Safety and effectiveness of electromyography-induced rehabilitation treatment after epidural electrical stimulation for spinal cord injury: study protocol for a prospective, randomized, controlled trial
Xiao-Pei Sun , Jie-Jian Shi , Yong Bao , Jie Zhang Hui-Juan Pan Dian-You Li Yu Liang Qing Xie
Abstract Epidural electrical stimulation is a new treatment method for spinal cord injury (SCI).Its efficacy and safety have previously been reported.Rehabilitation treatment after epidural electrical stimulation is important to ensure and improve the postoperative efficacy of epidural electrical stimulation in patients with SCI.Considering that electromyography (EMG)-induced rehabilitation treatment can accurately match the muscle contraction of patients with SCI, we designed a study protocol for a prospective, randomized controlled trial.In this trial, on the premise of adjusting the spinal cord electrical stimulator to obtain the maximum EMG signal of the target muscle, patients with SCI receiving epidural electrical stimulation will undergo EMG-induced rehabilitation treatment.Recovery of muscle strength of key muscles, quality of life, safety and therapeutic effects will be monitored.Twenty patients with SCI who are scheduled to undergo epidural electrical stimulation in Shanghai Ruijin Rehabilitation Hospital will be randomly divided into two groups with 10 patients per group.The control group will receive conventional rehabilitation treatment.The EMG-induced rehabilitation group will receive EMG-induced rehabilitation treatment of the target muscles of the upper and lower limbs based on conventional rehabilitation treatment.After rehabilitation treatment, follow up for all patients will occur at 2 weeks and 1, 3 and 6 months.The primary outcome measure of this trial will be evaluation of target muscle recovery using the Manual Muscle Testing grading scale.Secondary outcome measures will include modified Barthel Index scores, integrated EMG values, the visual analogue scale, Spinal Cord Independence Measure scores, and modified Ashworth scale scores.The safety indicator will be the incidence of adverse events.This trial will collect data regarding the therapeutic effects of EMG-induced rehabilitation in patients with SCI receiving epidural electrical stimulation for 6 months after rehabilitation treatment.Findings from this trial will help develop rehabilitation methods in patients with SCI after epidural electrical stimulation.This study protocol was approved by Ethics Committee of Shanghai Ruijin Rehabilitation Hospital (Approval No.RKIRB2022-12) on February 15, 2022 and was registered with Chinese Clinical Trial Registry (registration number: ChiCTR2200061674; date: June 30, 2022).Study protocol version: 1.0.
Key Words: electromyography-induced rehabilitation; epidural electrical stimulation; muscle strength; pain; quality of life; randomized controlled trial;recovery; spinal cord injury
Introduction
Spinal cord injury (SCI) is characterized by high incidence, high disability rate and high cost, and brings a huge burden to the patient’s family and to society (Agrawal and Joshi, 2015; Taweel and Seyam, 2015; Zhang et al.,2018; Islamov et al., 2021).Extraspinal cord electrical stimulation is a new treatment for SCI.Its safety has been fully confirmed, and postoperative rehabilitation treatment is important to ensure and improve the therapeutic effects of this operation.Effectiveness of epidural electrical stimulation on SCI has been reported (Angeli et al., 2018; Aslan et al., 2018; Gill et al., 2018;Harkema et al., 2018; Herrity et al., 2018; Straaten et al., 2018; Wagner et al., 2018; Watrin et al., 2018).The results of most clinical trials of epidural electrical stimulation were obtained from case series studies and case reports.However, a few randomized controlled trials have investigated epidural electrical stimulation.Rehabilitation methods (Angeli et al., 2014; Rejc et al.,2017; Formento et al., 2018; Moraud et al., 2018) and outcome measures differ greatly between studies, affecting the reliability of previous results(Calvert et al., 2019; Choi et al., 2021).
Xiang et al.(2021) and Zong et al.(2021) performed single-fiber electromyography (EMG) and found that the peripheral muscles of SCI patients may reinnervate, which may be related to the remodeling of the central nervous system after SCI.This may be the basis for the recovery of motor function in patients with SCI.EMG-induced rehabilitation exercise can accurately target the muscle contraction of patients.Yu (2020) reported that after EMG-induced rehabilitation exercise, sensory impairment and the ability of daily living improved to different degrees.Therefore, it is of great significance to investigate the safety and effectiveness of EMG-induced rehabilitation in patients with SCI receiving epidural electrical stimulation (De Biase et al., 2011; Middleauth et al., 2013; Sdrulla et al., 2018).In view of this, we design a randomized controlled trial to investigate the clinical efficacy of EMG-induced rehabilitation exercise versus conventional rehabilitation exercise in SCI patients receiving epidural electrical stimulation and to determine the optimal postoperative operative methods.Findings from this trial will provide scientific evidence for rehabilitation treatment of SCI.
