MicroRNAs as potential biomarkers in temporal lobe epilepsy and mesial temporal lobe epilepsy
Bridget Martinez , Philip V.Peplow
Abstract Temporal lobe epilepsy is the most common form of focal epilepsy in adults, accounting for one third of all diagnosed epileptic patients, with seizures originating from or involving mesial temporal structures such as the hippocampus, and many of these patients being refractory to treatment with anti-epileptic drugs.Temporal lobe epilepsy is the most common childhood neurological disorder and,compared with adults, the symptoms are greatly affected by age and brain development.Diagnosis of temporal lobe epilepsy relies on clinical examination, patient history, electroencephalographic recordings, and brain imaging.Misdiagnosis or delay in diagnosis is common.A molecular biomarker that could distinguish epilepsy from healthy subjects and other neurological conditions would allow for an earlier and more accurate diagnosis and appropriate treatment to be initiated.Among possible biomarkers of pathological changes as well as potential therapeutic targets in the epileptic brain are microRNAs.Most of the recent studies had performed microRNA profiling in body fluids such as blood plasma and blood serum and brain tissues such as temporal cortex tissue and hippocampal tissue.A large number of microRNAs were dysregulated when compared to healthy controls and with some overlap between individual studies that could serve as potential biomarkers.For example, in adults with temporal lobe epilepsy, possible biomarkers are miR-199a-3p in blood plasma and miR-142-5p in blood plasma and blood serum.In adults with mesial temporal lobe epilepsy, possible biomarkers are miR-153 in blood plasma and miR-145-3p in blood serum.However, in many of the studies involving patients who receive one or several anti-epileptic drugs, the influence of these on microRNA expression in body fluids and brain tissues is largely unknown.Further studies are warranted with children with temporal lobe epilepsy and consideration should be given to utilizing mouse or rat and non-human primate models of temporal lobe epilepsy.The animal models could be used to confirm microRNA findings in human patients and to test the effects of targeting specific microRNAs on disease progression and behavior.
Key Words: adults; biomarkers; blood plasma; blood serum; children; hippocampal tissue; mesial temporal lobe epilepsy; microRNA; temporal cortical tissue; temporal lobe epilepsy
Introduction
Epilepsy is a group of neurological disorders characterized by recurrent,unprovoked epileptic seizures (International League Against Epilepsy, 1993).Affecting around 50-60 million patients globally (Moshe et al., 2014; Wang et al., 2015a; World Health Organization, 2022), epilepsy is one of the most disabling medical disorders.Genetic and familial studies strongly suggest that a neurobiological basis may underlie the pathophysiology of epilepsy,but its etiology is not well understood (Pitkänen and Lukasiuk, 2011).While epilepsy can begin at any age, it most commonly begins in childhood or older adulthood.People over 65 years of age have the highest incidence of epilepsy of any age, accounting for nearly 25% of cases of new-onset epilepsy.Due to changing age demographics, there is an increasing number of older adults with epilepsy.There are many causes of epilepsy and seizures and in older people, these include brain insults such as stroke, traumatic brain injury, brain tumor, Alzheimer’s disease, and lifestyle risk factors such as alcohol, smoking,sleep deprivation, stress, and depression (No author listed, 2022).Worldwide it is estimated that 10.5 million children under 15 years have active epilepsy (Guerrini, 2006).In the adult, the conditions leading to or arising from epilepsy may result in a loss or interference with previously acquired functions.By contrast, in a child, there may be interference with development rather than a pronounced loss of function.A child may fail to develop a skill,no longer progressing normally along the developmental continuum; the child may have a decreased rate of acquiring a behavior; or the child may actually regress or lose previously acquired developmental skills (Smith, 2010).
The most frequent form of focal epilepsy in adults is temporal lobe epilepsy(TLE) (Coan et al., 2014; Bernhardt et al., 2015), accounting for one third of all diagnosed epileptic patients, with seizures usually originating from or involving mesial temporal structures such as the hippocampus, and with up to 70% of these patients being refractory to drug treatment with anti-epileptic drugs (AEDs) (Callaghan et al., 2007; de Boer et al., 2008; Yang et al., 2020).Seizures result from abnormal excessive neuronal discharges in the brain and can produce symptoms ranging in severity from a brief sensory experience to a major convulsive episode (Chang and Lowenstein, 2003).Status epilepticus(SE) occurs when seizures cannot be terminated by inhibitory mechanisms,(John Hopkins Medicine, 2022).It is one of the most frequent neurological emergencies with an incidence of about 20/100,000, with the potential to cause brain injury after 30-60 minutes, and a case fatality rate of around 15% (Knake et al., 2001; Trinke et al., 2015).A high risk of memory deficits is associated with TLE (Oyegbile et al., 2004) and up to 80% of patients with drug resistant TLE demonstrates episodic memory impairments on neuropsychological testing (Helmstaedter, 2002) that often negatively affect daily functioning and quality of life (Giovagnoli et al., 2014).TLE is the most common childhood neurological disorder (Gataullina et al., 2015) occurring in 33-82 children per 100,000 children per year (Wu et al., 2019).Compared with adults, the symptoms of TLE in children are greatly affected by age and brain development.The changing symptoms cause great challenges for doctors trying to identify and effectively treat children with TLE (Nickels et al., 2012).
The neuropsychological manifestations of early versus later onset of epilepsy are different.Neuropsychological and magnetic resonance imaging (MRI)measures in two groups of adults with TLE were compared.One group had a mean age of seizure onset at 7.8 years of age and the other group at 23 years of age.The analyses were controlled for the duration of epilepsy, presence of precipitating injury, secondary generalized epilepsy, depression, comorbid medical conditions, and the number of AEDs.The early-onset patients performed worse on all cognitive measures (intelligence, language, visual perception, memory, executive function) compared to late-onset patients and controls, with few differences between late-onset patients and controls.A difference in white matter volume was found between the two patient groups, with the decrease in brain tissue volume in the early-onset group not limited to the temporal lobe but observed both ipsilateral and contralateral to the side of seizure onset (Hermann et al., 2002).
A combination of clinical examination, patient history,electroencephalographic (EEG) recordings, and brain imaging is used in the diagnosis of TLE, which requires a range of technical expertise and is resourceintensive (Sidhu et al., 2018; Amin and Benbadis, 2019; Galovic et al., 2019).A correct diagnosis is critical and informs the choice of therapy among other factors (Moshé et al., 2015).EEG has only limited diagnostic gain unless performed within specialist epilepsy monitoring units where continuousvideo-EEG recording is available (Engel et al., 2013; Walker et al., 2016).Brain imaging is negative in many patients with epilepsy (Muhlhofer et al., 2017).Misdiagnosis or delay in diagnosis is common (Amin and Benbadis, 2019;Mathias and Bensalem-Owen, 2019).Up to 30% of adults are likely to be misdiagnosed with epilepsy (Zaldi et al., 2000; van Donselaar et al., 2006), and in pediatric cases, this number is higher with 39% of children in Denmark not receiving a correct diagnosis (Uldall et al., 2006).Psychogenic non-epileptic seizures and syncope are frequent causes of misdiagnosis (Zaldi et al.,2000; Petkar et al., 2012).Furthermore, patients with confusion or coma of unknown etiology often have non-convulsive SE, which can only be detected by EEG and is therefore frequently ignored (Meierkord and Holtkamp, 2007;Bauer and Trinka, 2010; Shavit et al., 2012).It is not yet possible to identify patients who are at risk of developing TLE following a potential epilepsyinciting event such as traumatic brain injury, stroke, or SE (Garner et al., 2019;Engel and Pitkänen, 2020; Klein and Tyrlikova, 2020; Lӧscher, 2020).Although effective treatment for epilepsy is available, only 10-40% of patients receive drug treatment and the offer of surgical treatment is still limited.In addition to all the difficulties imposed by the disease (Banerjee et al., 2009), a lack of treatment relates to higher morbidity and mortality associated with epilepsy(Engel et al., 2012).A molecular biomarker that could distinguish epilepsy and SE from other neurological conditions would allow for an earlier and more accurate diagnosis and appropriate treatment (Hegde and Lowenstein, 2014;Pitkänen et al., 2016; Walker et al., 2016).
