雯翔,陈思禹
综 述
肝脏分泌因子与代谢性疾病
梁佳琦,刘畅,张雯翔,陈思禹
中国药科大学生命科学与技术学院,南京 211198
代谢性疾病泛指代谢功能发生问题所引起的疾病,其主要症状包括中心性肥胖、胰岛素抵抗、血脂血糖异常、血压升高等。肝脏作为人体内一个重要的代谢器官,在调节全身葡萄糖和脂质代谢等许多生理过程中起关键作用。近年来的大量研究表明,肝脏可以合成和分泌多种生物信号分子,如FGF21、Fetuin-A以及ANGPTL8等,以自分泌/旁分泌的方式调节机体的代谢过程。干预相关肝脏分泌因子的表达可有助于预防、诊断和治疗代谢性疾病。然而,目前肝脏分泌因子与代谢稳态之间的互作机制仍不明确。本文对不同肝脏分泌因子与代谢性疾病的关系展开论述,以期为治疗代谢性疾病提供新的策略和参考。
肝脏分泌因子;代谢性疾病;诊断治疗
近年来,随着经济的腾飞与生活节奏的改变,不健康的饮食模式和久坐不动的生活方式导致代谢性疾病的患病率显著提高,已成为威胁全球人类健康的重要因素。代谢性疾病是由体内氨基酸、糖脂代谢紊乱引发的一类疾病,其症状主要包括中心性肥胖、胰岛素抵抗、血脂血糖异常、血压升高等,是诱发心脑血管疾病、内分泌系统疾病的重要危险因素[1]。肝脏作为体内重要的代谢器官,近年来已被证实具有重要的内分泌功能。它能通过产生多种功能性的肝脏分泌因子(表1),如FGF21、Fetuin-A以及ANGPTL4等,已陆续被证实具有重要的代谢调控功能,其与肝脂肪变性、炎症信号传导、胰岛素抵抗等有关[2]。它们能以内分泌、自分泌及旁分泌方式,建立起组织器官间的良性对话秩序。各个器官组织之间的通讯和相互调控对维持机体代谢稳态至关重要。本文对不同肝脏分泌因子与代谢性疾病的关系展开综述,以期为治疗代谢性疾病提供新的策略。
1 成纤维细胞生长因子家族(fibroblast growth factors, FGFs)
FGFs是一类多肽生长因子,分子量约为17~ 34 kDa,FGF家族由22个不同成员组成,具有13%~71%的氨基酸同源性。在脊椎动物中,FGFs在基因水平和氨基酸序列上都高度保守,其结构中具有两个保守的半胱氨酸残基,对肝素/硫酸肝素具有高亲和力[19]。FGFs控制胚胎发育和成体生物体中的多种生理反应。在发育过程中,FGFs通过控制细胞增殖、存活、迁移和分化在模式形成和形态发生中发挥关键作用[20~22]。在成体生物中,FGFs参与组织修复、维持能量及代谢稳态[23,24]。在FGF家族的22个成员中,FGF15/19、FGF21和FGF23构成了FGF19亚家族,它们作为内分泌激素调节胆汁酸、脂肪酸、葡萄糖和无机盐代谢。其中,FGF15/19是一种在小肠回肠肠上皮细胞中表达的肠道激素,并在餐后响应胆汁酸吸收而释放[25,26]。FGF23主要由骨组织中的成骨细胞和骨细胞分泌,主要调节全身磷酸盐稳态和维生素D代谢[27,28]。FGF21主要在肝脏中表达,作为一种肝脏分泌因子,在能量调节代谢过程发挥重要作用[29]。
1.1 FGF21
Nishimura等[30]于2000年首次在小鼠胚胎中分离出FGF21,其在小鼠和人类之间约有75%的氨基酸序列同源性。在生理条件下,血液中的FGF21主要来源于肝脏[31]。除此之外,FGF21在脂肪组织、胰腺、肌肉中也均有少量表达[29]。
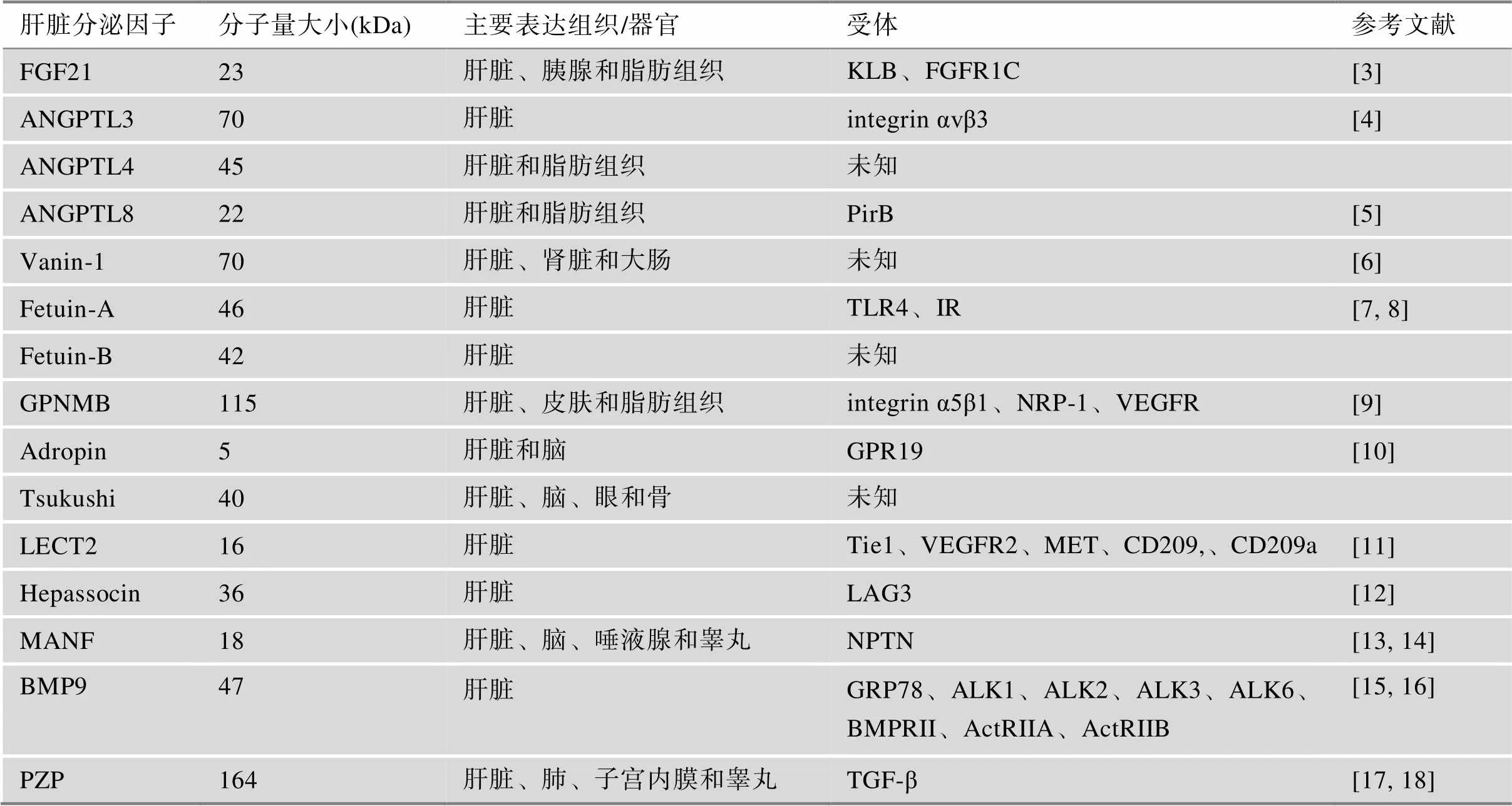
表1 肝脏分泌因子的主要特征
FGF21在调节能量平衡和维持葡萄糖和脂质稳态方面具有重要作用[32]。在肥胖和糖尿病动物模型中,FGF21可在不影响总热量摄入下导致体重减轻、血浆葡萄糖和甘油三酯(triglyceride, TG)水平降低,并提高机体胰岛素敏感性[33]。此外,在胰岛素抵抗状态下(如脂肪肝、肥胖和2型糖尿病),血清FGF21水平会升高。因此,FGF21也可作为糖尿病的预测因子或生物标志物[34]。
FGF21主要通过影响肝脏、脂肪组织和中枢神经系统发挥其内分泌作用。在肝脏中,FGF21降低胰岛素抵抗,增强脂肪氧化,降低TG水平及肝细胞中脂滴的积累[35,36]。在脂肪组织中,一方面,FGF21通过增强脂肪组织胰岛素敏感性来促进葡萄糖利用并增加能量消耗[37,38]。另一方面,暴露于寒冷或运动后,FGF21会诱导过氧化物酶体增殖物激活受体γ共激活因子-1α(peroxisome proliferator activated receptor γ coactivator-1α, PGC-1α)的上调,从而促进脂肪组织和骨骼肌的产热[39]。在中枢神经系统中,FGF21可以提高下丘脑促肾上腺皮质激素释放因子的表达,刺激交感神经活动,进而促进棕色脂肪组织中的能量消耗[40]。此外,FGF-21还具有抗炎作用。研究表明,在非酒精性脂肪性肝炎(nonalcoholic steatohepatitis, NASH)小鼠模型中,FGF21可通过调节脂联素抑制Th17细胞分化和IL-17A表达来缓解炎症[41]。
虽然FGF21可以对肥胖等相关代谢性疾病发挥较好的药理学作用。然而,由于其药代动力学和生物物理特性较差,天然的FGF21并不适合临床使用。目前,已经开发出大量针对FGFR1-β-klotho受体复合物的长效FGF21类似物和激动性单克隆抗体,一些FGF21类似物和模拟物已进入肥胖、2型糖尿病和NASH患者临床试验的早期阶段[24]。FGF21类似物的靶向递送,以及FGF21组织特异性受体激动剂的开发,将有助于提高基于FGF21的药物疗法的疗效和安全性。
2 血管生成素样蛋白家族 (angiopoietin- like proteins, ANGPTL)
ANGPTL是一种与血管生成素结构相似的蛋白质家族。迄今为止,已发现8个ANGPTL亚型,即ANGPTL1至ANGPTL8。其中,ANGPTL3、ANGPTL4和ANGPTL8主要在肝脏中表达。近年来,许多研究表明ANGPTLs在生理和病理过程中发挥着关键的调节作用,在糖脂代谢、炎症、造血和癌症的发生发展中都有一定的生物学功能[42~44]。
2.1 ANGPTL3
ANGPTL3仅在肝脏中产生,因此可以归类为真正的肝脏特异性分泌因子[45]。ANGPTL3作为一种血浆TG的调节剂,对脂蛋白脂肪酶(lipoprotein lipase, LPL)的活性具有抑制作用,该酶锚定在毛细血管内皮上,并催化TG水解生成脂肪酸[46]。小鼠中ANGPTL3的失活可降低血浆TG和游离脂肪酸水平并抑制动脉粥样硬化[47,48]。在人体内,ANGPTL3突变可导致低密度脂蛋白(low density lipoprotein, LDL)、高密度脂蛋白和TG的血浆水平降低,诱发家族联合性低血脂症[49]。此外,ANGPTL3功能丧失的突变杂合子携带者患冠状动脉疾病的风险低于非携带者[50,51]。
