IITZ-01 activates NLRP3 inflammasome by inducing mitochondrial damage
Wenxin Hu,and Wei Jiang
The CAS Key Laboratory of Innate Immunity and Chronic Disease,School of Basic Medical Sciences,Division of Life Sciences and Medicine,University of Science and Technology of China,Hefei 230027,China
Abstract: NLRP3 inflammasome can be activated by a variety of pathogen activators (including components of bacteria,viruses and fungi) or “danger signals” (including abnormal metabolites and environmental components),so its activation mechanism is extremely complex.IITZ-01 is a lysosomotropic molecule that can disrupt lysosomal functions.We found that IITZ-01 can activate inflammasome at a low concentration.Then,we determined that IITZ-01 is a specific activator of NLRP3 inflammasome through inflammasome stimulation,ELISA,Western blot and other experiments.Mechanistically,NLRP3 inflammasome activation induced by IITZ-01 is independent of direct binding and ion flow but dependent on mitochondrial damage and mROS accumulation.This study suggests that a lysosomotropic compound can activate NLRP3 inflammasome by impairing mitochondrial functions.
Keywords: NLRP3 inflammasome;IITZ-01;mitochondria;mROS
1 Introduction
NLRP3 inflammasome is a multiprotein complex composed of the receptor protein NLRP3,adaptor protein ASC and effector protein pro-caspase-1[1].After stimulated by PAMPs(pathogen-associated molecular patterns,such as pore-forming toxins,Nigericin,RNA of virus,β-glucans,etc.) or DAMPs (danger-associated molecular patterns,such as ATP,MSU,β-amyloid,dust,UV radiation,etc.),the priming signal will be initiated,leading to increased expression of NLRP3,pro-IL-1β and pro-IL-18[2-4].Subsequently,the NLRP3 protein undergoes conformational changes,and the exposed LRR domain binds to NEK7,which can promote oligomerization of the NLRP3 protein[5,6].The exposed Nterminal of NLRP3 recruits ASC,which also contains a PYD domain.ASC then recruits pro-caspase-1 and induces selfcleavage of pro-caspase-1,producing mature caspase-1.Activated caspase-1 cleaves precursors of inflammatory cytokines and GSDMD,mediating cell death known as pyroptosis.The mature inflammatory cytokines IL-1β and IL-18 are released outside cells in a GSDMD-dependent manner and then bind to cytokine receptors on the surface of other immune cells,causing signal cascade reactions and inflammatory reactions[7].Appropriate inflammasome activation is necessary for the host because it helps to eliminate infection and abnormal metabolic molecules in the body.Excessive inflammasome activation leads to various diseases,such as autoimmune diseases,metabolic diseases and neurodegenerative diseases[8-13].Because inflammasome disorders are associated with a variety of congenital and acquired inflammatory diseases,the study of the recognition and activation mechanism of inflammasome has important clinical significance[7].
NLRP3 inflammasome can be activated by a variety of agonists,such as bacteria,fungi,viruses and viral components,crystalline and particulate substances,metabolic molecules and environmental factors;therefore,the mechanism of NLRP3 inflammasome activation is extremely complex[14-24].It has been reported that Nigericin and ATP activate NLRP3 inflammasome by inducing potassium (K+) outflow[16].Particle-or crystal-like agonists (such as MSU,cholesterol crystals,β-amyloid,silica,asbestos,and aluminum) can enter cells through endocytosis and induce NLRP3 inflammasome activation by interrupting lysosomal functions[25-30].
Mitochondria can also produce mROS (mainly including superoxide anions and hydrogen peroxide) and activate NLRP3 inflammasome after sensing cellular stress[31,32].For example,CL097 can target mitochondria,leading to an increase in mROS.Then,mROS promote the binding of NEK7 with NLRP3,accelerating inflammasome assembly by binding to NQO2 or damaging the function of mitochondrial complex I[33].Another article pointed out that ROS can induce thioredoxin interacting protein (TXNIP) binding to the NLRP3 protein and activate NLRP3 inflammasome[34].Treatment with reactive oxygen inhibitors such as diphenylammonium iodide (DPI) or N-acetyl-L-cysteine (NAC) will block the transcriptional upregulation of NLRP3[35].Thus,reactive oxygen may be an important upstream signal of NLRP3 inflammasome assembly.However,not all agonists activate the inflammasome in a ROS-dependent manner.Serum amyloid A activates NLRP3 inflammasome through mROS-dependent or -independent pathways,suggesting that there may be other auxiliary pathways in cells that can replace ROS[36].In recent years,it has been found that mitochondrial components may be potential ligands of NLRP3 inflammasome.Extracellular ATP is a typical sterile agonist of NLRP3 inflammasome that can bind to the purine receptor P2X7,open ATP-gated potassium channels,and cause intracellular K+outflow.Other studies found that ATP can induce mitochondrial dysfunction and cause oxidative damage to mitochondrial DNA[37].When oxidized mitochondrial DNA is released into the cytoplasm,it activates NLRP3 inflammasome,suggesting that oxidized mitochondrial DNA is likely to be the ligand of NLRP3 inflammasome.The deletion of autophagic proteins (such as microtubule associated protein 1 light chain 3B or autophagy regulator Beclin 1),genetic variation or newly synthesized mitochondrial DNA will increase the amount of oxidized mitochondrial DNA in the cytoplasm,which may be the reasons why they can activate NLRP3 inflammasome[31,38-40].These results show that mitochondria play an important role in the assembly and activation of NLRP3 inflammasome.