Methods
Study design
This is a prospective, single-center, open, randomized, parallel, controlled trial.Twenty patients with SCI receiving epidural electrical stimulation will be divided into two groups, with 10 patients per group.This trial will investigate the effects of EMG-induced rehabilitation exercise on muscle strength,lower limb sensory function, and quality of life in patients with SCI receiving epidural electrical stimulation, evaluate the safety and effectiveness of EMG-induced rehabilitation exercise in patients with SCI receiving epidural electrical stimulation, and help establish optimal rehabilitation exercise parameters and standardized rehabilitation strategies for patients with SCI receiving epidural electrical stimulation.This study protocol was approved by Ethics Committee of Shanghai Ruijin Rehabilitation Hospital (Approval No.RKIRB2022-12) on February 15, 2022 (Additional file 1).All participants will provide written informed consent (Additional file 2).The study protocol will be performed in accordance with the Declaration of Helsinki (2008 edition).Operation procedures of epidural electrical stimulation will follow the Expert consensus on the treatment of chronic pain by spinal cord electrical stimulation formulated by Expert consensus group on spinal cord stimulation for chronic pain (2011).The safety and effectiveness obtained by non-blinded investigators will be monitored once every 3 months by Data and Safety Monitoring Board of Shanghai Ruijin Rehabilitation Hospital.The study protocol was registered with Chinese Clinical Trial Registry (registration number: ChiCTR2200061674; date: June 30, 2020).Study protocol version:1.0.Outcome measures and timing are shown in Table 1.A trial flow chart is shown in Figure 1.The study protocol was written following the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) checklist(Chan et al., 2013; Additional file 3).
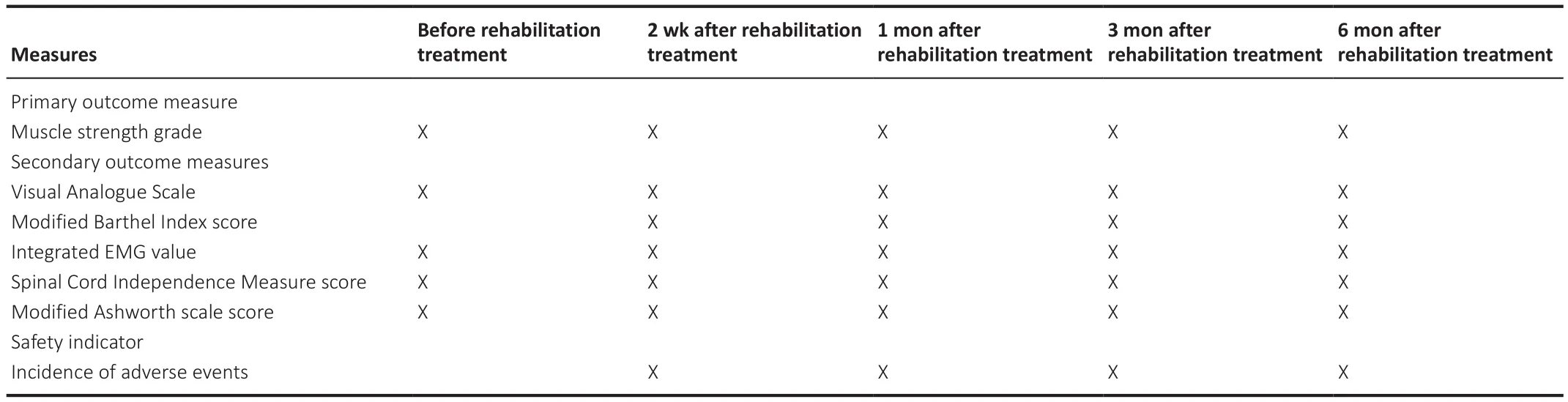
Table 1 |Outcome measures and timing
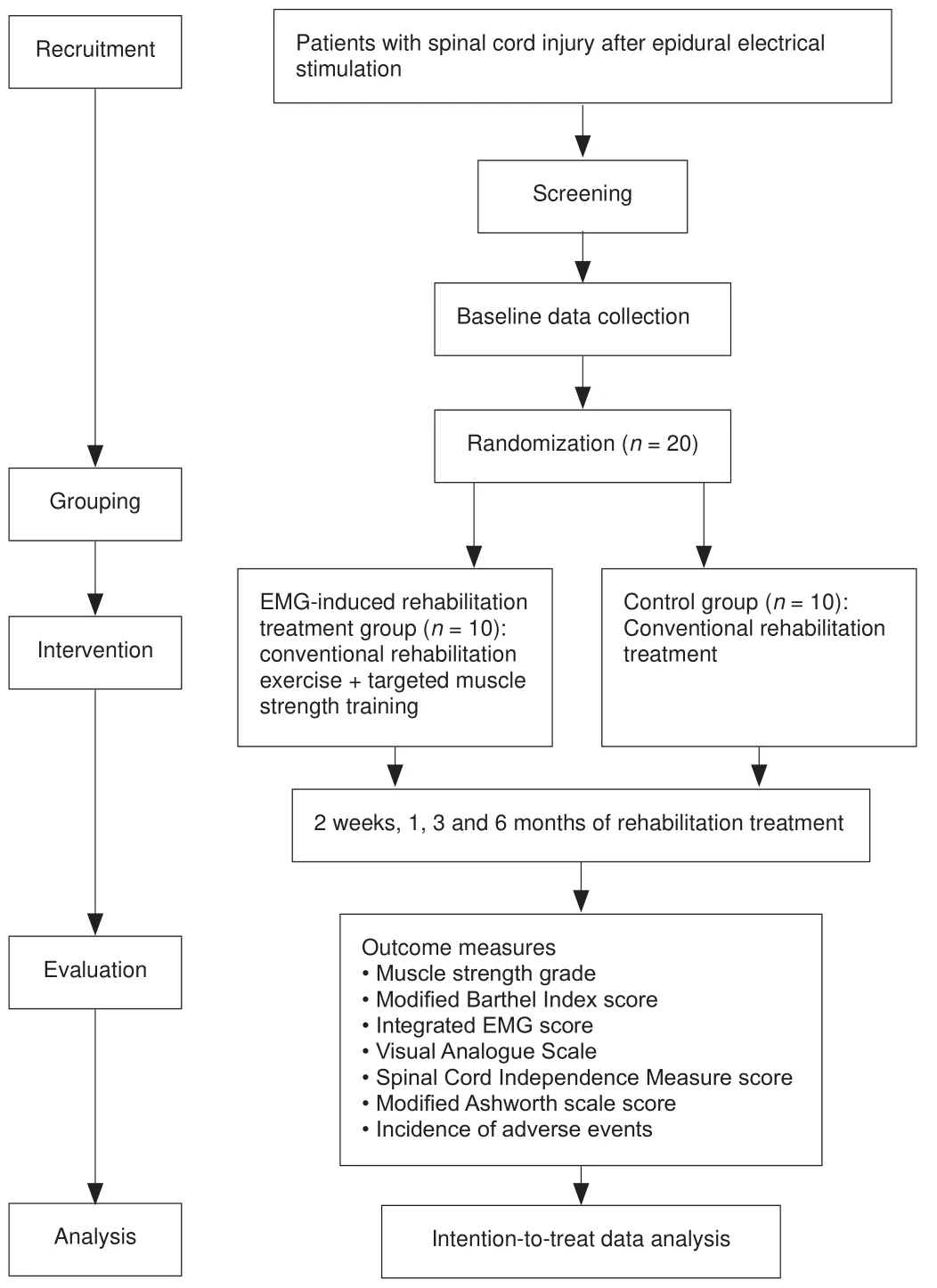
Figure 1|Trial intervention flow chart.
Recruitment