目前,美国Regeneron公司开发了一种靶向ANGPTL3的C末端LPL抑制结构域的单克隆抗体Evinacumab。在最近的临床三期试验中,高胆固醇血症患者使用24周的Evinacumab治疗后,显著降低了体内LDL和TG含量[52]。总之,ANGPTL3是血浆脂蛋白代谢的重要肝源性调节剂。未来抗ANGPTL3的治疗将成为降低特定患者血浆中LDL和TG的有效治疗策略。
2.2 ANGPTL4
与ANGPTL3一样,ANGPTL4在调节脂质储存和分解方面具有重要作用[53]。基因在人类等哺乳动物中高度保守,与小鼠具有77%的氨基酸序列相似性[54]。其通过依赖C端纤维蛋白原样结构域增加脂肪细胞的脂解作用,并通过N端卷曲螺旋结构域抑制LPL的活性[53,55]。ANGPTL4在肝脏和脂肪组织中的表达在进食和禁食后发生改变,表明ANGPTL4在调节脂肪代谢中发挥作用。它通过抑制LPL活性并增加脂肪细胞中TG的分解,从而减轻脂肪组织重量[56]。在小鼠中,腺病毒介导的ANGPTL4过表达可以改善葡萄糖耐量并减少肝葡萄糖输出[57]。
ANGPTL4在胰岛素功能及血糖控制方面的作用存在争议。一些研究认为尽管在ANGPTL4过表达的小鼠中有严重的肝脏脂肪变性,但它们的肝脏和全身胰岛素敏感性有所改善[57]。同时,ANGPTL4增强胰岛素介导的糖异生抑制过程从而降低原代肝细胞中的肝葡萄糖产生,并且在人体内,ANGPTL4水平与胰岛素敏感性正相关[57]。与此相反的是,另一些研究表明ANGPTL4基因敲除小鼠可降低血脂,减少肝脏和肌肉中的脂质积累,增强胰岛素信号传导,并改善血糖[58,59]。因此,未来需要进一步深入研究来探寻这些差异背后的原因,以确定ANGPTL4在调节血糖、血脂中的潜在治疗价值。
2.3 ANGPTL8
ANGPTL8,又称Betatrophin、RIFL、Lipasin或TD26,是一种由人基因(小鼠同源基因)编码的分泌蛋白,是ANGPTL蛋白家族的新成员,ANGPTL8参与机体脂质代谢、炎症、癌细胞侵袭和造血干细胞活性的调控[60~62]。与其他ANGPTLs相比,ANGPTL8缺乏纤维蛋白原/血管生成素样结构域[63]。在小鼠中,ANGPTL8主要在脂肪组织和肝脏中表达。在人体内,其主要表达部位是肝脏,在脂肪组织中仅有少量表达[64]。研究显示,ANGPTL8在各种代谢性疾病的发生发展中起关键作用。例如,1/2型糖尿病患者的血清ANGPTL8蛋白水平升高,并且重度肥胖患者和2型糖尿病患者的总胆固醇、LDL和载脂蛋白B的含量与ANGPTL8蛋白水平呈正相关[65]。在给予高脂进食后,小鼠的肝脏、棕色和白色脂肪组织中ANGPTL8的mRNA被显著诱导。同时,在小鼠体内用腺病毒过表达ANGPTL8会增加血清TG水平[66]。此外,ANGPTL8的表达还受禁食/再进食信号的调控。在空腹状态下ANGPTL8水平降低,而在再进食状态下显著增加[67]。本研究组发现,ANGPTL8作为肝脏分泌因子可重设小鼠肝脏时钟和代谢基因的昼夜节律,是一种响应食物信号的肝脏时钟关键调节器。从机制上讲,ANGPTL8的重置功能依赖于膜受体PirB的信号传递、激酶和转录因子的磷酸化,从而瞬时激活中枢时钟基因Per1[5]。这些结果提示肝脏分泌因子ANGPTL8能响应食物信号,以“自分泌”形式,参与肝脏糖脂代谢稳态的调节过程。
3 血管非炎性蛋白-1(Vanin-1)
Vanin-1是由肝小叶中肝实质细胞表达分泌的一种酶,作为机体氧化应激的感受器,其本质是一种泛酰巯基乙胺酶[68]。本研究组发现Vanin-1的表达和酶活在饥饿和db/db糖尿病小鼠肝脏中显著升高,并通过减弱肝实质细胞中Insulin/Akt信号通路活性,持续激活肝糖异生进程,诱发机体高血糖症和胰岛素抵抗[69]。这一结果提示肝脏Vanin-1是糖尿病药物研发的潜在新型靶标。为了拓展Vanin-1的病理生理学功能,本研究组进一步在白色脂肪组织中展开研究,发现Vanin-1表达和酶活在肥胖病人的白色脂肪中均降低,且与脂质水解调节基因甘油三酯脂酶(adipose triglyceride lipase, ATGL)和激素敏感性甘油三酯脂酶(hormone-sensitive lipase, HSL)的表达呈显著正相关性。更重要的是,Vanin-1的全身性缺失加剧了高脂食物诱发的小鼠腹部脂肪堆积和胰岛素抵抗进程[70]。这一结果提示白色脂肪中Vanin-1的降低是高脂食物诱发代谢性疾病的重要原因之一,且细胞内的非分泌Vanin-1同样具有重要的代谢调节功能。
相关研究表明Vanin-1还在许多代谢途径的调节中起作用。研究发现Vanin-1是小鼠肝脏中过氧化物酶体增殖物激活受体α(peroxisome proliferator activated receptor α, PPARα)的调控靶点之一,口服PPARα激动剂类药物能显着诱导Vanin-1的表达[71]。此外,Vanin-1还调节着肝脏的氧化应激。对乙酰氨基苯酚(APAP)是最常用的药物之一,其安全性较高。然而,当大量服用时,APAP会导致肝小叶中心肝细胞严重坏死,而Vanin-1在小鼠中可以保护肝脏免受APAP带来的肝毒性。与野生型小鼠相比,缺乏Vanin-1的小鼠对APAP造成的肝损伤更敏感,并且表现出血浆谷丙转氨酶浓度增加和更多的肝细胞坏死[72]。以上结果提示着Vanin-1在代谢稳态维持中的重要性,未来还需要进一步研究Vanin-1在不同器官中发挥何种(病理)生理作用。
4 胎球蛋白家族(Fetuin)
Fetuin家族是一种主要在肝脏分泌的糖蛋白,分为Fetuin-A和Fetuin-B。1944年Pedersen首次在牛血清中发现并分离得到一种酸性糖蛋白,命名为Fetuin-A。随后的半个世纪中陆续证明了Fetuin-A在骨骼重塑、神经系统发育等生物过程中都具有重要作用[73]。直到2000年,哺乳动物Fetuin家族的第2个成员Fetuin-B才被发现[74]。与Fetuin-A相比,Fetuin-B的氨基酸序列无显著改变,蛋白结构域具有高度同源性、半胱氨酸残基也具有整体保守性,故属于Fetuin-A的旁系同源物[74]。目前越来越多的研究显示,Fetuin家族在代谢性疾病的发生发展中扮演着重要的作用。
4.1 Fetuin-A
Fetuin-A,也称为alpha2-Heremans-Schmid,主要由肝实质细胞合成,然后释放到血液中,是一种大小约为46 kDa的肝脏分泌因子[75]。Fetuin-A水平受多种饮食因素的影响。据报道,膳食中的Omega-3脂肪酸摄入可能会增加Fetuin-A的浓度,而乳制品、姜黄素、烟酸、棕榈酸酯、咖啡和酒精等一些营养素会降低它的释放[76]。近年来,许多研究证明了Fetuin-A在代谢疾病中的重要作用。例如,有研究表明Fetuin-A可以作为代谢性疾病的标志物[77]。临床结果显示,体内较高的Fetuin-A水平与2型糖尿病、肥胖及其相关并发症之间存在密切联系[78~80]。此外,Fetuin-A在葡萄糖耐量、胰岛素抵抗和肝纤维化中发挥重要作用。Fetuin-A基因敲除小鼠的葡萄糖和胰岛素耐量试验表明葡萄糖清除率和胰岛素敏感性显着增强,并且可以防止高脂饮食(high fat diet, HFD)诱导的代谢紊乱。其机制与小鼠肝脏和骨骼肌中胰岛素受体及下游信号分子丝裂原活化蛋白激酶(mitogen-activated protein kinase, MAPK)和蛋白激酶B(protein kinase B, PKB/Akt)的磷酸化水平增加有关[81]。这些研究结果表明,Fetuin-A可能在调节葡萄糖代谢、胰岛素敏感性、体重增加和脂质堆积方面发挥重要作用,并且可能是治疗2型糖尿病、肥胖和其他胰岛素抵抗疾病的新型治疗靶点。