At present,many agonists of NLRP3 inflammasome have been found,but their activation mechanisms are different.To further explore the mechanism of NLRP3 inflammasome activation,it is urgent to find more agonists of NLRP3 inflammasome.IITZ-01 is an s-triazine analog that has lipophilic and ionizable basic groups,so it can specifically accumulate in lysosomes,leading to lysosome dysfunction[41].Due to dysfunction,lysosomes cannot combine with late endosomes to form autolysosomes,which leads to a decrease in autophagic activity.Therefore,IITZ-01 is an efficient inhibitor of autophagy.Meanwhile,a small amount of IITZ-01 will accumulate in mitochondria,disrupting mitochondrial membrane potential (MMP) and inducing apoptosis of MDA-MB-231(human breast cancer cells) in a caspase-3-dependent manner.However,whether IITZ-01 participates in inflammatory reactions has not been reported.
In this study,we found that IITZ-01 could activate inflammasome in BMDM cells at a low concentration and only specifically activated NLRP3 inflammasome.Mechanistically,IITZ-01 activated NLRP3 inflammasome by inducing mitochondrial damage and mROS accumulation.This study indicated that a lysosomotropic compound can activate NLRP3 inflammasome by impairing mitochondrial function,providing a new reference for clarifying the activation mechanism of NLRP3 inflammasome.
2 Materials and methods
2.1 Mice
C57BL/6J mice were obtained from Shanghai SLAC Laboratory Animal Limited Liability Company (Shanghai,China).Casp1-/-mice were donated by Dr.Richard A.Flavell Group.Asc-/-mice were donated from Dr.Vishva M.Dixit Group.Gsdmd-/-mice were donated by Dr.Shu Zhu Group (University of Science and Technology of China).Nlrp3-/-mice were donated by Dr.Jurg Tschopp Group (University of Lausanne).Ipaf-/-mice were donated by Dr.Vishva M.Dixit Group.Aim2-/-mice were donated by Shanghai Tenth People’s Hospital.Pyrin-/-mice were donated by Dr.Feng Shao (National Institute of Biological Sciences,Beijing).Mice were maintained in an SPF facility under a 12 h/12 h light/dark cycle (lights on at 07:00 and off at 19:00).All animal studies were performed in accordance with the guidelines of the Ethics Committee of University of Science and Technology of China.
2.2 Reagents
LPS (L2630) and Nigericin (N7143) were purchased from Sigma.MitoSOX (M36008),MitoTracker (M7512),and DAPI (P36935) were acquired from Invitrogen.A mouse IL-1β ELISA kit (DY401) was purchased from R&D Systems.A protein concentration determination kit (BL521A) was purchased from Biosharp.IITZ-01 was purchased from Selleck(S8764) or MedChemExpress (HY-112897).CY-09 was synthesized by Dr.Xianming Deng (Xiamen University,Xiamen,China).MCC950 (S7809) was purchased from Selleck.MnTBAP (sc-221954) was purchased from Santa Cruz Biotechnology.The anti-β-actin (66009-1-Ig),anti-mouse caspase-1(AG-20B-0042),anti-mouse IL-1β (AF-401-NA),anti-ASC(67824S),anti-GSDMD (ab219800),anti-NLRP3 (AG-20B-0014) and anti-NEK7 (ab133514) antibodies were acquired from Proteintech Group,AdipoGen,R&D Systems,Cell Signaling Technology,Abcam,AdipoGen and Abcam,respectively.Preparation and storage of antibodies were performed according to the manufacturer’s instructions.
2.3 Extraction and culture of BMDM cells
Mice were killed by cervical dislocation.The bone marrow in mouse legs was blown into DMEM medium.After red blood cells were lysed,fresh medium containing 0.025% M-CSF was added.Then,bone marrow-derived macrophages (BMDM) cells were cultured in a 37 °C CO2incubator for 4-5 d.
2.4 Inflammasome stimulation
Mature BMDM cells were digested with EDTA,counted in blood cell counting plates,and then inoculated into 12-well plates for overnight culture.On the morning of the second day,the cells were treated with Opti-MEM medium containing 1/10000 LPS.Then,the cells were stimulated by compounds according to the experimental needs.After stimulation,the cell supernatant was collected,and IL-1β was detected by ELISA.Western blot could also be used to detect the expression levels of proteins in the cell supernatant and cell lysate.