Study participants will be inpatients with SCI who will be scheduled to receive epidural electrical stimulation in Shanghai Ruijin Rehabilitation Hospital.Shanghai Ruijin Rehabilitation Hospital mainly treats patients with SCI by rehabilitation exercise.The patient source in this hospital is sufficient to ensure the number of patients required for the trial.This trial will recruit patients through delivering leaflets and issuing recruitment information on the bulletin board.Participants will be provided professional treatment and will be provided rehabilitation evaluation and EMG implementation free of charge.Potential participants or their family members can contact the project leader by telephone, email or Wechat through the attending physician.Participation in this trial is voluntary.Before the patient makes the decision to participate in the trial, they will be encouraged to ask the attending physician any relevant questions as far as possible until the patient fully understands the contents of this trial.Written informed consent will be provided before participation in this trial.The baseline data of the patient will be recorded in detail on admission.The patient’s medical history, symptoms, signs, imaging and pathological data will be collected and monitored by his or her attending physician.
Eligibility criteria Inclusion criteria
Patients who meet all of the following conditions will be considered for inclusion in this trial:
a) Those who meet the diagnostic criteria of SCI formulated by American Spinal Injury Association (Kang et al., 2019);
b) Those who receive epidural electrical stimulation within 6 months of injury and whose surgical wound has healed;
c) Age of 18-75 years, of either sex;
d) Provision of written informed consent.
Exclusion criteria
Patients who meet one or more of the following conditions will be excluded from this trial:
a) Those who have cognitive impairment, epilepsy or poor compliance;
b) Those who have received cardiac treatment equipment (such as a pacemaker or defibrillator);
c) Those who have unstable condition after epidural electrical stimulation or are unable to tolerate rehabilitation treatment (such as severe pneumonia,acute myocardial infarction, or blood and immune system diseases);
d) Those who participate in other clinical trials at the same time;
e) Pregnant women;
f) Those with depression or anxiety symptoms;
g) Those with intraspinal tumor;
h) Known or suspected drug or alcohol abusers;
i) Those who have severe dystonia after epidural electrical stimulation;
j) Those who have hearing or visual impairment after epidural electrical stimulation.