4.2 Fetuin-B
Fetuin-B是Fetuin家族的第二个成员,与Fetuin-A一样,主要由肝脏产生并抑制胰岛素受体酪氨酸激酶活性[74]。在小鼠中,Fetuin-B可以促进肝细胞产生胰岛素抵抗,导致小鼠葡萄糖耐受不良[82]。在人体内,患有脂肪肝和2型糖尿病的肥胖人群血浆Fetuin-B水平升高[83]。研究已表明,生理浓度的Fetuin-B会降低培养中的肌肉和肝细胞的胰岛素敏感性,并且在这种情况下,它不能激活促炎信号传导[84]。总之,以上这些发现为评估Fetuin-B在代谢性疾病的病理生理过程中的作用提供了理论依据,为疾病的早期诊断以及药物干预提供新的靶点并开辟新的视角。
5 非转移性黑色素瘤糖蛋白B(glycoprotein nonmetastatic melanoma protein B, GPNMB)
GPNMB是一种跨膜糖蛋白,于1995年首次发现在低转移特性的黑色素瘤细胞系中高度表达[85]。GPNMB由位于基因座7p15的基因编码,通过可变剪接形成2种亚型,分别包含572和560个氨基酸[86]。GPNMB锚定于细胞膜,其大部分结构位于胞膜外。GPNMB的细胞外部分具有12个糖基化位点、一个多囊肾病结构域(PKD)和一个整合素识别基序(RGD)[87]。GPNMB可被金属蛋白酶ADAM10切割,释放可与许多受体结合并引发细胞反应的可溶性片段[88]。
炎性疾病会大量诱导GPNMB的产生,而健康组织中GPNMB表达量非常低[89]。与正常人体内相比,2型糖尿病患者的血浆GPNMB水平升高并且GPNMB血清水平与肥胖及代谢参数(如臀围、体重指数和胰岛素抵抗)呈正相关[90, 91]。当小鼠全身性敲除基因后,除了会导致肥胖外,还会引起代谢综合征,如脂肪组织炎症升高、胰岛素抵抗和肝纤维化等[92]。武汉大学宋保亮课题组利用小鼠模型和人群样本,发现由肝脏分泌的GPNMB蛋白通过与CD44结合刺激脂肪细胞中的脂肪生成,激发PI3K-AKT-mTOR-SREBP1c信号通路从而参与肥胖及胰岛素抵抗的病程。而运用抗体中和等方法降低血液中的GPNMB可以有效抑制脂肪组织合成脂质的能力,提高机体能量消耗,减轻肥胖并改善胰岛素抵抗[90]。这些结果表明,GPNMB作为一种肝脏分泌因子可以调节脂肪生成作用,因此针对GPNMB的调控可能是一种治疗肥胖和糖尿病的策略。
6 Adropin
Adropin是一种高度保守的由76个氨基酸组成的激素,由能量稳态相关基因(energy homeostasis associated gene, ENHO)编码,主要由肝脏和大脑分泌,其表达会随着肝脏脂质利用率的升高而降低,可调节许多组织中的能量代谢稳态[93]。肥胖人群和2型糖尿病患者的血清Adropin水平较低,高脂进食会降低肝脏脂肪变性小鼠ENHO的表达[94, 95]。Adropin敲除小鼠的肝脏脂肪变性加剧,并伴有全身水平的葡萄糖耐量和胰岛素敏感性降低[96]。同时,在Adropin过表达的小鼠中高脂进食诱导的肝脂肪变性得到缓解,且全身胰岛素敏感性和葡萄糖耐量增强,脂肪酸氧化水平降低[93]。因此,Adropin的表达和分泌可以改善胰岛素敏感性和脂质代谢,同时抑制肝脂肪变性。这些研究表明,恢复肥胖患者肝脏和/或血液中的Adropin水平可能是治疗非酒精性脂肪肝(nonalcoholic fatty liver disease, NAFLD)和胰岛素抵抗的潜在方法。
7 Tsukushi
Tsukushi(TSK)是哺乳动物中高度保守的蛋白多糖,在肥胖小鼠和NASH模型中表达量显著增加[97]。相关研究表明,该分泌蛋白在小鼠代谢旺盛情况下(如低温环境或甲状腺激素处理)会被显著上调。当能量摄入过多时,TSK的敲除能够更好的调节体重和能量代谢平衡。TSK的缺失增加脂肪组织中交感神经支配和去甲肾上腺素的释放,介导脂肪组织的产热,改善饮食诱导的肥胖从而促进小鼠的能量消耗[98]。此外,TSK敲除小鼠减少肝脏的TG水平、缓解炎症及肝脏纤维化进程并抑制饮食诱导的肥胖,这可能是由于全身代谢的改善而不是针对促炎、促纤维化或胰岛素信号通路的直接作用来介导的[97]。然而,TSK在NAFLD中的调控作用仍存在争议。Mouchiroud[99]等的研究证明TSK对维持全身胆固醇稳态有重要作用,TSK能降低循环中HDL含量并减少肝脏中胆固醇向胆汁酸的转化,但TSK不会影响NAFLD的发展进程且不会改变饮食诱导的体重。未来的研究应寻找特定的TSK受体,以进一步明确TSK在NAFLD中的功能及其在外周组织脂质代谢和胰岛素信号传导中的作用。
8 白细胞衍生趋化因子2(LECT2)
LECT2最早由Yamagoe等[100]在PHA激活的人T细胞白血病SKW-3细胞中分离出来,是一种分子量大小为16 kDa的新型中性粒细胞趋化因子,由基因编码并主要在肝脏中表达。在小鼠体内,当肥胖小鼠从HFD转变为常规饮食(regular diet,RD)后,体重与血清LECT2水平下降。同时,从RD到HFD的转换迅速提高了小鼠血清LECT2水平。进一步研究发现,血清LECT2水平与肝脏TG含量呈正相关,但与脂肪组织重量无关[101]。此外,LECT2全身敲除小鼠骨骼肌胰岛素敏感性提高,其机制可能是增强了胰岛素刺激下AKT的磷酸化。相反,LECT2的回补则激活JNK信号通路,并通过增加胰岛素受体底物1(insulin receptor substrate 1, IRS1)的磷酸化来抑制胰岛素信号传导[102, 103]。总之,这些研究表明LECT2可能在代谢性疾病中发挥关键作用,它可能是代谢性疾病的潜在生物标志物和治疗靶点。
9 肝细胞衍生纤维蛋白原相关蛋白1(Hepassocin)
Hepassocin也称为HFREP1或纤维蛋白原样蛋白1,由肝细胞分泌,可促进细胞生长和增殖[104]。最近研究发现HFREP1与NAFLD以及全身胰岛素抵抗有关。HFREP1水平在NAFLD患者中升高,并且体内循环浓度与血浆葡萄糖水平和胰岛素抵抗正相关。HFREP1通过细胞外信号调节激酶ERK1/2依赖性途径抑制胰岛素信号传导以诱导胰岛素抵抗。此外,HFREP1肝脏敲低的小鼠改善了HFD小鼠和ob/ob小鼠的胰岛素抵抗[105]。HFREP1的水平还受到不同营养信号的调节。油酸可通过转录激活因子3信号传导来增加HepG2细胞中HFREP1表达,而棕榈酸处理的原代肝细胞可以通过C/EBPβ介导的转录激活诱导HFREP1表达,并进一步促进骨骼肌中的胰岛素抵抗进程[106,107]。综上所述,减少体内循环HFREP1的含量可能是治疗胰岛素抵抗的可行方法。