2.5 ELISA
Cell supernatant samples were collected into Eppendorf tubes and analyzed for mouse IL-1β with an ELISA kit (purchased from R&D Systems,DY401) according to the manufacturer’s instructions.
2.6 Western blot
The proteins in the cell supernatant were extracted by the methanol-chloroform method.The proteins in the cells were directly diluted with sample buffer.Then,the samples were placed in a constant temperature metal bath for 10 min at 101 °C.Polyacrylamide gel electrophoresis was performed,80 V was separated for 30 min,and 120 V was separated for 1 h.The gel concentrate was discarded,and the proteins were transferred from the gel to a PVDF membrane under 90 V for 1 h.The PVDF membrane was sealed in 5% skim milk for 30 min,the primary antibody was added,and the samples were incubated at 4 °C overnight.The next day,the primary antibody was recovered.After incubating with the secondary antibody for 2 h,the PVDF membrane was washed with PBST 3 times and developed with developer.The development results were observed with BioRad and analyzed with Image Lab software.
2.7 Detection of intracellular potassium
BMDM cells fromNlrp3-/-mice were inoculated into 6-well plates (1.5×106-2×106cells/well).Cells were incubated overnight in a CO2incubator.The next day,the cells were stimulated normally according to the experimental needs.After stimulation,the supernatant was removed,and K+-free PBS was used to rinse the cells 3 times.One milliliter of nitric acid was added to each well to fully lyse the cells.The lysate was transferred into a 25 mL small beaker,2 mL concentrated nitric acid was added to each hole for flushing,and the nitric acid flushing solution was incorporated into the small beaker.The small beaker was heated with a magnetic stirrer,and nitric acid was boiled until dry.An appropriate amount of concentrated nitric acid was added to the small beaker,heated and boiled several times until the powders were light yellow after boiling.After reaching a constant volume,the samples were sent to the Instruments Center for Physical Science of the University of Science and Technology of China to measure the potassium ion (K+) content by inductively coupled plasma (ICP).
2.8 Detection of mitochondrial damage and mROS production
The cell climbing sheets were transferred into the cell culture plates.Mature BMDM cells (1.5×105-2×105cells/mL) were inoculated into cell culture plates.The next day,the cells were stimulated normally as needed for experiments.Mito-Tracker (final concentration is 50 nmol/L) or MitoSOX (final concentration is 1 nmol/L) was added 20-30 min before the end of stimulation.After stimulation,the supernatant was removed,and 1×PBS was used to flush the cells 3 times.Then,4% paraformaldehyde was added away from light,and climbing sheets were fixed at room temperature for 20 min.The cell climbing sheets were washed with PBST 3 times and shaken at room temperature for 10 min each time.Then,the sheets were sealed with DAPI,kept away from light at room temperature and dried overnight.The next day,climbing sheets were observed and scanned with the DAPI channel and rhodamine channel of a laser confocal microscope.
2.9 DARTS assays
Mature BMDM cells were prestimulated with Opti-MEM medium containing 1/10000 LPS.Then,NP-40 cell lysate was added to fully lyse cells,and the protein concentration in the lysate was determined by a BCA protein concentration determination kit.The proteins were diluted to 0.2 mg/mL with NP-40 cell lysate.The solution was divided into several groups,and different doses of compounds were added according to the manufacturer’s instructions.The next day,each sample was divided into two groups.One group was used as the control group,and another group was digested with 1500 ng/mL streptomycin protease.After 20 min,3× sample buffer was added to terminate the reaction.Finally,the target proteins and β-actin were detected by Western blot.
2.10 Statistical analysis
The data in this study were analyzed by GraphPad Prism software.IfP≥ 0.05,it means that there is no significant difference between the data of each group,marked as “ns”;if 0.01 ≤P≤ 0.05,it indicates that there are differences between groups of data,marked as “*”;ifP≤ 0.01,it indicates that there is a significant difference between the data of each group,marked as “**”;ifP≤ 0.001,it indicates that there is a very significant difference between the data of each group,marked as “***”.
3 Results
3.1 IITZ-01 activates inflammasome in BMDM cells
To determine whether IITZ-01 could activate inflammasome,we first prestimulated BMDM cells with LPS for 3 h to increase the expression of proteins related to the inflammasome and then added different concentrations of IITZ-01 or treated cells with IITZ-01 for different times.Finally,we detected caspase-1 activation and IL-1β secretion by ELISA and Western blot.Western blot experiments showed that IITZ-01 could induce the self-cleavage of pro-caspase-1 in BMDM cells,causing the production and release of mature caspase-1(Fig.1c).ELISA and Western blot experiments showed that IITZ-01 could induce IL-1β production in a partly dose-dependent and time-dependent manner (Fig.1).The optimal conditions for IITZ-01 to stimulate BMDM cells were 2 μmol/L and 3 h.These results suggested that IITZ-01 might be an activator of the inflammasome.