Mesial temporal lobe epilepsy with hippocampal sclerosis (mTLE-HS) is the most common and well-defined form of symptomatic focal epilepsy.The precise diagnosis of mTLE-HS should be based on clinical history,electrophysiological findings, neuroimaging, and video-EEG.However,electrophysiological findings are often nonspecific or absent (Engel et al.,2013), and even high-resolution MRI may be negative in 20-30% of cases(Malmgren and Thom, 2012).The diagnosis should always be made based on a combination of factors and the semiological pattern of seizures is the only mandatory criterion (Wieser, 2004).Approximately 65% of all patients with drug-resistant epilepsy are patients with mTLE-HS (Cascino, 2004).For these cases, upon a careful assessment of the viability, surgical treatment is superior to drug treatment alone regarding seizure control, quality of life,employability rates, cognitive performance, and risk of sudden death (Wiebe,2003; Engel et al., 2012).Surgical intervention when combined with drug therapy may provide long-term total seizure control in 62-83% of patients(Mathon et al., 2015).The surgical outcome is usually evaluated using the Engel scale, with a favorable outcome being classified as Engel I or II, while an unfavorable surgical outcome corresponds to Engel III or IV (Engel et al.,1993).Approximately 10% of operated cases have little or no improvement in seizure control (Jeong et al., 2005; Vale et al, 2012; Savitr Sastri et al., 2014).
The underlying mechanisms of epileptogenesis are still unclear; hence, a better understanding of involved biological processes is fundamental for novel treatment strategies and therapeutics.Among potential therapeutic targets as well as possible biomarkers of pathological changes in the epileptic brain are microRNAs (miRNAs).MiRNAs have been implicated in various pathophysiological processes, including neuroinflammation and neurodegeneration (e.g., Alzheimer’s and Parkinson’s disease) (Hutvágner and Zamore, 2002; Pillai, 2005).The monitoring of miRNA expression might disclose the onset or progression of the disease, making miRNAs a useful biomarker.Numerous studies of miRNA dysregulation in TLE have provided evidence for their importance in epilepsy pathogenesis (Brennan and Henshall, 2018).Abnormal expression of miRNAs is related to pathways of inflammation, cell death, synaptic reorganization, and neuronal excitability,thereby being strongly associated with epileptogenesis (Chen et al., 2008).Mesial temporal lobe epilepsy with hippocampal sclerosis is associated with alterations in miRNA expression, particularly in the hippocampus.More than 20 miRNAs were differentially expressed in hippocampi and peripheral blood from both animal models and tissue resected specimens from patients with drug-resistant epilepsy (Korotkov et al., 2017).Several factors can influence the levels of miRNA expression in the blood such as motor phenomena,use of AEDs, and specific epileptic syndromes, among others, which makes identifying a specific biomarker for epilepsy challenging (Raoof et al, 2018).Thus, published data are often conflicting, and few studies specifically examine correlations between miRNA and mTLE-HS (Korotkov et al., 2017;Raoof et al, 2018).Few published studies have analyzed circulating miRNAs in patients with epilepsy in comparison to healthy subjects to identify potential biomarkers for this disease (Wang et al., 2015a, b; Avansini et al., 2017; Yan et al., 2017).We chose to analyze recent literature on the levels of miRNA expression in cerebrospinal fluid, blood, and brain tissue samples from TLE patients that could serve as diagnostic biomarkers to distinguish patients from controls, monitor disease severity, and potential therapeutic targets.
MicroRNAs in Epilepsy
We performed a PubMed search for original research articles published from January 2014 to November 2021 on possible miRNA biomarkers of epilepsy compared to healthy controls in cerebrospinal fluid, whole blood, blood plasma, blood plasma exosomes, blood serum, and brain tissue samples.The steps involved in the review and its contents are shown (Figure 1).A total of 22 articles were found for this review which had indicated that patients were diagnosed with temporal lobe epilepsy or mesial temporal lobe epilepsy.Of these articles, 9 had examined miRNAs in temporal lobe epilepsy in adults and 4 were studies in children.In addition, 9 articles reported on miRNA findings in mesial temporal lobe epilepsy of which 7 had been performed with adult patients (two articles did not indicate the ages of the subjects).
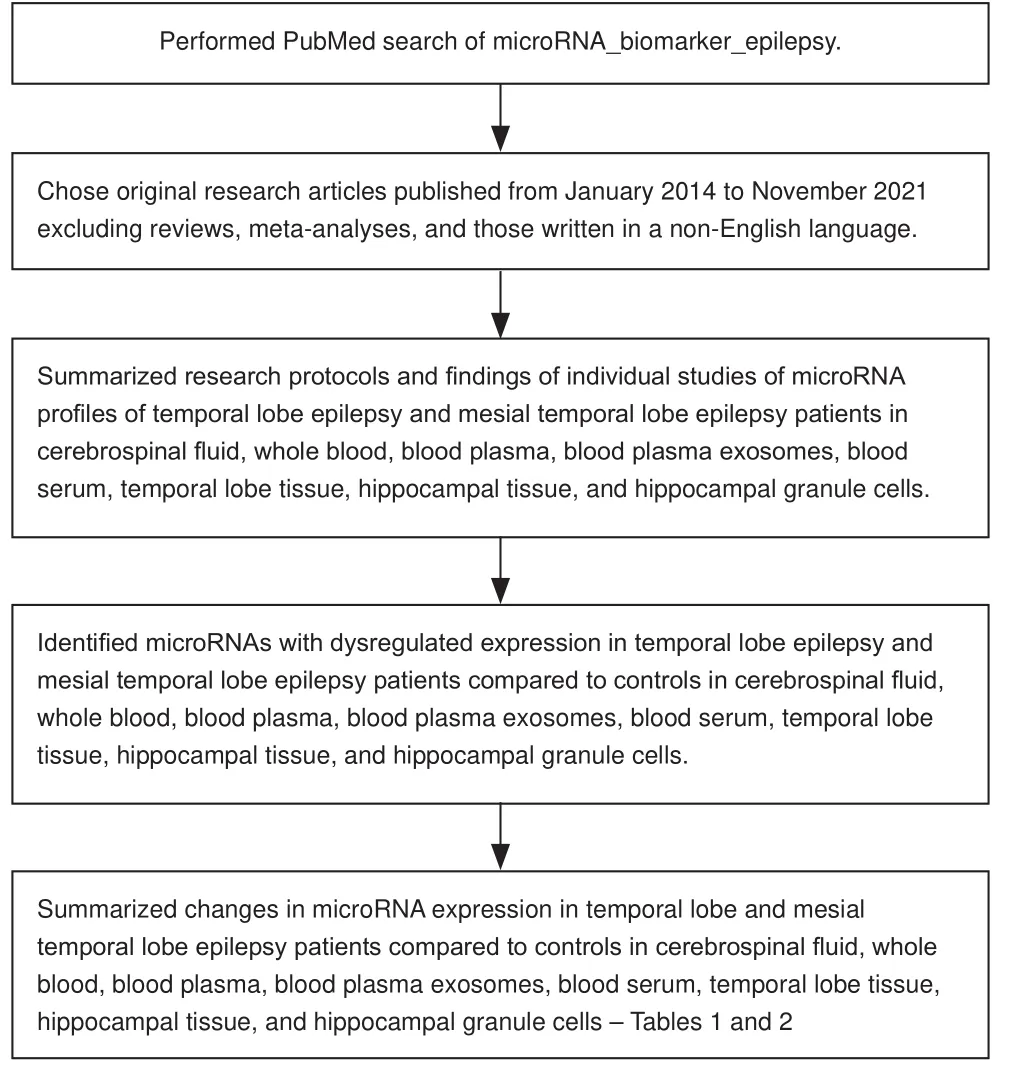
Figure 1|Flow diagram indicating how the review was performed and its contents.