10 中脑星形胶质细胞源性神经营养因子(mesencephalic astrocyte-derived neurotrophic factor, MANF)
MANF是一种新型保守的神经营养因子,能够通过内质网应激而分泌[108]。最新的研究表明,MANF在维持机体代谢稳态中发挥重要作用。研究显示,与对照组小鼠相比,10个月大的MANF杂合子小鼠表现为更为严重的肝脏炎症表型和肝损伤。相反,MANF的回补可以防止饮食引起的肝脂肪变性,改善衰老引起的小鼠的肝损伤、炎症和代谢功能障碍[109]。因此在代谢应激期间维持高MANF水平可以作为防止肝脏脂肪变性和炎症发展的保护性干预措施。此外,MANF在肝脏特异性过表达促进腹股沟皮下脂肪发生褐变并减轻了饮食诱导的肥胖、胰岛素抵抗和肝脏脂肪变性。从机制上讲,MANF通过p38-MAPK途径直接促进白色脂肪细胞发生褐变[110]。该研究揭示了MANF作为肝脏分泌因子在调节脂肪组织产热中的关键作用,有望成为肥胖和相关代谢紊乱的潜在治疗靶点。
11 骨形态发生蛋白9(bone morphogenic protein 9, BMP9)
BMP9,也称为生长分化因子2(growth differentiation factor 2, GDF2),主要在肝脏中表达,并分泌到小鼠和人类的血浆中,调节许多生物过程,如间充质干细胞分化、血管生成、神经发生、软骨形成和肿瘤发生等[111~113]。Um等[114]研究表明当小鼠冷暴露3周后,肝脏BMP9表达和血浆水平显着增加。冷暴露可通过激活cAMP反应元件结合蛋白(cAMP response-element binding protein, CREB)和CREB结合蛋白诱导肝脏BMP9表达从而加速脂肪细胞发生褐变。综上所述,BMP9作为一种在寒冷条件下发挥作用的新型肝脏分泌因子,有望成为预防和治疗肥胖以及代谢疾病的有力药理学靶标。
12 妊娠区带蛋白(pregnancy zone protein, PZP)
PZP是一种高度依赖雌激素的免疫血浆蛋白,含有多个蛋白酶的识别位点,在人类妊娠期间的表达显着升高[115]。Lin等[15]发现,小鼠和人体的血清和肝脏中PZP的蛋白表达量在进食后显著增加。研究显示,在饮食诱导下PZP与小鼠棕色脂肪(brown adipose tissue, BAT)中的受体GRP78蛋白(glucose regulated protein of 78 kDa)结合,形成器官间的信号传导。其通过激活BAT中的p38 MAPK-ATF2信号通路促进BAT的产热,减轻机体重量,并改善了相关的代谢紊乱。该研究揭示了间歇性饮食条件下肝脏与BAT之间相互作用的分子机制,为以调控产热为靶点而防治肥胖提供了重要的依据,有望得以实现临床转化,为治疗减重提供新策略。
13 结语与展望
长期以来,肝脏作为体内一个重要的代谢器官长期受到科学界重视,其在凝血、解毒、代谢、免疫、消化吸收等方面都发挥重要作用[116,117]。一些解剖、结构和功能特征同时也证明了肝脏是组织间通讯的重要器官。肝脏接受约25%的心输出量,提供大量血液,肝细胞和非实质细胞将产物分泌到肝窦中,这些产物通过中央静脉流向下腔静脉,最终流向心脏后重新分配到外周组织。由于肝脏控制调节全身葡萄糖和脂质代谢稳态,因此其在代谢性疾病扮演着关键作用。例如,在NAFLD发病过程中发生肝脏脂肪变性可引起肝脏胰岛素抵抗,从而导致血糖升高和2型糖尿病的发生发展。肝脏还通过释放肝脏分泌因子来调节其他组织中的葡萄糖代谢和胰岛素作用,而患有代谢性疾病后会改变肝脏蛋白质组的分泌,从而促进胰岛素抵抗和其他代谢并发症的发生(图1)。
越来越多的研究表明,肝脏分泌因子在机体代谢及能量调控方面发挥着重要作用,它们可以通过以下一项或多项来调节整个生物体的能量平衡过程:(1)增加胰岛素敏感性;(2)增加葡萄糖摄取;(3)降低血浆甘油三酯水平、调节胆固醇稳态;(4)降低食物摄入量、减轻体重;(5)激活脂肪细胞增加能量消耗。因此适当地干预相关肝脏分泌因子的表达,有助于预防、诊断和治疗代谢性疾病。目前,已有部分针对肝脏分泌因子开发的药物已用于临床试验。例如,IONIS-ANGPTL3-LRx作为一种靶向人ANGPTL3 mRNA编码序列内的反义寡核苷酸药物,可降低ANGPTL3蛋白表达量和相关脂蛋白水平,且没有发现治疗相关的不良事件[118]。此外,使用FGF21类似物LY2405319对肥胖和2型糖尿病患者进行治疗,发现该药物具有改善血脂异常、促进体重降低、降低空腹胰岛素水平等疗效[119]。但目前关于这类药物的安全性、耐受性、药代动力学和药效学的数据很少,因此未来应继续进行相关试验来探索未知领域从而促进临床转化。

图1 肝脏分泌因子通过运输到远端组织发挥系统作用
在胰腺中,Fetuin-A破坏β细胞功能成熟,而ANGPTL8促进β细胞增殖;FGF21调节腹内侧下丘脑的蔗糖摄入量;在血管和肺部,Fetuin-A抑制血管钙化和肺部肿瘤生长;在肌肉中,Adropin通过抑制平滑肌细胞增殖,LECT2、HFREP1和Fetuin-B诱导骨骼肌产生胰岛素抵抗;在脂肪组织中,PZP激活BAT促进产热,TSK抑制BAT活性减少产热,MANF和BMP9促进WAT褐变,ANGPTL4加速脂肪细胞中脂质的分解,GPNMB刺激脂肪细胞中的脂质生成。在心脏中,Adropin改善内皮细胞功能,Fetuin-A抑制心脏中钙盐的形成和积累;此外,Fetuin-A可以抑制肾结石的形成。
虽然已陆续发现数种肝脏分泌因子并在其与代谢性疾病的关系研究中取得了实质性的进展,但该领域仍面临一些挑战。第一个挑战是如何对靶细胞/靶器官的信号接收或响应能力进行评估。事实上,由于靶器官/靶细胞的差异,不同的肝脏分泌因子在发挥生理功能时存在极大的不同。因此,在临床上不仅要考虑特殊情况下肝脏分泌因子的变化差异,还需要对其运输途径及靶器官的信号接收或响应能力进行评估,尤其是在多种复杂的肝脏分泌因子共同作用的情况下,这一现象使得进一步研究工作变得十分艰难。第二个挑战是探究代谢性疾病如何调节肝脏分泌因子的变化。研究表明,脂肪变性过程中会发生转录重编程,但通过内质网和高尔基体调节蛋白质加工的机制尚不清楚。第三个也是最重要的临床挑战是能否确定一种或一组肝源性分泌因子来预测或者诊断代谢性疾病的发生发展过程。此外,上述分泌因子还存着肝脏是否是其唯一分泌器官的差异,如Adropin,也在大脑中分泌。且在不同生理/病理情况下,对单一因子在多器官分泌的调节往往更加难以操作。解决这些挑战与问题将加深人们对于代谢性疾病发病机制的理解,并从肝脏分泌因子的角度开发有效的治疗手段。
[1] Saklayen MG. The global epidemic of the metabolic syndrome., 2018, 20(2): 12.