Withdrawal criteria
Patients will be withdrawn from this trial if one or more of the following conditions occur:
a) Any clinical adverse events, abnormal examination results or other medical conditions that may lead to no longer benefiting from continuing treatment;b) Patient’s condition is not suitable for further treatment;
c) Participant voluntarily requests to suspend the treatment;
d) Marked protocol deviations, such as disqualification and noncompliance,after enrollment;
e) Upon the request of the investigator for safety reasons.
Termination criteria
The principal investigator has the right to terminate the study at any time.The reasons for terminating the study include but are not limited to the following:continuation of the trial may endanger the relevant rights and interests of a certain number of participants.
Randomization and blinded evaluation
SPSS 24.0 statistical software (SPSS, IBM, Armonk, NY, USA) will be used to generate a random number table.Patients included in this trial will be numbered 1-20.First, a random number of 0 or 1 will be generated for each participant using the RANUNI function (1 for EMG-induced rehabilitation treatment group and 0 for control group).Then, the random numbers will be sorted by PROC SORT and then grouped in parallel according to the ratio 1:1.Twenty patients will be divided into two groups, with 10 patients in each group.Group concealment will not be implemented during grouping.The persons who will collect and evaluate outcomes will not know the trial protocol.
Sample size estimation
According to the clinical experience of our department, it is hypothesized that the percentage of patients whose unarmed muscle strength was graded 3 in the EMG-induced rehabilitation treatment and control groups was 90% and 60%, respectively.According to the results calculated by PASS 15.0 software(PASS, Kaysville, UT, USA), a power of 80% and an alpha of 0.05 (bilateral test)were assumed and a 20% dropout rate was considered.According to the 1:1 parallel distribution principle, 39 participants will be included in each group,thus statistical difference of the rate between the two groups can be detected.
However, considering the number of patients with SCI who received epidural electrical stimulation in Shanghai Ruijin Rehabilitation Hospital in the past, 20 patients, 10 patients per group, will be included in phase I of this study for evaluation.No cases will be expected to be lost to 6-month follow up.
Rehabilitation treatment methods
Spinal cord electrical stimulation parameters will be recorded for each participant.The control group will receive conventional rehabilitation treatment according to lower and upper limb function.The EMG-induced rehabilitation treatment group will receive target muscle strength training using the optimal EMG-evoked potential of target muscles.All participants will be assessed before rehabilitation treatment, and follow up will occur at 2 weeks and 1, 3 and 6 months after rehabilitation treatment.
Preparation for epidural electrical stimulation
Each patient’s condition and pain will be evaluated before treatment(Expert consensus group on spinal cord stimulation for chronic pain, 2021).Notably, the therapeutic effects of electrical stimulation to the spinal cord on pain are affected by depression, anxiety and somatization (Celestin et al.,2009).Laboratory and imaging (magnetic resonance imaging, CT and X-ray)examinations will be performed to determine routine blood test results,routine urinary test results, blood biochemistry results and coagulation function, and to identify lamina space, epidural space and spinal cord condition in related segments to exclude spinal canal tumor.
Epidural electrical stimulation
(1) Epidural electrical stimulation will be performed using the St.Jude Medical Eon Mininerve stimulation system (Abbott, Plano, USA).16-contact grids for epidural electrical stimulation will be implanted on the dorsal surface of the spinal cord.The stimulation level will be adjusted to provide the optimal EMG-evoked potential.
(2) Selection of target muscles: upper limb muscles (biceps brachii, triceps brachii and extensor digitorum) and lower limb muscles (such as quadriceps femoris, gastrocnemius and hamstring).
(3) Adjustment of electrical stimulation parameters: stimulation frequency,20-60 Hz; pulse width, 200-300 μs; stimulation intensity, 0.1-1.0 mA;stimulation time, 24 hours per day (wireless chargers, 24 hours of electrical stimulation).
(4) Adjustment of optimal evoked potential: patients will lie in a comfortable and relaxed supine position in a quiet environment at room temperature(24°C).Patients will rest for 15 minutes after the change of body position.A surface EMG collector (Analyzing System for Surface Electomyography FlexComp 20, VISHEE, China) will be placed on the target muscle (Figure 1).The wireless regulator will be aligned to the controller.The current intensity,frequency and pulse width will be set from low to high.Electrical stimulation parameters for 16-contact grids will be adjusted to achieve the optimal EMG-evoked potential of the target muscle based on the EMG surface signal combined with patient feedback (e.g., no discomfort and no pain).