Temporal lobe epilepsy In adults
Cerebrospinal fluid (CSF): Raoof et al.(2017) collected CSF samples from three different centers and performed both a discovery and validation phase analysis.The discovery phase involved 15 TLE, 15 SE, and 15 controls, and with the OpenArray platform the most abundant miRNAs were miR-19b-3p,miR-24-3p, miR-30b-5p, miR-30c-5p, miR-150-5p, miR-204-5p, miR-223-3p,miR-320a and miR-483-5p.Overall, the most abundant and most consistently detected miRNAs in each of the three sets of samples were generally very similar.A number of significantly regulated miRNAs were detected which included 5 differentially expressed miRNAs in TLE compared to controls (1 downregulated and 4 upregulated) and 15 miRNAs in SE samples compared to controls (7 downregulated and 8 upregulated).The validation phase was performed with CSF samples from 14 TLE, 17 SE, and 25 controls.A fourth group of samples from 25 patients with other neurological diseases was included (the majority were patients with Alzheimer’s disease and multiple sclerosis).By RT-PCR assay, levels of miR-19b-3p were significantly lower in TLE samples compared to controls, SE, and patients with other neurological diseases, indicating that decreased CSF levels of miR-19b-3p may be a biomarker of TLE.Levels of miR-451a were higher in SE compared to controls,TLE, and other neurological disease samples, indicating increased CSF levels of miR-451a as a biomarker of SE.Expression of miR-21-5p was also significantly higher in SE samples compared to TLE and control samples, suggesting this may be a non-specific biomarker of brain metabolism due to seizure activity.Although miR-21-5p was at higher levels in SE compared to other neurological diseases, this was not significant.Levels of miR-886-3p were significantly higher during validation in TLE samples compared to samples from patients with other neurological diseases.The levels of miR-204-5p and miR-223-3p were not significantly different between the groups.While there was no influence of age on CSF levels of miR-19b-3p, miR-21-5p, or miR-451a, there was a strong correlation for miR-886-3p indicating the dysregulation of miR-886-3p could be biased by age.Receiver operating characteristics curve analysis (ROC) showed that miR-451a was the best performing single miRNA with the area under the curve (AUC) 0.91 for distinguishing between TLE and SE samples.MiR-21-5p also showed good distinction between SE and control samples with AUC 0.83.Levels of miR-19b-3p showed good separation between TLE and each other sample type with AUC 0.73-0.78.Combining all three miRNAs gave AUC 0.83 for TLE compared to all other groups.When the comparison was made between SE and other groups, the combination miR-21-5p and miR-451a (AUC 0.85) was as accurate as all three miRNAs combined.
Whole blood: 30 TLE patients were recruited by Xiao et al.(2018) of which 14 were normal and 9 had HS on MRI, 10 had disease course > 10 years and 20 had disease course < 10 years, and 10 were drug-resistant and 20 were drug-responsive.A control group consisted of 30 subjects.Peripheral venous blood was collected into an EDTA tube and stored at -80°C prior to analysis.DNA methylation profiles were constructed primarily focusing on lncRNAs and miRNAs.Differentially methylated miRNAs in TLE included novel miRNAs (45%) and known miRNAs (55%).Several miRNA families and clusters were differentially methylated between TLE and controls, including 3 miRNA families and 3 chromosomal clusters hypermethylated in TLE: one chromosomal cluster, miR-23b and miR27b, from chromosome 9; let 7 familycomprising of miRNAs from one chromosomal cluster, let-7a, let-7d, and let-7f, from chromosome 9; miR-515 family comprising of miRNAs from one chromosomal cluster, miR-517b, miR-518a, and miR-516a, from chromosome 19; and miR-548 family comprising miR-548j from chromosome 22 and miR-603 from chromosome 10.TLE patients can be divided into different subgroups: drug resistant or drug responsive, MRI negative or hippocampal sclerosis, and disease course > 10 years or < 10 years.A set of miRNAs and lncRNAs was found with significantly differential methylation in the different subgroups.Significantly differential methylation miRNAs include miR-30a,miR-219, miR-128, miR-203, and miR-302.MiR-219 was found differentially methylated in both drug resistant and drug responsive, MRI negative, and hippocampal sclerosis subgroups.Aberrantly methylated miRNAs and lncRNAs are related to ion channel activity, drug metabolism, MAPK signaling pathway,and neurotrophin signaling pathway.Aberrantly methylated ncRNA and pathway targets might be involved in TLE development and progression.
Blood plasma: Using RT-PCR, Brennan et al.(2020) analyzed miRNA expression in blood plasma samples from 13 TLE patients which included baseline (24 hours seizure-free) and post-seizure samples (24 hours post-seizure).All patients were on poly drug therapy.Blood plasma samples from 16 nonfasting healthy volunteers served as controls.MiR-93a-5p (up in pre- and post-seizure TLE), miR-199a-3p (up in pre- and post-seizure TLE), miR-574-3p(down in pre-seizure TLE) in blood plasma were all differentially expressed in TLE patients compared to controls with no differences between pre- and postseizure samples.Levels of most of the miRNAs did not differ in post-seizure samples compared to pre-seizure samples indicating they represent potential biomarkers of epilepsy rather than acute seizures.Levels of miR-142-5p were higher in post-seizure samples compared to baseline suggesting this miRNA may be transiently affected by acute seizure activity.Levels of miR-93-5p, miR-199a-3p, and miR-574-3p were not different from controls in plasma samples from patients diagnosed with non-epileptic attack disorder (baseline and 24 hours post seizure-like behavior).ROC analysis of each miRNA from both preand post-seizure samples of TLE patients indicated a strong predictive value of these miRNAs as biomarkers.MiR-93a-5p, miR-199a-3p, and miR-574-3p had AUC 0.77, 0.8 and 0.79 for pre-seizure samples.MiR-93a-5p, miR-199a,and miR-574-3p had AUC 0.83, 0.83 and 0.71 for post-seizure samples.The combination of these 3 miRNAs gave AUC 0.88 and 0.86 for pre- and postseizure samples, respectively.