Pro-caspase-1 is an essential component of inflammasome.In some cases,ASC also participates in the formation of inflammasome as a connector protein.After activation,the inflammasome punches holes in the cell membrane in a GSDMD-dependent manner with the release of the inflammatory cytokines IL-1β and IL-18.To further determine whether IITZ-01 could activate inflammasome,we isolated BMDM cells from wild-type (WT) mice andCasp1-/-,Asc-/-orGsdmd-/-mice,stimulated them with IITZ-01 under the same conditions,and finally detected caspase-1 and IL-1β by ELISA and Western blot.It was found that when caspase-1,ASC or GSDMD proteins were absent in cells,IL-1β release decreased significantly (Fig.2a,c,e),and caspase-1 activation was severely inhibited (Fig.2b,d,f).The above results showed that the activation and release of caspase-1 induced by IITZ-01 depended on ASC and GSMSD,while the production of IL-1β depended on caspase-1,ASC and GSDMD.In conclusion,IITZ-01 could induce IL-1β formation by activating inflammasome.
3.2 IITZ-01 specifically activates NLRP3 inflammasome
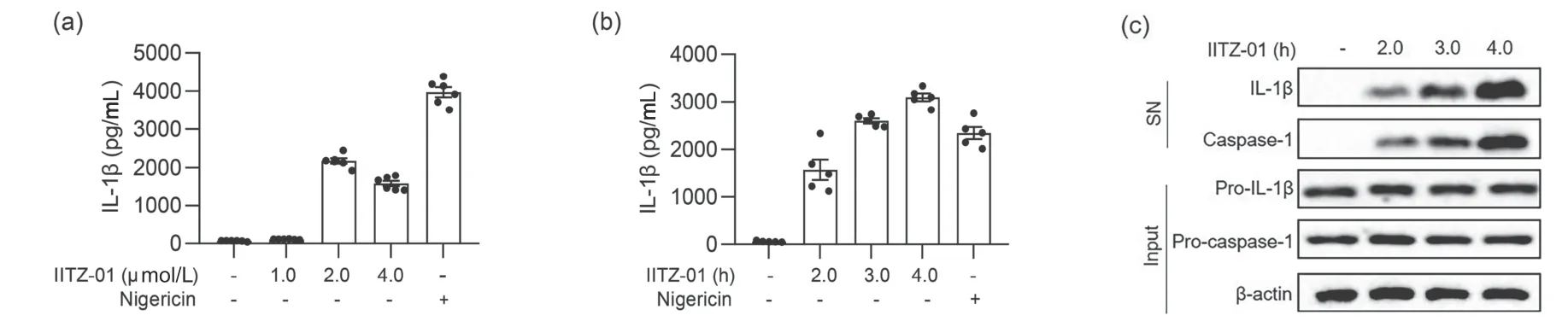
Fig.1.IITZ-01 induced caspase-1 activation and IL-1β production in BMDM cells.(a) ELISA of IL-1β in the supernatant from BMDM cells primed with LPS for 3 h and then stimulated with different doses of IITZ-01.(b,c) BMDM cells were primed with LPS for 3 h and then stimulated with IITZ-01(2 μmol/L) for different times.(b) ELISA of IL-1β in the supernatant.(c) Western blot of IL-1β,cleaved caspase-1 in culture supernatant (SN) and the precursors of IL-1β (pro-IL-1β),pro-caspase-1,β-actin in cell lysate (input).Nigericin was used as a positive control.Data are means ± SEMs (n=6 or 5).

Fig.2.IITZ-01 activated inflammasome in BMDM cells.(a,b) BMDM cells from WT or Casp1-/- mice were primed with LPS for 3 h and then stimulated with IITZ-01 (2 μmol/L) for different times.(a) ELISA of IL-1β in the supernatant.(b) Western blot of IL-1β and cleaved caspase-1 in SN and pro-IL-1β,pro-caspase-1,and β-actin in cell lysate.(c,d) BMDM cells from WT or Asc-/- mice were primed with LPS for 3 h and then stimulated with IITZ-01 (2 μmol/L) for different times.(c) ELISA of IL-1β in the supernatant.(d) Western blot of IL-1β and cleaved caspase-1 in SN and pro-IL-1β,pro-caspase-1,ASC,and β-actin in cell lysate.(e,f) BMDM cells from WT or Gsdmd-/- mice were primed with LPS for 3 h and then stimulated with IITZ-01(2 μmol/L) for different times.(e) ELISA of IL-1β in the supernatant.(f) Western blot of IL-1β and cleaved caspase-1 in SN and pro-IL-1β,pro-caspase-1,GSDMD,and β-actin in cell lysate.Nigericin was used as a positive control.Data are means ± SEMs (n=5).**P≤0.01,***P≤0.001.