Postoperative conventional rehabilitation procedures
Patients will implement the conventional drafting, passive joint movement and assignment task, five times a week.Four weeks of training will be considered as one treatment cycle.The precise rehabilitation treatment plan is as follows:
(1) Maintenance of joint range of motion and prevention of hand and foot joint contracture: range of motion exercises of the shoulder, elbow, wrist,metacarpophalangeal and interphalangeal joints of the upper limbs, and the hip, knee and ankle joints of the lower limbs will each be performed 30 times in all directions.The metacarpophalangeal joint will be flexed and the lateral ligament will be pulled using the hand rest brace to prevent the hand joint contracture from affecting hand function in the future.The Achilles tendon will be pulled using the foot rest position brace to prevent ankle contracture.
(2) Muscle stretching training: for muscles with increased muscle tension or event spasm, low-intensity continuous passive stretching training will be performed and braces will be used when necessary.
(3) Turn over training: the patients will be guided to turn over in the left and right directions.Those with quadriplegia will need to be assisted to varying degrees.Turn over should be performed once at least every 2 hours.
(4) Balance function training: balance training in sitting and standing positions.
(5) Transfer function training: bed-to-wheelchair transfer function training.
(6) Wheelchair function training: indoor wheelchair movement training.
(7) Hand function training: assignment task will be designed according to relevant activities of daily living to improve hand function, including fingertip grasp task, finger belly grasp task, coarse grasp task, reverse grasp task, hook grasp task, cylindrical grasp task, spherical grasp task and finger side power grasp task.
(8) Sensory training: the strap sense below the damage plane will be improved by local compression.
(9) Use of assistive devices combined with daily living ability training:according to different injury locations and different degrees of injury, as well as the possible functional results, daily living ability training will be performed with the help of braces and assistive devices.Training will include activities such as eating, bathing or showering, dressing, toileting and walking.
Muscle strength training
(1) Muscle strength training
Selection of target muscles: Upper limbs (biceps brachii, triceps brachii and extensor digitorum) and lower limbs (such as quadriceps femoris,gastrocnemius and hamstring).
Posture selection: Muscle strength will be evaluated and the patient will be placed in an appropriate posture.In principle, the patient will be placed in a comfortable position.
Exercise intensity: According to muscle strength, passive training (muscle strength 0-I), assistance training (muscle strength II-III) or resistance muscle strength training (muscle strength III) will be performed to strive to achieve the maximum muscle strength contraction of target muscles.
Training frequency: Under the induction of peripheral surface EMG, each training will last 20 minutes, and participants will be required to complete more than 30 full-range activities of the target joint.Training frequency will be five times a week.Four weeks of training will be one treatment course.
(2) Muscle training methods
Quadriceps femoris muscle strength training: when the muscle strength is grade 0 or 1, the patient will be placed in a lying position on one side, and the contralateral lower limb will be suspended with a suspension device so that the thigh and lower leg are always at the same level during knee flexion and extension.When muscle strength is grade 2 or 3, the patient will be asked to sit beside the bed and the bed will be raised until the patient’s sole leaves the ground.When muscle strength is greater than 3, the patient will be asked to sit beside the bed and the bed will be raised until the patient’s sole leaves the ground; sandbags of different weights will be used at the ankle as appropriate.
Hamstring muscle strength training: when muscle strength is grade 0 or 1,the patient will be in a lying position on one side, and the lower limb on the opposite side will be suspended with suspension equipment so that the thigh and lower leg will always be at the same level during knee flexion and extension.When muscle strength is grade 2 or 3, the patient will be placed in the prone position.When muscle strength is greater than grade 3, the patient will be asked to lie in prone position, and sandbags of different weights will be used at the ankle as appropriate.
Outcome measures Primary outcome measure
Evaluation of muscle strength recovery of target muscle in patients with SCI using the MMT grading scale: The MMT grading scale is a muscle strength assessment method that assesses the ability of the tested muscle to produce the maximum voluntary contraction under the premise of exerting resistance with the help of gravity or bare hands (de Padua et al., 2020).During theMMT test, patients will be asked to execute different body positions to evaluate the ability of target muscles to complete the tested actions and to judge the contraction strength of targeted muscles, according to the function of the patient’s muscle or muscle group.The muscle strength will be scored as 0, 1-, 1, 1+, 2-, 2, 2+, 3-, 3, 3+, 4-, 4, 4+, 5-, 5, 5+.Higher scores indicate better muscle strength recovery of the targeted muscle.
Evaluation time points: before, and 2 weeks, 1, 3 and 6 months after rehabilitation treatment.