[2] Kuang H, Lin JD. Gpnmb: expanding the code for liver-fat communication., 2019, 1(5): 507–508.
[3] Kilkenny DM, Rocheleau JV. The FGF21 receptor signaling complex: Klothoβ, fgfr1c, and other regulatory interactions., 2016, 101: 17–58.
[4] Jiang S, Qiu GH, Zhu N, Hu ZY, Liao DF, Qin L. ANGPTL3: a novel biomarker and promising therapeutic target., 2019, 27(8): 876–884.
[5] Chen SY, Feng MY, Zhang SY, Dong ZW, Wang YF, Zhang WX, Liu C. ANGPTL8 mediates food-driven resetting of hepatic circadian clock in mice., 2019, 10(1): 3518.
[6] Bartucci R, Salvati A, Olinga P, Boersma YL. Vanin 1: Its physiological function and role in diseases., 2019, 20(16): 3891.
[7] Sardana O, Goyal R, Bedi O. Molecular and pathobiological involvement of fetuin-A in the pathogenesis of NAFLD., 2021, 29(4): 1061–1074.
[8] Stefan N, Häring HU. The role of hepatokines in metabolism., 2013, 9(3): 144–152.
[9] Taya M, Hammes SR. Glycoprotein non-metastatic melanoma protein B (GPNMB) and cancer: a novel potential therapeutic target., 2018, 133: 102–107.
[10] Stein LM, Yosten GLC, Samson WK. Adropin acts in brain to inhibit water drinking: potential interaction with the orphan g protein-coupled receptor, gpr19., 2016, 310(6): R476– R480.
[11] Xie Y, Fan KW, Guan SX, Hu Y, Gao Y, Zhou WJ. Lect2: A pleiotropic and promising hepatokine, from bench to bedside., 2022, 26(13): 3598–3607.
[12] Liu XH, Qi LW, Alolga RN, Liu Q. Implication of the hepatokine, fibrinogen-like protein 1 in liver diseases, metabolic disorders and cancer: the need to harness its full potential., 2022, 18(1): 292–300.
[13] Yagi T, Asada R, Kanekura K, Eesmaa A, Lindahl M, Saarma M, Urano F. Neuroplastin modulates anti- inflammatory effects of manf., 2020, 23(12): 101810.
[14] Yang S, Li SH, Li XJ. Manf: a new player in the control of energy homeostasis, and beyond., 2018, 9: 1725.
[15] Lin J, Jiang XX, Dong M, Liu XM, Shen QW, Huang YY, Zhang HL, Ye RC, Zhou HQ, Yan CL, Yuan SL, Wu XN, Chen L, Wang YF, He M, Tao Y, Zhang ZY, Jin WZ. Hepatokine pregnancy zone protein governs the diet- induced thermogenesis through activating brown adipose tissue., 2021, 8(21): e2101991.
[16] Jiang QQ, Liu BB, Xu KS. New insights into BMP9 signaling in liver diseases., 2021, 476(10): 3591–3600.
[17] Kumar R, Kuligina E, Sokolenko A, Siddiqui Q, Gardi N, Gupta S, Varma AK, Hasan SK. Genetic ablation of pregnancy zone protein promotes breast cancer progression by activating TGF-β/SMAD signaling., 2021, 185(2): 317–330.
[18] Philip A, Bostedt L, Stigbrand T, O'Connor-McCourt MD. Binding of transforming growth factor-beta (TGF-beta) to pregnancy zone protein (PZP). Comparison to the TGF-beta-alpha 2-macroglobulin interaction., 1994, 221(2): 687–693.
[19] Mossahebi-Mohammadi M, Quan MY, Zhang JS, Li XK. FGF signaling pathway: a key regulator of stem cell pluripotency., 2020, 8: 79.
[20] Savchenko E, Teku GN, Boza-Serrano A, Russ K, Berns M, Deierborg T, Lamas NJ, Wichterle H, Rothstein J, Henderson CE, Vihinen M, Roybon L. FGF family members differentially regulate maturation and proliferationof stem cell-derived astrocytes., 2019, 9(1): 9610.
[21] Wang J, Xiang B, Dolinsky VW, Kardami E, Cattini PA. Cardiac FGF-16 expression supports cardiomyocyte survival and increases resistance to doxorubicin cytotoxicity., 2018, 37(11): 866–877.
[22] Blitz E, Matsuda H, Guenther S, Morikawa T, Kubota Y, Zada D, Lerer-Goldshtein T, Stainier DYR, Appelbaum L. Thyroid hormones regulate goblet cell differentiation and FGF19-FGFR4 signaling., 2021, 162(5): bqab047.
[23] Farooq M, Khan AW, Kim MS, Choi S. The role of fibroblast growth factor (FGF) signaling in tissue repair and regeneration., 2021, 10(11): 3242.
[24] Geng LL, Lam KSL, Xu AM. The therapeutic potential of FGF21 in metabolic diseases: from bench to clinic., 2020, 16(11): 654–667.
[25] Morton GJ, Kaiyala KJ, Foster-Schubert KE, Cummings DE, Schwartz MW. Carbohydrate feeding dissociates the postprandial FGF19 response from circulating bile acid levels in humans., 2014, 99(2): E241–E245.
[26] Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo GZ, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis., 2005, 2(4): 217–225.
[27] Yoshiko Y, Wang H, Minamizaki T, Ijuin C, Yamamoto R, Suemune S, Kozai K, Tanne K, Aubin JE, Maeda N. Mineralized tissue cells are a principal source of FGF23., 2007, 40(6): 1565–1573.
[28] Quarles LD. Skeletal secretion of FGF-23 regulates phosphate and vitamin d metabolism., 2012, 8(5): 276–286.
[29] Tezze C, Romanello V, Sandri M. fgf21 as modulator of metabolism in health and disease., 2019, 10: 419.
[30] Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver., 2000, 1492(1): 203–206.
[31] Markan KR, Naber MC, Ameka MK, Anderegg MD, Mangelsdorf DJ, Kliewer SA, Mohammadi M, Potthoff MJ. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding., 2014, 63(12): 4057–4063.
[32] Xu S, Dai J, Tong Y. Research progress of FGF21 and disease treatment., 2022, 29(2): 209– 216.
徐赛, 戴佳, 童玥. FGF21与疾病治疗的研究进展. 药物生物技术, 2022, 29(2): 209–216.
[33] Keinicke H, Sun G, Mentzel CMJ, Fredholm M, John LM, Andersen B, Raun K, Kjaergaard M. FGF21 regulates hepatic metabolic pathways to improve steatosis and inflammation., 2020, 9(8): 755–768.
[34] Guo C, Zhao L, Li YY, Deng X, Yuan GY. Relationship between FGF21 and drug or nondrug therapy of type 2 diabetes mellitus., 2021, 236(1): 55–67.
[35] Gao Y, Zhang W, Zeng LQ, Bai H, Li J, Zhou J, Zhou GY, Fang CW, Wang F, Qin XJ. Exercise and dietary intervention ameliorate high-fat diet-induced NAFLD and liver aging by inducing lipophagy., 2020, 36: 101635.
[36] Xu J, Stanislaus S, Chinookoswong N, Lau YY, Hager T, Patel J, Ge HF, Weiszmann J, Lu SC, Graham M, Busby J, Hecht R, Li YS, Li Y, Lindberg R, Véniant MM. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models—association with liver and adipose tissue effects., 2009, 297(5): E1105-E1114.
[37] Wang N, Sun B, Guo HN, Jing YY, Ruan Q, Wang MJ, Mi Y, Chen H, Song L, Cui W. Association of elevated plasma FGF21 and activated FGF21 signaling in visceral white adipose tissue and improved insulin sensitivity in gestational diabetes mellitus subtype: a case-control study., 2021, 12: 795520.