Raoof et al.(2018) performed a discovery and validation phase analysis using blood plasma samples in a multi-center study.The discovery cohort consisted of 32 patients with refractory focal epilepsy on poly drug therapy (majority with refractory TLE, 16 from Marburg, Germany, and 16 from Dublin, Ireland),and 32 non-fasting healthy volunteers as controls (16 from Marburg and 16 from Dublin).On admission, a baseline sample (epilepsy baseline sample, EBS)was taken from each patient.At 24 hours after experiencing an electro-clinical seizure an epilepsy after-seizure sample (EAS) was collected.The validation phase involved an extended cohort of patients and controls from the two centers in addition to samples from Erlangen, Germany.It included 102 TLE patients (29 with both EBS and EAS samples) and 110 controls; samples from 10 patients with frontal lobe epilepsy, 23 patients with genetic generalized epilepsy, 6 patients who experienced SE, and 16 patients with psychogenic non-epileptic seizures.RT-PCR was used to confirm the discovery results,selecting the 5 differentially expressed miRNAs identified by OpenArray profiling and 10 miRNAs identified by RNA-seq analysis of pooled plasma samples.miR-122-5p was omitted as it is a liver-specific, highly abundant miRNA in plasma, and 12 of 14 miRNAs selected from the profiling studies for validation as biomarkers of TLE or seizure or both, were confirmed.The levels of miR-27a-3p and miR-328-3p were lower in samples collected after an electro-clinical seizure (higher Ct value) than controls (n
= 32/group), indicating a potential value in diagnosing seizure episodes.Plasma levels of miR-654-3p were a promising biomarker of TLE as levels were significantly higher for EBS(lower Ct value) compared to control or EAS.A similar trend was seen for miR-543.The validation phase identified differential expression of 5 other miRNAs that had been selected for confirmation in post-seizure plasma samples.These were miR-199a-3p, miR-126-5p, miR-339-5p, miR-654-3p, miR-4433b-3p which displayed higher expression in EBS samples compared to controls and EAS.Three miRNAs, miR-27a-3p, miR-328-3p and miR-654-3p, were chosen to be validated in a larger cohort of 102 TLE patients and 110 controls.The validation phase confirmed the previously observed dysregulation of miR-27a-3p and miR-328-3p in TLE patient plasma compared to controls.In addition,levels of miR-27a-3p and miR-654-3p were also significantly different in plasma from genetic generalized epilepsy patients (higher Ct values) compared to controls.None of the miRNAs were differentially expressed in psychogenic non-epileptic seizures samples compared to controls.The levels of the three miRNAs were not significantly different in samples from frontal lobe epilepsy and SE patients compared to controls or between epilepsy patient groups.There were no significant differences in plasma levels of miR-27a-3p, miR-328-3p and miR-654-3p between TLE patients with or without hippocampal sclerosis.By ROC analysis, the highest performing miRNA for distinguishing EBS samples from controls was miR-654-3p (AUC 0.87).The best performing miRNAs as seizure biomarkers for distinguishing EAS from EBS were miR-328-3p (AUC 0.90), and miR-27a-3p (AUC 0.88).Fifty-nine new-onset epilepsy patients and 20 subjects without epileptic seizures and matched with the epileptic patients by gender and age were recruited by Wang et al.(2017).24 patients were treated with phenytoin daily and 15 other patients were treated with gabapentin daily and 1 patient with carbamazepine daily.19 patients were only treated with valproate daily for 2 weeks.Patients did not receive any medication for 2 weeks before blood sampling to exclude the effect of AEDs on miRNA expression profiles.The severity of epileptic seizures was categorized by a seizure severity questionnaire and each of the 16 items scored on a 7-point Liker scale,with lower scores representing lower impact.Overall assessment of seizure severity was measured with the last two items for which the total score ranges from 0 to 7.Patients with a score of < 3 were allocated into the mild group, 3-5 into the moderate group, and > 5 into the severe group.By qRTPCR, the expression of miR-134 in the patients with new-onset severe epilepsy(n
= 19) was significantly higher than that of controls.However, no significant difference was found between the patients with mild (n
= 20) and moderate epilepsy (n
= 20) and healthy controls.After treatment with valproate acid, the mean plasma miR-134 level of patients with severe epilepsy was significantly decreased from 5.62 ± 1.60 to 3.26 ± 2.80.A significant positive correlation was observed between the severity of seizure symptoms and plasma miR-134 levels in the moderate and severe seizure groups, whereas there was no correlation in the mild seizure group.Raoof et al.(2017) collected blood plasma samples from a subgroup of TLE patients on the same day as CSF collection (see entry under CSF).Using RTPCR, a check was made for a correlation between the CSF levels of miR-19b-3p, miR-21-5p, and miR-451a and the levels in the plasma of TLE patients.No correlation was observed for miR-19b-3p or miR-21-5p between CSF and plasma levels.The levels of miR-451a obtained from plasma showed a trend toward a negative correlation with the levels in CSF in the same individual,although not reaching statistical significance.
Blood plasma samples were obtained by Sun et al.(2016) from 25 refractory TLE patients and 25 healthy controls (age and gender matched) with no history of seizures or exposure to AEDs and included 8 patients who underwent surgery for intracranial hematoma.By RT-qPCR, miR-129-2-3p expression was significantly increased in plasma samples from the refractory TLE patients compared to controls.The expression of miR-935 was not significantly increased in plasma samples from refractory TLE patients.Using ROC analysis, the expression level of miR-129-2-3p in plasma was a useful biomarker for differentiating refractory TLE patients from healthy controls with AUC 0.778.There were no significant correlations between miR-129-2-3p and age, gender, age of epilepsy onset, side of lesion, and history, but it was correlated with seizures frequency and Engel classification.With the increase in seizures frequency as the prognosis worsened, the value of miR-129-2-3p in plasma was significantly increased.
Blood serum: De Benedittis et al.(2021) studied a group of 27 TLE patients and 20 age and gender matched controls who were Caucasian, unrelated individuals with no neurological or psychiatric disease and no history of seizures.All patients were on mono or polytherapy AEDs at their last clinical visit.Patients were subsequently divided into two groups according to their response to AED treatment and seizure frequency: (1) drug responsive epilepsy, defined as seizure freedom for at least 24 months, and (2) drug resistant TLE, based on the ILAE criteria for drug resistant epilepsy as seizures persisting despite trials of two or three adequately tolerated and appropriate anti-epileptic drugs (Kwan et al., 2010).Two miRNAs, miR-138-5p and miR-298, were excluded from subsequent analyses, as they did not show any significant change in their expression.By qRT-PCR, the relative expressions of miR-142, miR-146a, and miR-223 were significantly upregulated in serum samples of TLE patients compared to healthy controls.Additionally, miR-132 was upregulated in sera from TLE patients compared to controls, but the difference was not significant.The relative expressions of miR-142 and miR-223 were significantly upregulated in drug resistant patients (n
= 10)compared to drug responsive patients (n
= 17).Both miR-146a and miR-132 were downregulated in sera of drug resistant TLE patients, but the difference with drug responsive TLE patients was not significant.Correlation analysis revealed a significant correlation in drug resistant subjects between miR-146a and gender and miR-223 and age of onset.MiR-142 and miR-223 and right hippocampal volume showed a correlation coefficient of 0.56 and 0.58,respectively; miR-146a and left hippocampal volume showed a negative correlation coefficient of -0.6.The lack of significance was probably due to the reduced numerosity of the population.In the drug responsive group,there was a significant positive association between miR-142 and miR-223,miR-146a and miR-132, age and age of onset, disease duration and left hippocampal volume, and right hippocampal volume and left hippocampal volume.In all patients, including drug resistant patients and drug responsive patients, there was a significant positive association between miR-142 and miR-223, miR-146a and miR-132, miR-142 and pharmaco-resistance, and miR-223 and pharmaco-resistance.Correlation analysis suggested a positive correlation between age and disease duration in drug resistant patients, and a negative correlation between age of onset and disease duration.In the drug responsive group, there was a significant negative correlation between age of onset and disease duration.ROC analysis showed an AUC of 0.80 for miR-142,an AUC of 0.75 for miR-223, and an AUC of 0.80 for combined miR-142 and miR-223 to discriminate drug responsivevs
.nonresponsive TLE patients.Temporal lobe tissue: Busch et al.(2020) examined miRNA expression in left(language dominant) temporal lobe tissue resected from 23 drug resistant TLE patients for treatment of their seizures.Given the known association betweenAPOE
polymorphisms and memory performance [i.e.,APOEe4
detrimental,APOEe2
protective (Small et al., 2004)], only patients who were homozygous forAPOEe3
allele (wildtype) were included in the study.All patients completed a comprehensive neuropsychological evaluation approximately 2 months prior to surgery that included an assessment of verbal episodic memory.Patients were then separated into two memory groups based on amean composite delayed memory score (combined delayed story recall and word-list learning tasks) (Banks et al., 2019; Jonaitis et al., 2019).Patients with mean scores < 85 were classified as having “impaired” memory (n
= 10)and those with mean scores > 85 were classified as having “intact” memory(n
= 13).The two memory groups were well matched on demographic and disease-related variables.The impaired memory group demonstrated significantly delayed verbal memory scores than the intact memory group.Using small RNA sequencing followed by differential transcript analysis, 4 differentially expressed miRNAs were identified in brain tissues of patients with impaired memory compared to those from patients with intact memory.Both miR-1237-5p and miR-6805-5p were downregulated, whereas miR-3939 and miR-6750-5p were upregulated in brain tissues derived from patients with impaired memory compared to those from patients with intact memory.Sun et al.(2016) used microarray to analyze miRNAs in the temporal cortex collected from nine refractory TLE patients who underwent surgical removal of the lesion in the first phase of the study.Among the differentially expressed miRNAs, miR-129-2-3p and miR-935 were much more strongly expressed in temporal cortex tissue of TLE patients compared to temporal lobe tissue obtained from eight patients with hypertension who required emergency surgery to clear intracranial hematoma.In the second phase, qRT-PCR was used to validate the differentially expressed miRNAs in tissue samples from 13 refractory TLE patients and 13 controls (both groups included patients from the first phase).Consistent with the microarray results, miR-129-2-3p was significantly increased in tissue samples from the refractory TLE patients compared to controls.The expression of miR-935 in tissue samples of the refractory TLE patients was not significantly increased.By ROC analysis, the expression of miR-129-2-3p in temporal lobe tissue was a useful biomarker for differentiating TLE patients from controls with AUC 0.929.