Secondary outcome measures
(1) MBI scale will be used to evaluate patient quality of life.The normal score of quality of life is 100 points, ≥ 60 points indicates the patient has the basic ability to take care of himself/herself in daily life, 41-59 points indicates that the patient has moderate dysfunction that needs help in daily life, and 21-40 points indicates that the patient’s daily life relies on others (Abu Baker et al.,2020).
(2) Integrated EMG value will be used to detect changes in EMG parameters.The value refers to the total number of motor units in the muscle that are activated within a certain period of time.
Evaluation time points: before, and 2 weeks, 1, 3 and 6 months after rehabilitation treatment.
(3) VAS score will be used to evaluate pain in the affected limbs.The score ranges from 0 to 100 points, where a higher VAS score indicates more severe pain in the affected limbs (Lefaucheur et al., 2004).
Evaluation time points: before, and 2 weeks, 1, 3 and 6 months after rehabilitation treatment.
(4) SCIM will be used to evaluate the patient’s self-management ability.SCIM consists of self-care, respiration, sphincter management and transfer subscales (Kumar et al., 2020).The self-care subscale covers feeding, bathing,dressing and grooming.The respiration and sphincter management subscales cover respiration, sphincter management-bladder, sphincter managementbowel and use of toilet.The transfer subscale covers transfer indoors and outcomes and transfer in room and toilet.
Evaluation time points: before, and 2 weeks, 1, 3 and 6 months after rehabilitation treatment.
(5) MAS will be used to evaluate the patient’s muscle spasm.MAS is scored as 0, 1, 1+, 2, 3, 4.A score of 1+ will be designated as 1.5 points.A higher MAS score indicates more severe muscle spasm (Baunsgaard et al., 2016).
Evaluation time points: before, and 2 weeks, 1, 3 and 6 months after rehabilitation treatment.
Safety indicators
The safety indicator will be the incidence of adverse events.Incidence of adverse events = number of patients having adverse events/total number of patients × 100%.
Evaluation time points: 2 weeks, 1, 3 and 6 months after rehabilitation treatment.
(1) Adverse events of epidural electrical stimulation: epilepsy and convulsion,local tingling, burning sensation, rash, allergic reaction, implant infection.Partial laminectomy may lead to spinal alignment deformity and instability.
(2) Adverse reactions of rehabilitation treatment: stretching training may cause local soft tissue pain, local hematoma and fracture in patients with severe osteoporosis.Assignment task may cause local blood circulation disorder in the long-term application of assistive devices.Transfer training may lead to falls and ankle sprains.Some patients may have abnormal autonomic dysfunction, such as postural hypotension.At present,rehabilitation technology is mature and relevant rehabilitation treatment personnel are skilled, which greatly ensures the safety of the rehabilitation treatment process.
(3) Adverse events of EMG-induced muscle strength training: surface EMG electrodes may lead to adverse reactions such as local skin rash and allergic reaction.The currently used material for electrodes is conductive silica gel that has good skin compatibility and low incidence of allergic reactions.Muscle strength training may lead to autonomic nervous response such as an increase in blood pressure.During muscle strength training, blood pressure and heart rate will be monitored.Patients will be asked not to hold their breath to reduce the incidence of adverse reactions.
Definition, identification method and management system of adverse events and adverse reactions Definition of adverse events
Adverse events refer to adverse medical events that occur in subjects after clinical rehabilitation treatment, but they do not necessarily have a causal relationship with treatment.An adverse event can be any unexpected,adverse symptom, sign, disease or abnormal examination result, whether or not it is related to the material.
Judgment criteria for adverse events
Adverse events include all unexpected clinical manifestations.As long as these events occur after surgery, they should be reported as adverse events regardless of whether they are related to rehabilitation training or muscle strength training.
(1) During the treatment period, any patient self-reported adverse reactions or abnormal changes in the objective laboratory examination indicators will be truthfully recorded.The severity, duration, treatment measures and outcomes of the adverse event will be recorded at the same time.
(2) Clinicians will also comprehensively determine the relationship between adverse events and rehabilitation training or muscle strength training,and evaluate the possible relationship between adverse events and trial medication using the five classification levels “definitely related, probably related, possibly unrelated, definitely unrelated or unable to determine”.All adverse events, except those classified as “unable to determine” will be considered adverse reactions.
Records and reports of adverse events
Any adverse events and all related symptoms will be recorded in detail,including occurrence time, severity, duration, measures taken and final outcomes (e.g., disappearance, remission and persistence).
Statistical analysis method Main analysis and sensitivity analysis
Main analysis will be conducted among the intention-to-treat population,including all patients.Sensitivity analysis will be performed in the population after treatment according to the treatment plan.Multiple imputation will be conducted to handle missing follow-up data.
Data description
Trial results will be analyzed following the intention-to-treatment principle.Interim analyses will not be performed.Statistical analysis will be performed by statisticians using SPSS 24.0 software.The measurement data will be statistically described using mean, standard deviation, median, minimum and maximum values, and interquartile ranges.The count data will be statistically described using case numbers and percentages.