[38] Schlein C, Talukdar S, Heine M, Fischer AW, Krott LM, Nilsson SK, Brenner MB, Heeren J, Scheja L. FGF21 lowers plasma triglycerides by accelerating lipoprotein catabolism in white and brown adipose tissues., 2016, 23(3): 441–453.
[39] Cuevas-Ramos D, Mehta R, Aguilar-Salinas CA. Fibroblast growth factor 21 and browning of white adipose tissue., 2019, 10: 37.
[40] Owen BM, Ding XS, Morgan DA, Coate KC, Bookout AL, Rahmouni K, Kliewer SA, Mangelsdorf DJ. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss., 2014, 20(4): 670–677.
[41] Bao LC, Yin J, Gao W, Wang Q, Yao WB, Gao XD. A long-acting FGF21 alleviates hepatic steatosis and inflamemation in a mouse model of non-alcoholic steatohepatitis partly through an FGF21-adiponectin-IL17A pathway., 2018, 175(16): 3379–3393.
[42] Carbone C, Piro G, Fassan M, Tamburrino A, Mina MM, Zanotto M, Chiao PJ, Bassi C, Scarpa A, Tortora G, Melisi D. An angiopoietin-like protein 2 autocrine signalingpromotes EMT during pancreatic ductal carcinogenesis., 2015, 6(15): 13822–13834.
[43] Abu-Farha M, Sriraman D, Cherian P, AlKhairi I, Elkum N, Behbehani K, Abubaker J. Circulating ANGPTL8/betatrophin is increased in obesity and reduced after exercise training., 2016, 11(1): e0147367.
[44] Chen HA, Kuo TC, Tseng CF, Ma JT, Yang ST, Yen CJ, Yang CY, Sung SY, Su JL. Angiopoietin-like protein 1 antagonizes MET receptor activity to repress sorafenib resistance and cancer stemness in hepatocellular carcinoma., 2016, 64(5): 1637–1651.
[45] Conklin D, Gilbertson D, Taft DW, Maurer MF, Whitmore TE, Smith DL, Walker KM, Chen LH, Wattler S, Nehls M, Lewis KB. Identification of a mammalian angiopoietin-related protein expressed specifically in liver., 1999, 62(3): 477–482.
[46] Kersten S. Physiological regulation of lipoprotein lipase., 2014, 1841(7): 919–933.
[47] Wang Y, Gusarova V, Banfi S, Gromada J, Cohen JC, Hobbs HH. Inactivation of ANGPTL3 reduces hepatic VLDL-triglyceride secretion., 2015, 56(7): 1296– 1307.
[48] Gusarova V, Alexa CA, Wang Y, Rafique A, Kim JH, Buckler D, Mintah IJ, Shihanian LM, Cohen JC, Hobbs HH, Xin YR, Valenzuela DM, Murphy AJ, Yancopoulos GD, Gromada J. ANGPTL3 blockade with a human monoclonal antibody reduces plasma lipids in dyslipidemic mice and monkeys., 2015, 56(7): 1308–1317.
[49] Robciuc MR, Maranghi M, Lahikainen A, Rader D, Bensadoun A, Öörni K, Metso J, Minicocci I, Ciociola E, Ceci F, Montali A, Arca M, Ehnholm C, Jauhiainen M. ANGPTL3 deficiency is associated with increased insulin sensitivity, lipoprotein lipase activity, and decreased serum free fatty acids., 2013, 33(7): 1706–1713.
[50] Stitziel NO, Khera AV, Wang X, Bierhals AJ, Vourakis AC, Sperry AE, Natarajan P, Klarin D, Emdin CA, Zekavat SM, Nomura A, Erdmann J, Schunkert H, Samani NJ, Kraus WE, Shah SH, Yu B, Boerwinkle E, Rader DJ, Gupta N, Frossard PM, Rasheed A, Danesh J, Lander ES, Gabriel S, Saleheen D, Musunuru K, Kathiresan S, PROMIS and Myocardial Infarction Genetics Consortium Investigators. ANGPTL3 deficiency and protection against coronary artery disease., 2017, 69(16): 2054–2063.
[51] Dewey FE, Gusarova V, Dunbar RL, O'Dushlaine C, Schurmann C, Gottesman O, McCarthy S, Van Hout CV, Bruse S, Dansky HM, Leader JB, Murray MF, Ritchie MD, Kirchner HL, Habegger L, Lopez A, Penn J, Zhao A, Shao WP, Stahl N, Murphy AJ, Hamon S, Bouzelmat A, Zhang R, Shumel B, Pordy R, Gipe D, Herman GA, Sheu WHH, Lee IT, Liang KW, Guo XQ, Rotter JI, Chen YDI, Kraus WE, Shah SH, Damrauer S, Small A, Rader DJ, Wulff AB, Nordestgaard BG, Tybjaerg-Hansen A, van den Hoek AM, Princen HMG, Ledbetter DH, Carey DJ, Overton JD, Reid JG, Sasiela WJ, Banerjee P, Shuldiner AR, Borecki IB, Teslovich TM, Yancopoulos GD, Mellis SJ, Gromada J, Baras A. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease., 2017, 377(3): 211–221.
[52] Raal FJ, Rosenson RS, Reeskamp LF, Hovingh GK, Kastelein JJP, Rubba P, Ali S, Banerjee P, Chan KC, Gipe DA, Khilla N, Pordy R, Weinreich DM, Yancopoulos GD, Zhang Y, Gaudet D, Investigators EH. Evinacumab for homozygous familial hypercholesterolemia., 2020, 383(8): 711–720.
[53] McQueen AE, Kanamaluru D, Yan K, Gray NE, Wu L, Li ML, Chang A, Hasan A, Stifler D, Koliwad SK, Wang JC. The c-terminal fibrinogen-like domain of angiopoietin-like 4 stimulates adipose tissue lipolysis and promotes energy expenditure., 2017, 292(39): 16122–16134.
[54] Zhu PC, Goh YY, Chin HFA, Kersten S, Tan NS. Angiopoietin-like 4: a decade of research., 2012, 32(3): 211–219.
[55] Robal T, Larsson M, Martin M, Olivecrona G, Lookene A. Fatty acids bind tightly to the N-terminal domain of angiopoietin-like protein 4 and modulate its interaction with lipoprotein lipase., 2012, 287(35): 29739–29752.
[56] Mandard S, Zandbergen F, van Straten E, Wahli W, Kuipers F, Müller M, Kersten S. The fasting-induced adipose factor/angiopoietin-like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity., 2006, 281(2): 934–944.
[57] Xu AM, Lam MC, Chan KW, Wang Y, Zhang JL, Hoo RLC, Xu JY, Chen BY, Chow WS, Tso AWK, Lam KSL. Angiopoietin-like protein 4 decreases blood glucose and improves glucose tolerance but induces hyperlipidemia and hepatic steatosis in mice., 2005, 102(17): 6086–6091.
[58] Singh AK, Aryal B, Chaube B, Rotllan N, Varela L, Horvath TL, Suárez Y, Fernández-Hernando C. Brown adipose tissue derived ANGPTL4 controls glucose and lipid metabolism and regulates thermogenesis., 2018, 11: 59–69.
[59] Aryal B, Singh AK, Zhang XB, Varela L, Rotllan N, Goedeke L, Chaube B, Camporez JP, Vatner DF, Horvath TL, Shulman GI, Suárez Y, Fernandez-Hernando C. Absence of ANGPTL4 in adipose tissue improves glucose tolerance and attenuates atherogenesis., 2018, 3(6): e97918.
[60] Li YC, Teng CB. Angiopoietin-like proteins 3, 4 and 8: Regulating lipid metabolism and providing new hope for metabolic syndrome., 2014, 22(8): 679–687.
[61] Vatner DF, Goedeke L, Camporez JPG, Lyu K, Nasiri AR, Zhang DY, Bhanot S, Murray SF, Still CD, Gerhard GS, Shulman GI, Samuel VT. ANGPTL8 antisense oligonucleotide improves adipose lipid metabolism and prevents diet-induced nafld and hepatic insulin resistance in rodents., 2018, 61(6): 1435–1446.
[62] Zheng JK, Umikawa M, Cui CH, Li JY, Chen XL, Zhang CZ, Huynh H, Kang XL, Silvany R, Wan X, Ye JX, CantóAP, Chen SH, Wang HY, Ward ES, Zhang CC. Inhibitory receptors bind ANGPTLs and support blood stem cells and leukaemia development., 2012, 485(7400): 656–660.