According to different receptor proteins,inflammasomes mainly include NLRP3,NLRC4,AIM2,Pyrin and other inflammasomes.To explore which inflammasome was activated by IITZ-01,we used IITZ-01 to stimulate BMDM cells from WT orNlrp3-/-,Ipaf-/-,Aim2-/-,andPyrin-/-mice.It was found that IITZ-01 could not induce caspase-1 activation(Fig.3b),and IL-1β could not be detected in the cell supernatant (Fig.3a) when BMDM cells lost the NLRP3 protein.After NLRC4 (also known as IPAF),AIM2 or Pyrin protein was absent,there was no significant difference in IL-1β production or caspase-1 activation compared with WT cells(Fig.4).The above experimental results showed that IL-1β generation induced by IITZ-01 was mainly derived from NLRP3 inflammasome activation.
To further determine whether IITZ-01 activated NLRP3 inflammasome,we used IITZ-01 to stimulate BMDM cells for 3 h after pretreatment with LPS for 3 h.Different doses of the specific NLRP3 inflammasome inhibitors CY-09 or MCC950 were added to the cell supernatant 1 h before stimulation with IITZ-01.After stimulation,we used ELISA and Western blot to detect caspase-1 activation and IL-1β generation.It was found that both CY-09 (Fig.3c,d) and MCC950 (Fig.3e,f)could inhibit inflammasome activation caused by IITZ-01 in a dose-dependent manner.In conclusion,IITZ-01 could specifically activate NLRP3 inflammasome.
3.3 NLRP3 inflammasome activation induced by IITZ-01 is independent of direct binding and ion flow
Next,we explored how IITZ-01 activated NLRP3 inflammasome.The NLRP3 protein is a member of the NLR family.Its C-terminal LRR domain is considered to have the function of recognizing and binding to PAMPs or DAMPs.However,to date,no agonist that can directly bind to the NLRP3 protein has been found.NLRP3 inflammasome is composed of NLRP3,NEK7,ASC and pro-caspase-1 proteins.In the experiments,we first harvested the lysate of BMDM cells,added IITZ-01,and incubated tubes on the turntable,allowing IITZ-01 to fully react with proteins.Then,proteins were hydrolyzed with the appropriate concentration of streptomycin protease,and finally,samples were detected by specific antibody and Western blot to determine whether IITZ-01 could bind to these specific proteins.The DARTS assays showed that IITZ-01 could not prevent proteins related to NLRP3 inflammasome from degrading,meaning that IITZ-01 did not directly bind to the protein components of NLRP3 inflammasome (Fig.5a).

Fig.3.IITZ-01 activated NLRP3 inflammasome in BMDM cells.(a,b) BMDM cells from WT or Nlrp3-/- mice were primed with LPS for 3 h and then stimulated with IITZ-01 (2 μmol/L) for different times.(a) ELISA of IL-1β in the supernatant.(b) Western blot of IL-1β and cleaved caspase-1 in SN and pro-IL-1β,pro-caspase-1,NLRP3,and β-actin in cell lysate.(c,d) BMDM cells were primed with LPS for 2 h,treated with different doses of CY-09 for 1 h,and then stimulated with IITZ-01 (2 μmol/L) for 3 h.(c) ELISA of IL-1β in the supernatant.(d) Western blot of IL-1β and cleaved caspase-1 in SN and pro-IL-1β,pro-caspase-1,and β-actin in cell lysate.(e,f) BMDM cells were primed with LPS for 2 h,treated with different doses of MCC950 for 1 h,and then stimulated with IITZ-01 (2 μmol/L) for 3 h.(e) ELISA of IL-1β in the supernatant.(f) Western blot of IL-1β and cleaved caspase-1 in SN and pro-IL-1β,pro-caspase-1,and β-actin in cell lysate.Nigericin was used as a positive control.Data are means ± SEMs (n=6,5 or 5).*0.01 ≤ P ≤ 0.05,***P ≤ 0.001,ns,not significant.