Hippocampal granule cells: 14 drug resistant TLE patients, who were candidates to epilepsy surgery, were recruited by Zucchini et al.(2014).All patients underwent tailored temporal lobe resection to remove the epileptogenic area according to the findings during pre-surgical investigation.Surgery consisted of removing the temporal pole, the anterior neocortical lateral cortex, the uncus-entorhinal area, and the hippocampus and parahippocampal gyrus.The main surgical specimens (hippocampus and/or temporal pole) were removed “en bloc”.Specimens were spatially oriented, formalin fixed and paraffin embedded.They were dewaxed and stained with hematoxylin and eosin.Neuropathological evaluation was performed using the most recent classifications of HS, granule cell pathology(GCP), and focal cortical dysplasias (Blümcke et al., 2009, 2011, 2013),applying the recommended histochemical and immunohistochemical stains.Specimens either displayed no GCP or GCP type 2.Four different types of neuropathological features can define GCP type 2.(1) Dispersion: rows of granule cells spread into the molecular layer and the distance between granule cells is increased; (2) ectopic granule cells: single ectopic granule cells are dispersed into the molecular layer; (3) clusters: ectopic granule cells form clusters within the molecular layer; (4) bilaminar: two granule cell layers,separated by a cell-free gap (Blümcke et al., 2009).While patterns of granule cell loss (thinning and/or cell free gaps, GCP 1) occur isolated, patterns of architectural abnormalities (GCP 2) can appear with cell loss.Therefore,only sections in which no cell loss was detected (based on neuronal nuclei staining) were included in the analysis.10 μm thick sections were cut using a microtome and the dentate granule layer of the dentate gyrus was laser dissected.Microdissected cells were captured in microcut transfer film.Granule cells were collected from at least 3-4 slices per patient to obtain an adequate quantity of RNA.Materials from all sections of the same patient were pooled together.Ten patients were selected of which the majority were on poly-drug therapy, and neuropathological examination showed that these patients had HS type 1 (Blümcke et al., 2013), which was associated with no granule cell pathology (no GCP) in five patients and with granule cell pathology (GCP type 2) in the other five.GCP consisted of granule cell dispersion in four cases and bilaminar granule cell layer in one case.By miRNA microarray, 12 miRNAs were differentially expressed in patients without GCP compared to patients with GCP type 2.Of these, six had relatively higher expression in tissue from patients without GCP and six were higher in those with GCP type 2.Differential expression of a subset of three miRNAs (miR-338-5p, miR-219-5p, and miR-487a) was validated in an extended cohort of patients (the 10 original patients plus another 2 per group).Using qRTPCR, expression levels of all these miRNAs were different in the two groups,confirming microarray findings.The levels of miR-338-3p and miR-219-5p were decreased in GCP type 2 but did not reach significance.In contrast,miR-487a expression was significantly lowered in GCP type 2.Using qRT-PCR,ANTXR1 mRNA levels were increased in the GCP type 2 group.An increase of ANTXR1 immunoreactivity was observed in the granule cell layer of patients with GCP type 2, as compared with those with no GCP.
In children
Blood plasma: MiRNA expression in blood plasma samples from 59 children diagnosed with TLE and 63 healthy controls was determined by Niu et al.(2021) using qRT-PCR.The expression of miR-194-5p in blood plasma was significantly lower in children with TLE compared to the control group.By ROC analysis, using the expression level of miR-194-5p in plasma gave AUC 0.896, with sensitivity of 0.810 and specificity of 0.881 at the cut-off value of 0.859, indicating the diagnostic value of miR-194-5p to the clinical diagnosis of children with TLE.
Blood serum: Yu et al.(2021) examined miRNA expression in blood serum samples from 98 TLE children and 72 healthy, age and gender matched children.By qRT-PCR, the level of miR-148a-3p expression in serum was significantly increased in TLE children compared with healthy controls.TLE children were divided into two groups according to the median expression value of miR-148a-3p, giving a low miR-148a-3p expression group (n
= 45) and a high miR-148a-3p expression group (n
= 53).MiR-148a-3p expression was significantly correlated with febrile seizure (FS) history and seizure frequency(per month) in TLE children.No significant associations were shown between miR-148a-3p expression and other clinical features, including age, gender,course of the disease, interictal EEG, unilateral temporal, bilateral temporal,and seizure duration(s).TLE children were divided into the FS-positive group(n
= 38) and the FS-negative group (n
= 60).qRT-PCR showed that the level of miR-148a-3p expression was significantly increased in FS-positive children compared to non-FS children.By ROC analysis, using the level of miR-148a-3p expression for distinguishing TLE from controls gave AUC 0.928, sensitivity 0.837, and specificity 0.917 at the cut-off value of 1.015, which indicated high diagnostic accuracy and specificity of miR-148a-3p in TLE.ROC analysis showed that the level of miR-148a-3p expression could distinguish TLE children with FS history from non-FS children giving AUC of 0.891, sensitivity of 0.842, and specificity of 0.883 at the cut-off value of 1.485.Using qRT-PCR assay, Wu et al.(2021) found the level of miR-29a in blood serum to be significantly lower in 65 TLE children compared with 70 healthy,age and gender matched children.11 of the TLE cases developed mesial sclerosis.Using ELISA, significantly higher concentrations of tumor necrosis factor-α, interleukin-6, and interferon-γ were detected in the serum of TLE patients compared to the control group.Correlation analysis revealed that the levels of serum miR-29a were negatively correlated with the levels of tumor necrosis factor-α, interleukin-6, and interferon-γ.HMGB1
was significantly higher in the serum of TLE patients and was shown to be a target gene of miR-29a, and negatively correlated with miR-29a level.ROC analysis for diagnostic evaluation based on the serum levels of miR-29a in the TLE children and control group gave AUC 0.898, with sensitivity 0.862 and specificity 0.771, at a cut-off value of 0.810Li et al.(2020) used qRT-PCR to examine miRNA expression in blood serum samples of 63 TLE children and 63 healthy, age and gender matched controls.According to the test results, 11 cases had developed mesial sclerosis.TLE children had significantly lower expression of miR-15a-5p compared with the control group.Furthermore, the level of miR-15a-5p expression was compared in the clinical groups of TLE patients according to the results of EEG and MRI.There was no significance for miR-15a-5p expression in bilateral temporal patients (n
= 16) compared with unilateral temporal patients (n
= 47).Also, there was no significance for miR-15a-5p expression in mesial sclerosis patients (n
= 11) compared to no abnormality (n
= 52).These data indicated that miR-15a-5p expression did not vary in different subgroups of TLE patients.ROC analysis to determine the diagnostic value of serum miR-15a-5p for TLE gave AUC 0.908, sensitivity 0.825, and specificity 0.881 at the cut-off of 1.650, indicating that serum miR-15a-5p might be a sensitive biomarker to distinguish TLE patients from healthy controls.Mesial temporal lobe epilepsy In adults
Blood plasma: Using RT-qPCR, Gong et al.(2018) showed that blood plasma miR-153 expression in 22 patients with refractory mesial temporal lobe epilepsy (mTLE) was significantly lower compared to 20 controls, who had never been diagnosed with epilepsy or seizures and had undergone surgical treatment for head trauma or cerebral hemorrhage.