[63] Kersten S. New insights into angiopoietin-like proteins in lipid metabolism and cardiovascular disease risk., 2019, 30(3): 205–211.
[64] Catalano-Iniesta L, Sánchez Robledo V, Iglesias-Osma MC, Galán Albiñana A, Carrero S, Blanco EJ, Carretero- Hernández M, Carretero J, García-Barrado MJ. Evidences for expression and location of ANGPTL8 in human adipose tissue., 2020, 9(2): 512.
[65] Espes D, Lau J, Carlsson PO. Increased circulating levels of betatrophin in individuals with long-standing type 1 diabetes., 2014, 57(1): 50–53.
[66] Zhang R. Lipasin, a novel nutritionally-regulated liver- enriched factor that regulates serum triglyceride levels., 2012, 424(4): 786–792.
[67] Dang FB, Wu R, Wang PF, Wu YT, Azam MS, Xu Q, Chen YQ, Liu Y. Fasting and feeding signals control the oscillatory expression of ANGPTL8 to modulate lipid metabolism., 2016, 6: 36926.
[68] Rommelaere S, Millet V, Gensollen T, Bourges C, Eeckhoute J, Hennuyer N, Baugé E, Chasson L, Cacciatore I, Staels B, Pitari G, Galland F, Naquet P. Pparalpha regulates the production of serum vanin-1 by liver., 2013, 587(22): 3742–3748.
[69] Chen SY, Zhang WX, Tang CQ, Tang XL, Liu L, Liu C. Vanin-1 is a key activator for hepatic gluconeogenesis., 2014, 63(6): 2073–2085.
[70] Chen SY, Zhang WX, Sun C, Song MM, Liu S, Xu MY, Zhang XJ, Liu L, Liu C. Systemic nanoparticle-mediated delivery of pantetheinase vanin-1 regulates lipolysis and adiposity in abdominal white adipose tissue., 2021, 8(12): e2101789.
[71] van Diepen JA, Jansen PA, Ballak DB, Hijmans A, Hooiveld GJ, Rommelaere S, Galland F, Naquet P, Rutjes FPJT, Mensink RP, Schrauwen P, Tack CJ, Netea MG, Kersten S, Schalkwijk J, Stienstra R. PPAR-alpha dependent regulation of vanin-1 mediates hepatic lipid metabolism., 2014, 61(2): 366–372.
[72] Ferreira DW, Goedken MJ, Rommelaere S, Chasson L, Galland F, Naquet P, Manautou JE. Enhanced hepatotoxicity by acetaminophen in vanin-1 knockout mice is associated with deficient proliferative and immune responses., 2016, 1862(4): 662–669.
[73] Icer MA, Yıldıran H. Effects of fetuin-A with diverse functions and multiple mechanisms on human health., 2021, 88: 1–10.
[74] Olivier E, Soury E, Ruminy P, Husson A, Parmentier F, Daveau M, Salier JP. Fetuin-B, a second member of the fetuin family in mammals., 2000, 350(Pt 2): 589–597.
[75] Brown WM, Saunders NR, Møllgård K, Dziegielewska KM. Fetuin——an old friend revisited., 1992, 14(11): 749–755.
[76] Icer MA, Yıldıran H. Effects of nutritional status on serum fetuin-A level., 2020, 60(11): 1938–1946.
[77] Chung HS, Lee HJ, Hwang SY, Choi JH, Yoo HJ, Seo JA, Kim SG, Kim NH, Choi DS, Baik SH, Choi KM. Relationship of circulating fetuin-A levels with body size and metabolic phenotypes., 2018, 2018: 7918714.
[78] Sujana C, Huth C, Zierer A, Meesters S, Sudduth-Klinger J, Koenig W, Herder C, Peters A, Thorand B. Association of fetuin-A with incident type 2 diabetes: results from the monica/kora augsburg study and a systematic meta- analysis., 2018, 178(4): 389–398.
[79] Guo VY, Cao B, Cai CY, Cheng KKY, Cheung BMY. Fetuin-A levels and risk of type 2 diabetes mellitus: a systematic review and meta-analysis., 2018, 55(1): 87–98.
[80] Trepanowski JF, Mey J, Varady KA. Fetuin-A: a novel link between obesity and related complications., 2015, 39(5): 734–741.
[81] Mathews ST, Singh GP, Ranalletta M, Cintron VJ, Qiang XL, Goustin AS, Jen KLC, Charron MJ, Jahnen-Dechent W, Grunberger G. Improved insulin sensitivity and resistance to weight gain in mice null for the ahsg gene., 2002, 51(8): 2450–2458.
[82] Shi J, Fan JG, Su Q, Yang Z. Cytokines and abnormal glucose and lipid metabolism., 2019, 10: 703.
[83] Li LN, Spranger L, Stobäus N, Beer F, Decker AM, Wernicke C, Brachs S, Brachs M, Spranger J, Mai K. Fetuin-B, a potential link of liver-adipose tissue cross talk during diet-induced weight loss-weight maintenance., 2021, 11(1): 31.
[84] Meex RC, Hoy AJ, Morris A, Brown RD, Lo JCY, Burke M, Goode RJA, Kingwell BA, Kraakman MJ, Febbraio MA, Greve JW, Rensen SS, Molloy MP, Lancaster GI, Bruce CR, Watt MJ. Fetuin B is a secreted hepatocyte factor linking steatosis to impaired glucose metabolism., 2015, 22(6): 1078–1089.
[85] Weterman MA, Ajubi N, van Dinter IM, Degen WG, van Muijen GN, Ruitter DJ, Bloemers HP. Nmb, a novel gene, is expressed in low-metastatic human melanoma cell lines and xenografts., 1995, 60(1): 73–81.
[86] van der Lienden MJC, Gaspar P, Boot R, Aerts JMFG, van Eijk M. Glycoprotein non-metastatic protein B: an emerging biomarker for lysosomal dysfunction in macrophages., 2018, 20(1): 66.
[87] Abdelmagid SM, Barbe MF, Rico MC, Salihoglu S, Arango-Hisijara I, Selim AH, Anderson MG, Owen TA, Popoff SN, Safadi FF. Osteoactivin, an anabolic factor that regulates osteoblast differentiation and function., 2008, 314(13): 2334–2351.
[88] Rose AAN, Annis MG, Dong ZF, Pepin F, Hallett M, Park M, Siegel PM. ADAM10 releases a soluble form of the gpnmb/osteoactivin extracellular domain with angiogenic properties., 2010, 5(8): e12093.
[89] Ripoll VM, Irvine KM, Ravasi T, Sweet MJ, Hume DA. Gpnmb is induced in macrophages by IFN-gamma and lipopolysaccharide and acts as a feedback regulator of proinflammatory responses., 2007, 178(10): 6557–6566.
[90] Gong XM, Li YF, Luo J, Wang JQ, Wei J, Wang JQ, Xiao T, Xie C, Hong J, Ning G, Shi XJ, Li BL, Qi W, Song BL. Gpnmb secreted from liver promotes lipogenesis in white adipose tissue and aggravates obesity and insulin resistance., 2019, 1(5): 570–583.
[91] Katayama A, Nakatsuka A, Eguchi J, Murakami K, Teshigawara S, Kanzaki M, Nunoue T, Hida K, Wada N, Yasunaka T, Ikeda F, Takaki A, Yamamoto K, Kiyonari H, Makino H, Wada J. Beneficial impact of GPNMB and its significance as a biomarker in nonalcoholic steatohepatitis., 2015, 5: 16920.
[92] Nickl B, Qadri F, Bader M. Anti-inflammatory role of GPNMB in adipose tissue of mice., 2021, 11(1): 19614.
[93] Kumar KG, Trevaskis JL, Lam DD, Sutton GM, Koza RA, Chouljenko VN, Kousoulas KG, Rogers PM, Kesterson RA, Thearle M, Ferrante AW, Mynatt RL, Burris TP, Dong JZ, Halem HA, Culler MD, Heisler LK, Stephens JM, Butler AA. Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism., 2008, 8(6): 468–481.
[94] Butler AA, Tam CS, Stanhope KL, Wolfe BM, Ali MR, O'Keeffe M, St-Onge MP, Ravussin E, Havel PJ. Low circulating adropin concentrations with obesity and aging correlate with risk factors for metabolic disease and increase after gastric bypass surgery in humans., 2012, 97(10): 3783–3791.
[95] Chen S, Zeng K, Liu QC, Guo Z, Zhang S, Chen XR, Lin JH, Wen JP, Zhao CF, Lin XH, Gao F. Adropin deficiency worsens HFD-induced metabolic defects., 2017, 8(8): e3008.