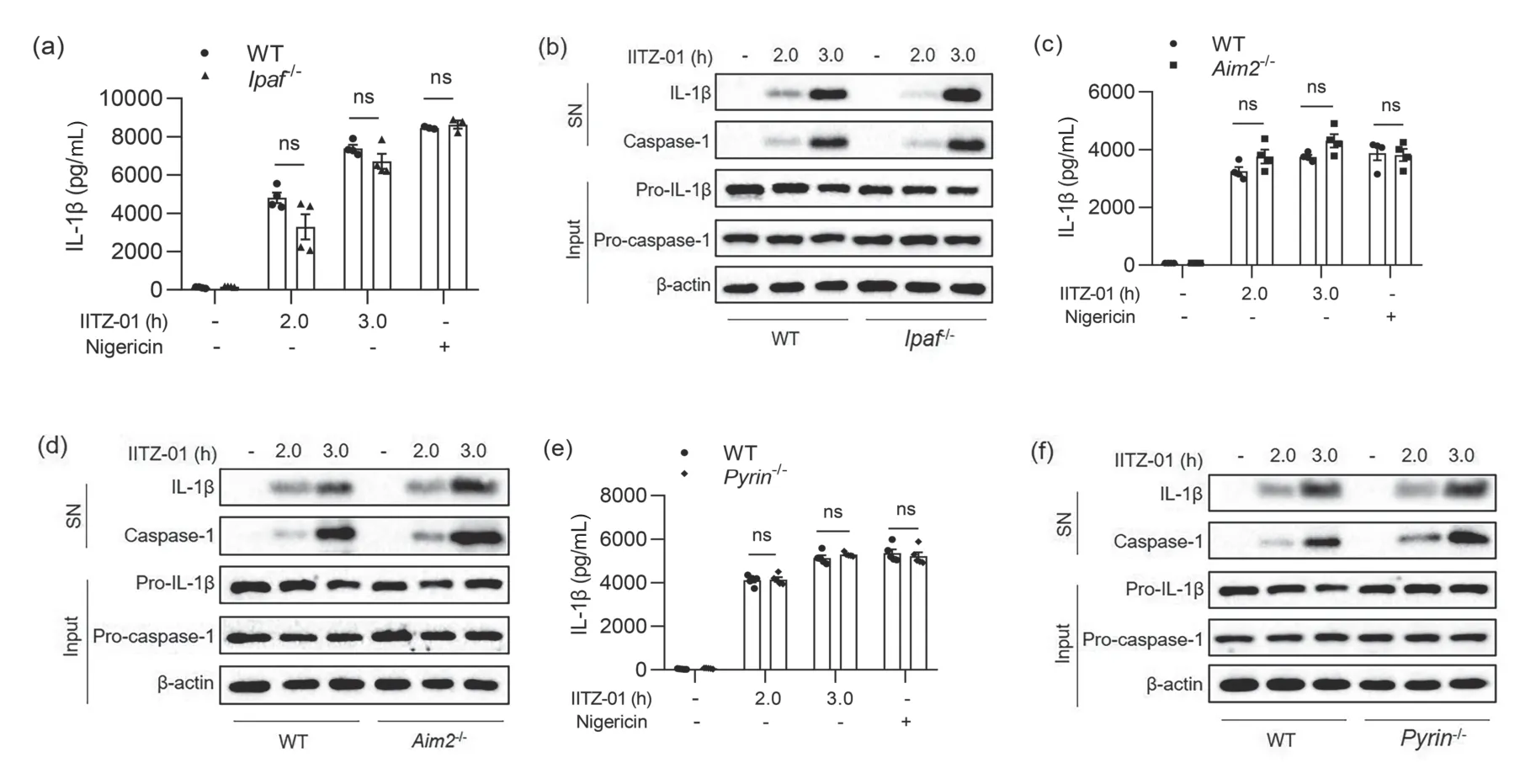
Fig.4.Inflammasome activation induced by IITZ-01 was independent of NLRC4,AIM2 and Pyrin.(a,b) BMDM cells from WT or Ipaf-/- mice were primed with LPS for 3 h and then stimulated with IITZ-01 (2 μmol/L) for different times.(a) ELISA of IL-1β in the supernatant.(b) Western blot of IL-1β and cleaved caspase-1 in SN and pro-IL-1β,pro-caspase-1,and β-actin in cell lysate.(c,d) BMDM cells from WT or Aim2-/- mice were primed with LPS for 3 h and then stimulated with IITZ-01 (2 μmol/L) for different times.(c) ELISA of IL-1β in the supernatant.(d) Western blot of IL-1β and cleaved caspase-1 in SN and pro-IL-1β,pro-caspase-1,and β-actin in cell lysate.(e,f) BMDM cells from WT or Pyrin-/- mice were primed with LPS for 3 h and then stimulated with IITZ-01 (2 μmol/L) for different times.(e) ELISA of IL-1β in the supernatant.(f) Western blot of IL-1β and cleaved caspase-1 in SN and pro-IL-1β,pro-caspase-1,and β-actin in cell lysate.Nigericin was used as a positive control.Data are means ± SEMs (n=4,4 or 5).ns,not significant.

Fig.5.NLRP3 inflammasome activation induced by IITZ-01 was independent of direct binding and ion flow.(a) Western blot of NLRP3,NEK7,procaspase-1,ASC and β-actin in BMDM cells treated with DARTS assays.(b,c) ELISA of IL-1β in the supernatant from BMDM cells primed with LPS for 3 h,treated with different doses of KCl,(b) and then stimulated with IITZ-01 (2 μmol/L) for 3 h or (c) stimulated with Nigericin (5 μmol/L) for 15 min.(d) ICP of intracellular potassium in BMDM cells of Nlrp3-/- mice primed with LPS for 3 h and then stimulated with IITZ-01 (2 μmol/L) for 3 h or Nigericin (5 μmol/L) for 15 min.Data are means ± SEMs (n=6,5 or 6).