Avansini et al.(2017) performed both a discovery and validation phase analysis.The discovery phase utilized blood plasma samples from 14 mTLE, 13 focal cortical dysplasias type II patients, and 16 healthy control subjects without epilepsy.Using qRT-PCR, only miR-134 was significantly downregulated in plasma of patients with mTLE compared to controls.No difference was found in plasma levels of miR-134 in patients with focal cortical dysplasias compared to controls.The expression levels of miR-23a and miR-31 were not different among the groups analyzed.In the validation phase, miR-134 was significantly downregulated in the plasma of 65 mTLE patients when compared to 83 healthy controls without epilepsy.miR-134 was downregulated both in drug responsive mTLE patients (n
= 27) and drug resistant mTLE patients (n
= 38) when compared to controls.No difference was observed in plasma miR-134 levels between drug responsive and drug resistant mTLE patients.In addition, plasma miR-134 was not statistically different between mTLE patients with (n
= 48) or without (n
= 17) the presence of signs indicating HS on MRI.Seizure frequency was also found to have no statistically significant effect on plasma miR-134 levels using a score of up to 1 seizure per month to define two groups of patients.By ROC analysis, in the discovery phase, plasma levels of miR-134 could discriminate patients with mTLE from controls with AUC of 0.75, sensitivity of 0.65 and specificity of 0.75.In the validation phase, the accuracy for identifying patients with mTLE was indicated by AUC 0.671 with a sensitivity of 0.75 and specificity of 0.58.Blood plasma miRNA expression was examined in mTLE patients in both a discovery and validation phase by Li et al.(2016).In the discovery phase,miR-153 expression was significantly decreased in the plasma of 32 surgical patients with mTLE compared with 18 age-matched surgical controls.No significant difference in miR-494 expression was observed between these surgical patients and controls.The mTLE patients had undergone anterior temporal lobectomy, while the controls had undergone surgical treatment forhead trauma or cerebral hemorrhage.Downregulation of miR-153 in plasma was validated by RT-qPCR in 56 mTLE patients with/without surgery compared to 101 age-matched healthy non-surgical controls, while no significant difference in plasma miR-494 level was found.In a univariate linear regression model, plasma miR-153 level was negatively correlated with mTLE in a significant manner.In a multivariate linear regression model, after controlling for age and gender, low plasma miR-153 level was an independent risk factor for mTLE.The results remained robust even after considering all clinical characteristics such as fasting glucose and alanine aminotransferase.
Blood plasma exosomes: Yan et al.(2017) isolated exosomes from blood plasma samples of patients with mTLE-HS and age and gender matched healthy volunteers.By microarray of plasma exposomes from 3 mTLE-HS patients and 3 healthy controls, differential expression of 50 exosomal miRNAs was shown in mTLE-HS patients compared to healthy controls.Among these,2 miRNAs were significantly increased and 48 were significantly decreased(fold change > 1.2,P
< 0.05) in mTLE-HS patients compared to controls.qRTPCR was used to validate the microarray measurements.The expression levels of 6 exosomal miRNAs selected from the microarray results were determined in a cohort of 40 mTLE-HS patients and 40 healthy controls.Among these,the level of miR-3613-5p expression was significantly increased, while the expression levels of miR-4668-5p, miR-4322, miR-8071, miR-6781-5p, and miR-197-5p were significantly decreased in mTLE-HS patients.The qRT-PCR results were consistent with the microarray data.By ROC analysis, AUC values for miR-3613-5p, miR-4668-5p, miR-8071, and miR-197-5p were 0.844, 0.789,0.932, and 0.802, respectively, for discriminating cases of mTLE-HS from healthy controls.The AUC for miR-4322 and miR-6781-5p were > 0.7 but did not reach statistical significance.The results showed that miR-8071 was the most valuable biomarker for discriminating mTLE-HS patients from healthy individuals, and the best cut-off of miR-8071 was 0.450 with a sensitivity of 0.833 and specificity of 0.967.The association was examined between exosomal miR-8071 expression and clinicopathological characteristics in mTLEHS patients.The expression levels of exosomal miR-8071 were categorized as low or high in relation to the median value.The results demonstrated that there was no significant correlation between exosomal miR-8071 expression and clinicopathological features, such as age, gender, family history, history of epilepsy, seizure time, laterality of the epileptogenic zone, AED therapy at the last clinic visit, sampling time from the last seizure, and comorbid conditions.However, exosomal miR-8071 level was significantly associated with disease duration or seizure frequency.Blood serum: MiRNA expression was examined by Ioriatti et al.(2020) in blood sera of 28 patients who underwent anterior temporal lobectomy + amygdalohippocampectomy as a consequence of drug resistant mTLE-HS, and with 11 healthy volunteers as controls.Blood collection occurred upon admission to the operating room.All patients had at least 18 months of postoperative follow-up, 14 with the favorable surgical response (Engel I) and 14 with the unfavorable response (Engel III-IV).By qRT-PCR assay, no significant difference was found in miR-27a-3p expression between the groups, even if Engel I was grouped with Engel III-IV to compare epilepsy patients (regardless of prognosis) to the control group.The miR-328-3p was differentially expressed between groups according to the analysis of variance results.The Bonferroni post hoc test showed that the control group had a significantly lower mean miR-328-3p level than the Engel I or Engel III-IV groups.Comparing the control group to the Engel grouped categories (Engel I + Engel III-IV), the ANOVA results were also significant with the control group presenting a significantly lower mean than the Engel I + Engel III-IV group.The miR-654-3p analyses indicated a significant difference between the groups, and the control group had a significantly lower mean than the Engel I group.There was no difference between the control group and Engel I + Engel III-IV.By ROC analysis, comparing the control group to the Engel I group, miR-27a-3p gave AUC of 0.721.MiR-328-3p for comparing controls versus Engel I showed AUC 0.903, with sensitivity 0.786 and specificity 1.000.MiR-654-3p for comparing controls versus Engel I gave AUC 0.747, with sensitivity 0.571 and specificity 1.000.When the control group was compared to the unfavorable surgical prognosis group (Engel III-IV), miR-27a-3p showed no relevant discriminatory power (AUC 0.617).Comparing miR-328-3p between controls and Engel III-IV showed an AUC of 0.968, with a sensitivity of 1.000 and specificity of 0.909.MiR-654-3p showed no relevant discriminatory power with AUC 0.517.The combination of Engel I + Engel III-IV aimed to generate a group of epilepsy patients despite the surgical prognosis.Thus, when comparing epilepsy patients to controls, miR-27a-3p had non-relevant discriminatory power with AUC 0.669.MiR-328-3p in this same comparison had AUC of 0.935, with a sensitivity of 0.893 and specificity of 0.909.MiR-654-3p showed non-relevant discriminatory power with AUC 0.636.Comparing Engel I with Engel III-IV,miR-27a-3p and miR-328-3p had non-relevant discriminatory power.MiR-654-3p presented AUC of 0.736 for this comparison with a sensitivity of 0.571 and specificity of 0.846.