Analysis methods
The incidence of adverse events will be compared between groups using the Fisher’s exact test.The VAS score, MBI scale score, SCIM score, and MAS score will be compared between groups using a two-samplet
test for normally distributed data or Mann-WhitneyU
test for non-normally distributed data.Muscle strength grading indicators will be compared between the two groups using the Wilcoxon rank-sum test.The paired data will be evaluated using the Wilcoxon signed rank test.Correlation between outcomes will be analyzed using Pearson correlation analysis for normally distributed data or Spearman’s rank correlation test for non-normally distributed data.Repeated measure analysis of variance and covariance analysis will be performed to compare the scores between groups with different follow-up times, investigate the group effect and time effect on outcomes and analyze the differences in main effects and interaction effects.In clinical trials, the effects of two methods on patient outcome measures with follow-up time are often compared.Therefore, a generalized estimating equation will be used to fit a logistic regression model or a general linear regression model to screen the risk factors of different variables affecting outcome measures at different time points.The inspection level will beα
= 0.05 (bilateral).Statistical analysis data set
All baseline demographic data will be analyzed based on a full analysis set(FAS).Safety evaluation will be performed on a safety set.Effectiveness will be analyzed based on an FAS and per-protocol set (PPS).As for the population corresponding to PPS analysis, the point estimates and 95% confidence intervals of effectiveness indicators will be calculated for each group.The effectiveness indicators will be calculated for each group to determine effectiveness and clinical benefit.
Data collection and management Data collection
Patient’s medical records will be used as the original file of the clinical trial and will be kept complete.The data in the case report form (CRF) will be from, and consistent with, the original file.The investigators must ensure that the data are true, complete and accurate.All items on the case report file will be filled in without any blank or missing items (a slash will be used for items without records).Contents that are corrected will be underlined.Corrected contents and comments will be placed at the sides of the page, signed and dated.The original records will not be erased or overwritten.
Data management
All original data, documents, test reports, summary reports and the results of clinical trials will be stored in the electronic data capture system.They will be stored in an orderly manner by the archive room.All original data,test reports, test protocols and summary reports will be quickly and easily retrievable.A specially assigned person will be responsible for managing the archives.No one will be allowed to enter the archives room without approval.To protect the privacy of the participants, the names of the participants will not appear on the CRF.The investigators will confirm participant identifiers according to their codes and record them.
Data monitoring
The data monitor will be responsible for monitoring whether the trial isperformed in accordance with relevant regulations, good clinical practice and the study protocol of this trial; whether all CRFs are filled correctly and completely; whether all CRFs are consistent with the original files; and whether there are errors or omissions in the data.The monitor will cross check the contents of electric CRFs with those of the original file to ensure consistency between the data in electric CRFs and the original files.This process is also called source data verification.
Protocol violation
All requirements specified in the study protocol must be strictly implemented.Any intentional or unintentional deviation or violation of the study protocol and good clinical practice will be assigned to deviation from or violation of the protocol.In the case of any deviation from the protocol, the investigator or the supervisor will fill out a protocol violation record.Details of the time at which this event is found, the process and reasons of the event, and the corresponding treatment measures will be signed by the investigator and reported to the ethics committee.
Trial results publication
All articles and reports related to the trial will not be published until approval by the principle investigator.
Ethical considerations Ethical approval
This study protocol was approved by Ethics Committee of Shanghai Ruijin Rehabilitation Hospital (Approval No.RKIRB2022-12) on February 15,2022.During the clinical trial, study protocol will be reported to the ethics committee and filed after certain modifications.After the trial is completed,the investigator will notify the ethics committee that the trial has been completed.
Informed consent
Patients and their family members should voluntarily participate in the trial.All patients will provide informed consent to the trial process and sign the“informed consent form” on the premise of fully understanding the treatment plan.The clinical trial protocol is based on the principle of maximizing the protection of the rights, interests, safety and health of the participants.
Revision of the protocol
No one except the principle investigator can modify the study protocol.Any necessary changes in the study protocol will be carried out in the form of a protocol revision, and will be submitted to the ethics committee for approval or filling after obtaining the signature and consent of the principle investigator.The details of previous modifications will be described in the protocol.
Possible risks and precautions of the trial
The relevant rehabilitation assessment and trial methods involved in the trial process have been widely used in clinical practice.The adopted motion monitoring equipment and instruments will be mature products with sufficient conclusions on safety.The trial will not increase relevant risks.During the trial, if the patient has any discomfort symptoms, the patient’s attending physician should be immediately informed to address the discomfort as soon as possible.The trial will not disclose the privacy of patients and will not promote any home-based rehabilitation products to patients.If the functional improvement after treatment does not fully meet the expectations of patients, the trial will take corresponding follow-up treatment measures according to different levels of functional recovery.