[96] Ganesh Kumar K, Zhang JY, Gao S, Rossi J, McGuinness OP, Halem HH, Culler MD, Mynatt RL, Butler AA. Adropin deficiency is associated with increased adiposity and insulin resistance., 2012, 20(7): 1394–1402.
[97] Xiong XL, Wang QY, Wang S, Zhang JL, Liu TY, Guo L, Yu YH, Lin JD. Mapping the molecular signatures of diet-induced NASH and its regulation by the hepatokine tsukushi., 2019, 20: 128–137.
[98] Wang QY, Sharma VP, Shen H, Xiao YY, Zhu Q, Xiong XL, Guo L, Jiang L, Ohta K, Li SM, Shi HF, Rui LY, Lin JD. The hepatokine tsukushi gates energy expenditure via brown fat sympathetic innervation., 2019, 1(2): 251–260.
[99] Mouchiroud M, Camiré É, Aldow M, Caron A, Jubinville É, Turcotte L, Kaci I, Beaulieu MJ, Roy C, Labbé SM, Varin TV, Gélinas Y, Lamothe J, Trottier J, Mitchell PL, Guénard F, Festuccia WT, Joubert P, Rose CF, Karvellas CJ, Barbier O, Morissette MC, Marette A, Laplante M. The hepatokine tsukushi is released in response to NAFLD and impacts cholesterol homeostasis., 2019, 4(15): e129492.
[100] Yamagoe S, Yamakawa Y, Matsuo Y, Minowada J, Mizuno S, Suzuki K. Purification and primary amino acid sequence of a novel neutrophil chemotactic factor LECT2., 1996, 52(1): 9–13.
[101] Chikamoto K, Misu H, Takayama H, Kikuchi A, Ishii KA, Lan F, Takata N, Tajima-Shirasaki N, Takeshita Y, Tsugane H, Kaneko S, Matsugo S, Takamura T. Rapid response of the steatosis-sensing hepatokine LECT2 during diet-induced weight cycling in mice., 2016, 478(3): 1310–1316.
[102] Lan F, Misu H, Chikamoto K, Takayama H, Kikuchi A, Mohri K, Takata N, Hayashi H, Matsuzawa-Nagata N, Takeshita Y, Noda H, Matsumoto Y, Ota T, Nagano T, Nakagen M, Miyamoto KI, Takatsuki K, Seo T, Iwayama K, Tokuyama K, Matsugo S, Tang H, Saito Y, Yamagoe S, Kaneko S, Takamura T. LECT2 functions as a hepatokine that links obesity to skeletal muscle insulin resistance., 2014, 63(5): 1649–1664.
[103] Hirosumi J, Tuncman G, Chang LF, Görgün CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance., 2002, 420(6913): 333–336.
[104] Gao M, Zhan YQ, Yu M, Ge CH, Li CY, Zhang JH, Wang XH, Ge ZQ, Yang XM. Hepassocin activates the EGFR/ERK cascade and induces proliferation of l02 cells through the Src-dependent pathway., 2014, 26(10): 2161–2166.
[105] Wu HT, Ou HY, Hung HC, Su YC, Lu FH, Wu JS, Yang YC, Wu CL, Chang CJ. A novel hepatokine, HFREP1, plays a crucial role in the development of insulin resistance and type 2 diabetes., 2016, 59(8): 1732–1742.
[106] Cheng KP, Ou HY, Hung HC, Li CH, Fan KC, Wu JS, Wu HT, Chang CJ. Unsaturated fatty acids increase the expression of hepassocin through a signal transducer and activator of transcription 3-dependent pathway in HepG2 cells., 2018, 53(9): 863–869.
[107] Jung TW, Chung YH, Kim HC, Abd El-Aty AM, Jeong JH. Hyperlipidemia-induced hepassocin in the liver contributes to insulin resistance in skeletal muscle., 2018, 470: 26–33.
[108] Glembotski CC, Thuerauf DJ, Huang CQ, Vekich JA, Gottlieb RA, Doroudgar S. Mesencephalic astrocyte-derived neurotrophic factor protects the heart from ischemic damage and is selectively secreted upon sarco/endoplasmic reticulum calcium depletion., 2012, 287(31): 25893–25904.
[109] Sousa-Victor P, Neves J, Cedron-Craft W, Ventura PB, Liao CY, Riley RR, Soifer I, van Bruggen N, Kolumam GA, Villeda SA, Lamba DA, Jasper H. MANF regulates metabolic and immune homeostasis in ageing and protects against liver damage., 2019, 1(2): 276–290.
[110] Wu T, Liu QH, Li YP, Li H, Chen L, Yang XP, Tang Q, Pu SY, Kuang JY, Li R, Huang Y, Zhang JH, Zhang ZJ, Zhou J, Huang CY, Zhang GR, Zhao YN, Zou M, Jiang W, Mo L, He JH. Feeding-induced hepatokine, MANF, ameliorates diet-induced obesity by promoting adipose browning via p38 mapk pathway., 2021, 218(6): e20201203.
[111] Jensen GS, Leon-Palmer NE, Townsend KL. Bone morphogenetic proteins (BMPs) in the central regulation of energy balance and adult neural plasticity., 2021, 123: 154837.
[112] David L, Mallet C, Keramidas M, Lamandé N, Gasc JM, Dupuis-Girod S, Plauchu H, Feige JJ, Bailly S. Bone morphogenetic protein-9 is a circulating vascular quiescence factor., 2008, 102(8): 914–922.
[113] Majumdar MK, Wang E, Morris EA. BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of Il-1., 2001, 189(3): 275–284.
[114] Um JH, Park SY, Hur JH, Lee HY, Jeong KH, Cho Y, Lee SH, Yoon SM, Choe S, Choi CS. Bone morphogenic protein 9 is a novel thermogenic hepatokine secreted in response to cold exposure., 2022, 129: 155139.
[115] Ekelund L, Laurell CB. The pregnancy zone protein response during gestation: a metabolic challenge., 1994, 54(8): 623–629.
[116] Kubes P, Jenne C. Immune responses in the liver., 2018, 36: 247–277.
[117] Adeva-Andany MM, Pérez-Felpete N, Fernández-Fernández C, Donapetry-García C, Pazos-García C. Liver glucose metabolism in humans., 2016, 36(6): e00416.
[118] Graham MJ, Lee RG, Brandt TA, Tai LJ, Fu WX, Peralta R, Yu R, Hurh E, Paz E, McEvoy BW, Baker BF, Pham NC, Digenio A, Hughes SG, Geary RS, Witztum JL, Crooke RM, Tsimikas S. Cardiovascular and metabolic effects of ANGPTL3 antisense oligonucleotides., 2017, 377(3): 222–232.
[119] Gaich G, Chien JY, Fu HD, Glass LC, Deeg MA, Holland WL, Kharitonenkov A, Bumol T, Schilske HK, Moller DE. The effects of ly2405319, an FGF21 analog, in obese human subjects with type 2 diabetes., 2013, 18(3): 333–340.
Interaction between hepatokines and metabolic diseases
Jiaqi Liang, Chang Liu, Wenxiang Zhang, Siyu Chen
Metabolic diseases are broadly defined as diseases caused by problems in metabolic function, including central obesity, insulin resistance, lipid glucose abnormalities, and elevated blood pressure. As an important metabolic organ, the liver plays a key role in regulating many physiological processes such as systemic glucose and lipid metabolism. Numerous studies in recent years have shown that the liver can synthesize and secrete a variety of hepatokines, including FGF21, Fetuin-A and ANGPTL8, which regulate the metabolism in an autocrine/paracrine manner. Intervention of hepatokines expression may contribute to the prevention, diagnosis and treatment of metabolic diseases. However, further studies are needed to be investigated as the mechanism of hepatokines and metabolic homeostasis is still elusive. In this review, we summarize the relationships between hepatokines and metabolic diseases in order to provide new strategies for the treatment of metabolic diseases.
hepatokines; metabolic diseases; diagnosis and therapy
2022-06-27;
2022-09-06;
2022-09-20
国家自然科学基金(编号:31800992)和江苏省自然科学基金(编号: BK20180554)资助[Supported by the Natural Science Foundation of China (No. 31800992) and the Natural Science Foundation of JiangSu (No. BK20180554)]
梁佳琦,在读硕士研究生,专业方向:病理生理学,细胞生物学。E-mail: 3221030939@stu.cpu.edu.cn
陈思禹,博士,副教授,研究方向:代谢性疾病的干预靶标及先导药物发现。E-mail: siyuchen@cpu.edu.cn
10.16288/j.yczz.22-218
(责任编委: 孟卓贤)