In a study by Surges et al.(2016), patients with medically intractable mTLE with qualitative MRI features of unilateral HS without other pathologies on cranial MRI were admitted for evaluation of epilepsy surgery.After inclusion of the patients, blood samples were taken at the time of inclusion (baseline,pre-seizure sample) and at several time points after a bilateral convulsive seizure (BCS) had occurred during video-EEG monitoring (within 30 minutes,3-6 hours, 20-28 hours, and 3-6 days after a BCS) and the serum separated.A total of 51 patients were enrolled in the study of which 19 exhibited 23 BCS during video-EEG monitoring.In one patient the baseline sample was missing and in three patients only 50% of postictal serum samples were available.These four patients were excluded from further analysis.Overall,serum samples of 16 seizures in 15 patients were included in the final miRNA profiling using qPCR.Depending on the time-point, 49-51% of the assessed 752 miRNAs passed the detection test and were classified as detectable.Out of these, about 90% were detectable in the baseline sample as well as postictally, while the rest was detectable at one time-point only.Detectable miRNAs were analyzed for differential expression.Comparison of each timepoint after BCS with the baseline level revealed that when considering data of all 15 patients, differential expression of miRNAs was only statistically significant at the first postictal time-point, i.e., within 30 minutes after the BCS.Overall, 215 miRNAs were significantly altered as compared to baseline levels at this time-point.In contrast, at all later time-points (3-6 hours, 20-28 hours, and 3-6 days) no significant differences were observed, indicating that miRNA expression at 30 minutes after BCS is indeed seizure-related and of transient nature.20 miRNAs were selected for validation by qPCR based on their Ct-values.Validation timepoints were baseline and 30 minutes.For these time points, samples from 14 patients were available.Regarding the whole group, miR-143-3p, miR-145-3p, miR-365a-3p, and miR-532-5p showed a significant increase of expression 30 minutes postictally compared to the preictal levels.Linear regression analysis of these miRNAs revealed a significant correlation between total seizure duration and relative changes in miRNA expression levels in two of the four miRNAs (miR-143-3p, 145-5p).For these two miRNAs, seizure duration was inversely correlated with the relative increase of the miRNA in blood.Examination of miRNA expression in a subgroup of mTLE patients with seizures occurring during sleep demonstrated that in four mTLE patients miRNA expression levels were clearly higher as compared to the remaining mTLE patients 30 minutes after the BCS.Further analysis of miRNA expression focusing on these four patients revealed significantly deregulated miRNAs not only at the 30 minutes time-point postseizure but also at the 3-6 hours and 20-28 hours time-points.At 3-6 days following the BSC no significantly deregulated miRNAs were observed.In contrast, the remaining 11 patients showed no significantly altered miRNAs expression levels at 30 minutes, 3-6 hours, 20-28 hours, and 3-6 days.Thirteen miRNAs were selected for qPCR validation in these four patients based on significance levels and robust pre-seizure expression in the miRNA expression profiling.Ten of them were differentially expressed at 30 minutes,3-6 hours, and 20-28 hours, another three at 30 minutes and 3-6 hours.One candidate, miRNA-663b, was significantly increased 3-6 hours after the BSC.
Temporal cortex tissue: Gong et al.(2018) investigated miRNA expression in temporal cortex tissues resected from 22 patients with refractory mTLE who had undergone anterior temporal lobectomy.Temporal neocortical tissues without abnormal pathological changes were obtained from 20 controls, who had never been diagnosed with epilepsy or seizures.They had undergone surgical treatment for head trauma or cerebral hemorrhage.By qRT-PCR assay, the level of miR-153 expression was significantly downregulated in the temporal cortex of patients with mTLE compared with controls.By qRT-PCR and Western blot analysis, the expression level of hypoxia-inducible factor-1α was significantly upregulated in the temporal cortex of mTLE patients.A negative correlation between hypoxia-inducible factor-1α and miR-153 was shown by Pearson’s correlation test.
MiRNA analysis was performed by Li et al.(2016) using temporal cortex tissues resected from 32 patients with mTLE who had undergone anterior temporal lobectomy.All patients were taking 2 or more AEDs and met the definition of mTLE (Engel et al., 1996).18 samples of temporal neocortical tissues without abnormal pathological changes served as controls and included 5 head trauma and 13 cerebral hemorrhage cases.None of the controls were diagnosed with epilepsy or seizures.By qRT-PCR, expression of miR-153 in the temporal cortex was significantly downregulated in mTLE patients compared to controls.Expression of miR-494 in the temporal cortex was not significantly different in mTLE patients compared to controls.The expression level of hypoxia-inducible factor-1α was significantly upregulated in the temporal cortex of mTLE patients compared to controls.
The King said, How did the spirit get over the water, and where did it go after it had eaten the pear? The gardener answered, Some one came in a snow-white garment from heaven who made a dam, and kept back the water, that the spirit might walk through the moat
Hippocampal tissue: Baloun et al.(2020) studied miRNA expression in hippocampal tissue resected from 16 patients diagnosed with mTLE-HS.The diagnosis of unilateral mTLE was based on historical data, ictal and interictal EEG findings, ictal semiology, neuropsychology, and neuroimaging findings(MRI and positron emission computed tomography).Unilateral HS concordant with the EEG lateralization of the epileptogenic zone was confirmed by visual inspections of the MRI scans.None of the patients revealed other brain structural lesions on MRI scans or had undergone previous intracranial surgery.Hippocampal tissue was collected from 8 postmortem cases without hippocampal aberrations and served as control.Surgically resected and autopsy tissues were fixed in 10% neutral buffered formalin, grossly inspected, carefully oriented, and measured.Hippocampal tissue specimens were dissected into 2-3 mm-thick tissue slices along the anterior-posterior axis and paraffin embedded.Formalin-fixed paraffin-embedded tissue sections were stained with hematoxylin and eosin and evaluated under light microscopy; additionally, the presence of neuronal depletion and gliosis in HS tissue samples was confirmed using neuronal nuclei and glial fibrillary acidic protein immunohistochemistry.For miRNA analysis, paraffin-embeddedtissue slices showing the presence of hippocampal complex were selected.An analysis of age- and gender-related miRNAs in the dataset was performed to prevent bias arising from uneven distribution of age and gender in patient and control groups.These analyses did not uncover any gender-specific miRNA with an altered expression level in mTLE/HS within the data, while miR-7110-3p upregulated in controls > 60 years of age was removed from followup analyses.The basic concept suggests that the presence of seizures in childhood and adolescence could affect the miRNA expression profile and thuspotentially introduce a defect in brain development (Henshall, 2014; Rao and Pak, 2016).For this reason, patient data were categorized and analyzed in 4 categories based on their epilepsy onset age: (a) all patients together; (b) the first unprovoked seizure before the age of 10 (childhood); (c) between 10 and 19 years of age (adolescent); (d) at the age of 20 years or later (adult onset).Massive parallel sequencing data processing and differential expression analysis were performed.A search of the massive parallel sequencing datasets for differentially expressed miRNAs in patients with the TLE onset between the ages of 2 and 9 years discovered 123 miRNAs significantly dysregulated in patients.In the adolescent-onset category, 130 miRNAs were significantly dysregulated in TLE.The category with onset age above 20 contained 80 dysregulated miRNAs.49 miRNAs were significantly dysregulated in all age categories.Since all patients underwent surgery in adulthood, the period between epilepsy onset and sample collection differed among childhoodonset (mean 33.2 years), adolescent-onset (mean 23 years), and adult-onset(mean 11 years).The duration of epilepsy might have affected the expression of detected miRNA, and correlations were found between the expression of miR-142-3p, miR-135a-5p, and miR-484 (all upregulated) with the duration of epilepsy, but their effects were low.qPCR assay validated the upregulation of miR-129-2-3p and miR-142-3p across all onset categories in patients, and let-7b-3p, miR-135a-5p, and miR-140-5p increased in patients with TLE onset in adolescence.In addition, qPCR confirmed increased expressions of miR-142-5p, miR-193a-5p, and miR-203a-3p in one or more onset groups in patients along with the group without onset categorization.