Possible benefits, expenses, compensation and indemnification for participating in the trial
The patients participating in this trial will receive examination and related rehabilitation evaluation (muscle strength grading) without charge, but they will not receive any additional remuneration for participation in this trial.If any serious adverse events related to the trial occur, the sponsor will pay the patient’s follow-up medical expenses.In the case of hospitalization due to serious adverse reactions, the sponsor will also provide appropriate nutrition expenses, salary for missing work and compensation for bonus.However,if the patient needs examination and treatment for other diseases during the same period, the resulting expenses will not be within the scope of compensation.
Subject privacy
All information about the participants will be strictly confidential during the study.When necessary, relevant administrative departments, ethics committees or sponsors will be able to consult participant data.However, the investigators will not use the participant information for other purposes or disclose it to other groups without permission.Any electronically transmitted information will be renamed to ensure the confidentiality of the information.The information in all computers will be protected with passwords.The results of this trial may be reported at medical conferences and published in scientific journals.However, any information that can identify an individual will not be used.All trial results (including personal data and test receipts)appearing in the original medical records will be fully confidential within the scope permitted by law.
Discussion
Trial significance
SCI can lead to different degrees of sensory and motor dysfunction (Courtine and Sofroniew, 2019; Rowald et al., 2022).After SCI, damage to spinal cord morphology and function can lead to motor dysfunction (muscle strength weakening or even loss) and sensory dysfunction (sensory loss or abnormality,even neuropathic pain) at the level of injury, and urination and defecation dysfunction, which greatly affect the patient’s quality of life.Motor assistance and functional rehabilitation after SCI is a worldwide problem (Qiu, 2009;Cripps et al., 2011; Taweel and Seyam, 2015).However, there are still no effective interventions to repair injured spinal cord and restore its function.
On the basis of preliminary research results of other research groups(Xiang et al., 2021; Zong et al., 2021), we believe that SCI recovery may be related to the activation of subfunctional neural networks (silent fibers) and closely related to the activity-dependent reorganization caused by targeted rehabilitation training.For example, activation of subfunctional neural networks (silent fibers) coexists with changes in peripheral muscles.It is necessary to find and evaluate the electrophysiological changes of these peripheral muscles in the early stage.Therefore, we designed a protocol for a prospective, randomized controlled trial.This trial will recruit patients with SCI receiving epidural electrical stimulation, perform rehabilitation training of target muscles using EMG-induced rehabilitation training, monitor muscle strength recovery and the improvement in quality of life, and thereby provide reference evidence for formulating a postoperative rehabilitation protocol for patients receiving implantation of a spinal cord electrical stimulator.
Limitations and prospects of the trial
This is a randomized controlled trial without blind evaluation.The sample size included in this trial is too small to carry out subgroup analysis of outcome evaluation.The main reason why it is difficult to recruit participants is that the medical cost of spinal cord electrical stimulation is high, and only a small number of patients can bear the treatment cost in this hospital.The follow-uptime of the trial is only 6 months, and the long-term follow-up results of > 6 months will not be obtained.These limitations may affect the reliability of the results to some extent.However, we hope that findings from this randomized controlled trial will provide preliminary evidence for whether EMG-induced rehabilitation training provides improved muscle strength, quality of life, pain and spasm in patients with SCI who receive epidural electrical stimulation and whether the method can better maintain the therapeutic effects of epidural electrical stimulation compared with simple conventional rehabilitation training.Furthermore, the findings will establish whether the rehabilitation protocol is safe and reliable.If the findings of this trial indicate enhanced therapeutic effects, a multi-center, large-sample, randomized controlled trial will be performed to confirm the therapeutic effects and safety of this trial.
Trial status
Recruitment date: August 1, 2022
Study start date: August 1, 2022
Recruitment completion date: December 30, 2022
Data analysis date: July 1, 2023
Author contributions:
YB designed the trial and was responsible for the manuscript.All authors participated in recruitment, data collection and analysis and approved the final version of this manuscript.
Conflicts of interest:
The authors have no conflicts of interest to declare.
Availability of data and materials:
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Ethical Approval Documentation (Chinese).
Informed consent form (Chinese).
SPIRIT checklist.
- 中国神经再生研究(英文版)的其它文章
- Potential physiological and pathological roles for axonal ryanodine receptors
- Roles of constitutively secreted extracellular chaperones in neuronal cell repair and regeneration
- Melatonin, tunneling nanotubes, mesenchymal cells,and tissue regeneration
- MicroRNAs as potential biomarkers in temporal lobe epilepsy and mesial temporal lobe epilepsy
- Notice of Retraction
- Emerging roles of GPR109A in regulation of neuroinflammation in neurological diseases and pain