Most agonists of NLRP3 inflammasome can cause potassium outflow after stimulating cells.To investigate whether NLRP3 inflammasome activation caused by IITZ-01 was dependent on K+,we added KCl to the medium to increase the concentration of extracellular potassium or chloride ions and then added IITZ-01 or Nigericin.It was found that the increase in extracellular potassium and chloride ions significantly inhibited NLRP3 inflammasome activation caused by Nigericin (Fig.5c) but did not affect NLRP3 inflammasome activation induced by IITZ-01 (Fig.5b).We then used ICP experiments to directly detect the potassium ion content in cells.The experiments showed that the proportion of intracellular K+in BMDM cells decreased significantly after stimulation with Nigericin.However,there was no significant difference in intracellular K+content between IITZ-01-stimulated cells and control cells,indicating that there was no K+outflow in the process of NLRP3 inflammasome activation induced by IITZ-01 (Fig.5d).In conclusion,IITZ-01-induced activation of NLRP3 inflammasome was independent of direct binding and ion flow.
3.4 IITZ-01 activates NLRP3 inflammasome by inducing mitochondrial damage and reactive oxygen species accumulation
According to the literature[41],IITZ-01 can cause mitochondrial damage and destroy the mitochondrial membrane potential(MMP) in human breast cancer cells and normal human mammary epithelial cells,thus leading to apoptosis.Therefore,we hypothesized that IITZ-01 would also induce mitochondrial damage in BMDM cells,resulting in an increase in intracellular mROS,which could activate NLRP3 inflammasome.To verify the above hypothesis,after the cells were stimulated with IITZ-01,we stained the living cells with MitoTracker dye or MitoSOX dye and observed the morphological changes in mitochondria or mROS production,respectively.It was found that treatment with IITZ-01 in BMDM cells from WT orNlrp3-/-mice for 1 h caused significant mitochondrial damage,while MitoTracker could not label the morphology of mitochondria due to a decrease in MMP (Fig.6a).At the same time,treatment with IITZ-01 in BMDM cells from WT orNlrp3-/-mice led to an increase in mROS,which was shown by the enhancement of red fluorescence (Fig.6b).Since mROS are mainly located around mitochondria,it can be observed that mitochondria change from normal scattered lines to broken points or rods.When BMDM cells from mice were treated with Nigericin,mitochondrial damage was also observed,but the mitochondrial membrane potential did not change.Therefore,morphological changes in mitochondria could be observed.It was found that after stimulation with Nigericin,the mitochondria in BMDM cells changed from normal scattered lines to broken points or rods (Fig.6a).Treatment with Nigericin in BMDM cells from WT orNlrp3-/-mice also resulted in an increase in mROS (Fig.6b).
MnTBAP is a synthetic analog of superoxide dismutase(SOD),which can effectively eliminate reactive oxygen in the cytoplasm.We added MnTBAP 1 h before the cells were stimulated with IITZ-01 or Nigericin.ELISA and Western blot experiments showed that MnTBAP inhibited NLRP3 inflammasome activation induced by IITZ-01 in a dosedependent manner (Fig.6c).After mROS were removed,caspase-1 activation and IL-1β production were inhibited (Fig.6c,d).The above results suggested that mitochondrial damage and mROS accumulation in the cytoplasm were the reasons why IITZ-01 could activate NLRP3 inflammasome.
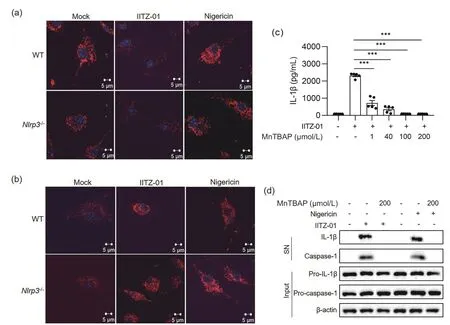
Fig.6.IITZ-01 activated NLRP3 inflammasome by inducing mitochondrial damage and mROS accumulation.(a) Immunofluorescence of mitochondrial morphology in BMDM cells from WT and Nlrp3-/- mice primed with LPS for 3 h and then stimulated with IITZ-01 (2 μmol/L) for 1 h or Nigericin (5 μmol/L) for 20 min.(b) Immunofluorescence of mROS in BMDM cells from WT and Nlrp3-/- mice primed with LPS for 3 h and then stimulated with IITZ-01 (2 μmol/L) for 3 h or Nigericin (5 μmol/L) for 20 min.(c) ELISA of IL-1β in the supernatant from BMDM cells primed with LPS for 2 h,treated with different doses of MnTBAP for 1 h,and then stimulated with IITZ-01 (2 μmol/L) for 5 h.(d) Western blot of IL-1β and cleaved caspase-1 in the SN of BMDM cells primed with LPS for 2 h,treated with different doses of MnTBAP for 1 h,and then stimulated with IITZ-01 (2 μmol/L) for 3 h or Nigericin (5 μmol/L) for 15 min.Western blot of pro-IL-1β,pro-caspase-1 and β-actin in the lysate of those cells.Data are means ± SEMs (n=5).***P≤0.001.
4 Conclusion and discussion
In this study,we found that IITZ-01,as a highly effective inhibitor of autophagy,can specifically activate NLRP3 inflammasome.Mechanistically,IITZ-01 activates NLRP3 inflammasome by inducing mitochondrial damage and mROS accumulation.Therefore,we identified a new activator of NLRP3 inflammasome.
In experiments,we found that IITZ-01 could induce BMDM cells from adherent spindles to expanded vacuole spheres morphologically.With the extension of time,round vacuoles expanded continuously,accompanied by the growth of cell size.After activation for 3 h or more,the vacuoles in the cells disappeared and were replaced by small circular bright spots in the middle of the cells,and the cells decreased.Nigericin could also induce BMDM cells to form round small bright spots after activating NLRP3 inflammasome.After further detection by ELISA and Western blot,it was found that only when BMDM cells showed this morphology could they have a high IL-1β level.This result suggested that the changes in cell morphology are inextricably related to inflammasome activation and the release of inflammatory cytokines.However,what are the bright spots? Is it a common phenotype of NLRP3 inflammasome activation? This requires further exploration with the help of structural biology.
IITZ-01 caused an obvious increase in intracellular mROS within 30 min (results not shown).However,ELISA and Western blot showed that IITZ-01 could not induce caspase-1 activation or IL-1β generation in BMDM cells within 2 h.This result indicated that NLRP3 inflammasome could be activated only when the intracellular accumulated mROS exceeded a certain threshold.After immunofluorescence staining with MitoTracker and MitoSOX,cells treated with IITZ-01 exhibited mitochondrial damage,which changed the mitochondria from lines to broken points.Therefore,we believe that mROS production mainly came from mitochondrial dysfunction.However,whether there are other factors that could cause mROS increases remains to be explored.
In this study,we found that IITZ-01 activated NLRP3 inflammasome by targeting mitochondria.However,it was unclear how IITZ-01 caused mitochondrial damage.We found that when IITZ-01 was added to the cell culture supernatant,many small vacuoles appeared in the cells after 15 min of stimulation.According to the literature,the formation of these vacuoles is related to lysosomal dysfunction.Can IITZ-01 activate NLRP3 inflammasome by targeting lysosomes and then affecting mitochondria? Generally,there are two methods of interaction between organelles:indirect contact through small molecules or proteases and direct contact.Next,we will explore the molecular mechanism of NLRP3 inflammasome induced by IITZ-01 by detecting lysosome-related proteases and observing the interaction between lysosomes and mitochondria.
In conclusion,we identified IITZ-01 as a new specific agonist of NLRP3 inflammasome.To further explore the signaling pathway and regulatory mechanism of NLRP3 inflammasome activation,more agonists of NLRP3 inflammasome need to be found.Therefore,we need to obtain more small molecule compound libraries and explore whether they can activate NLRP3 inflammasome and the mechanism of inflammasome activation.At the same time,mass spectrometry or CRISPR-Cas9 screening technology will be used to test the changes in small molecules and proteins in cells after NLRP3 inflammasome is stimulated with different agonists,which is expected to determine the key common upstream signals of NLRP3 inflammasome activation.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2020YFA0509101).
Conflict of interest
The authors declare that they have no conflict of interest.
Biographies
Wenxin Huis currently a master’s student under the supervision of Prof.Wei Jiang at the University of Science and Technology of China.Her research mainly focuses on NLRP3 inflammasome activation.
Wei Jiangreceived her Ph.D.degree from the University of Science and Technology of China in 2007,and did postdoctoral research at the Ludwig Institute of Oncology in Switzerland from 2007 to 2011.She is currently a Professor at the Division of Life Sciences and Medicine of the University of Science and Technology of China.Her research interests include the role and mechanism of pattern recognition receptors in innate immune recognition,signal transduction and related diseases.
- 中国科学技术大学学报的其它文章
- Sphingosine-1-phosphate induces Ca2+ mobilization via TRPC6 channels in SH-SY5Y cells and hippocampal neurons
- Comprehensive bioinformatic analysis of key genes and signaling pathways in glioma
- Evolutionary game analysis of promoting the development of green logistics under government regulation
- Self-supervised human semantic parsing for video-based person re-identification
- The surrounding vehicles behavior prediction for intelligent vehicles based on Att-BiLSTM