Hippocampal tissue samples from 20 patients who underwent amygdalohippocampectomy due to drug resistant mTLE-HS were analyzed for miRNA expression by Antônio et al.(2019).Patients were evaluated and classified into two groups in terms of surgical prognosis: one group consisted of 10 patients with favorable postoperative outcomes (Engel I), and the other group consisted of 10 patients with unfavorable postoperative outcomes (Engel IIIIV).Hippocampal tissue samples from nine autopsied individuals were used as controls.The autopsied individuals had no neurological or psychiatric medical history, and the samples were removed within 15 hours after death.By microarray analysis, a comparison of the samples from the two groups of patients (satisfactory outcomevs.
unsatisfactory outcome) revealed that some miRNAs were overexpressed in the hippocampal samples.Four of these miRNAs were selected for further investigation: miR-145, miR-181c, miR-199a, and miR-1183.By RT-qPCR, only miR-145 expression was significantly different between the mTLE-HS and control groups being decreased in hippocampal samples from mTLE-HS patients.Comparisons of the Engel groups (Ivs
.III-IV) revealed no significant difference in levels of expression of the four miRNAs.Bencurova et al.(2017) performed miRNA analysis on hippocampal tissue samples from 19 patients with left-sided mTLE and 14 patients with rightsided mTLE.All patients were referred for their medical intractability and fulfilled the diagnostic criteria for mTLE-HS.The diagnosis was made according to the ILAE criteria.Visual inspections of the MRI scans revealed unilateral HS concordant with the EEG lateralization of the epileptogenic zone in all patients.None of the patients revealed other brain structural lesions on MRI scans or had undergone previous intracranial surgery.Paraffin-embedded control hippocampal tissue samples were obtained from 10 postmortem cases without hippocampal aberrations.Surgically resected and autopsy tissues were identically treated: they were fixed in 10% neutral buffered formalin,grossly inspected, and carefully oriented, and their proportions measured.Hippocampal specimens were dissected into 2-3-mm-thick tissue slices along the anterior-posterior axis.Representative tissue samples were routinely processed and paraffin embedded.Formalin-fixed paraffin-embedded tissue sections were stained with hematoxylin and eosin and evaluated using light microscopy.The presence of neuronal depletion and gliosis in HS tissue samples was confirmed using neuronal nuclei and glial fibrillary acidic protein immunohistochemistry.Total RNA was isolated from formalinfixed paraffin-embedded tissue sections.Next-generation sequencing was carried out on 16 mTLE-HS patients and 8 post-mortem controls.Samples were evaluated by cluster analysis and searched for miRNAs with altered expression in mTLE-HS using differential expression analysis.The lack of a clear segregation suggested that very few miRNAs are differentially expressed between these patients and controls and that the miRNAs are expressed at very low levels (Nassirpour et al., 2014).Two differential expression analyses namely DESeq2 and edgeR were employed to identify miRNAs with altered expression in mTLE-HS.Differential expression analysis revealed no genderspecific miRNA with altered expression level in mTLE-HS.Only miR-7110-3p was upregulated in controls > 60 years of age and removed from follow-up analyses.No autopsy delay-related decrease in miRNA levels was observed,suggesting that the levels of miRNAs in the samples remain highly stable and autopsy delay did not have an effect on miRNA expression in control samples.qPCR expression analysis was performed on the validation cohort of an additional 17 mTLE-HS brain tissue samples.The control cohort was brain tissue samples from 10 patients.This analysis validated the expression of 26 miRNAs, whereas miR-184, miR-6131, and miR-19b-3p with altered expression identified by miRNA-Seq and verified by technical validation were not confirmed in the validation cohort.Conversely, miR-134-5p showed an altered expression level only in the validation cohort.MiR193a-5p, miR-320e, and miR-490-3p, already reported in the literature, were identified by qPCR as upregulated in both cohorts of patients with a fold-change of 1.7 and higher, despite not showing an altered expression in miRNA-Seq.On stringent analysis, 20 miRNAs displayed an altered expression pattern in mTLE-HS with 19 upregulated (miR-1260a, -1260b, -1275, -129-1-3p, -129-2-3p, -142-3p, -142-5p, -144-5p, -150-5p, -191-5p, -193b-3p, -339-5p, -374b-5p,-4443, -4454, -451a, -4792, -6087, -874-3p) and 1 downregulated (miR-1298-5p) in patients’ hippocampus.A differential expression fold-change of 1.8 or higher was observed by both next-generation sequencing and qPCR in nine miRNAs newly associated with epilepsy: miR-1260a, -1260b, -1275, -1298-5p,-339-5p, -4443, -4454, -4792, -6087.In six miRNAs selected from literature,no significant change in expression was detected between patients and controls (except for miR-132-5p and miR-301a-3p, which were significant only in DESeq2).
Those miRNAs found to have altered expression in CSF, blood plasma, blood plasma exosomes, blood serum, and brain tissues/cells in TLE and mTLE are summarized in Tables 1 and 2.
Discussion
Temporal lobe epilepsy, a common neurological disorder in both adults and children, can prove difficult and challenging to diagnose.There are two types of TLE (Klein and Joshi, 2019).mTLE involves the medial or inner part of the temporal lobe, manifesting in the hippocampus, parahippocampal gyrus,and the amygdala, and accounts for almost 80% of all temporal lobe seizures(Tatum, 2012).Neocortical or lateral temporal lobe epilepsy involves the outer part of the temporal lobe, and this type of TLE is found in approximately 10% of cases (Bercovici et al., 2012).
The majority of the different studies reviewed had performed miRNA profiling in blood plasma in adults with TLE (four studies) and mTLE (three studies),in blood serum in children with TLE (three studies), and in hippocampaltissue in adults with mTLE (three studies).Similar findings were found in two separate studies for some miRNAs.With regard to findings in blood plasma in adults with TLE (Table 1), miR-199a-3p was upregulated in TLE pre-seizurevs.
control (Raoof et al., 2018; Brennan et al., 2020), TLE post-seizurevs
.control (Brennan et al., 2020), and TLE pre-seizurevs
.TLE post-seizure (Raoof et al., 2018).Furthermore, in blood plasma in adults with TLE, miR-142-5p was upregulated in TLE post-seizurevs
.TLE pre-seizure (Brennan et al., 2020)while in blood serum in adults with TLE, miR-142-5p was upregulated in TLEvs.
control and in TLE drug resistantvs
.TLE drug responsive (De Benedittis et al., 2021).In blood plasma and temporal lobe tissue in adults with TLE, miR-129-2-3p was upregulated in TLEvs
.control (Sun et al., 2016).With regard to findings in adults with mTLE (Table 2), miR-153 was downregulated in blood plasma and temporal cortex tissue of mTLEvs.
control (Li et al., 2016; Gong et al., 2018).Furthermore, in adults with mTLE, miR-145-3p was upregulated in blood serum of mTLE-HS post-seizure at 30 minutesvs
.mTLE-HS pre-seizure(Surges et al., 2016) and downregulated in hippocampal tissue of mTLEvs
.control (Antônio et al.2019).Interestingly, upregulation of miR-129-2-3p,miR-142-3p, and miR-142-5p occurred in hippocampal tissue of mTLEvs.
control (Bencurova et al., 2017; Baloun et al., 2020).Dysregulation of certain miRNAs occurred in TLE compared to SE or other neurological diseases as a control.For instance, miR-19b-3p, miR-451a, and miR-21-5p were downregulated in CSF of TLEvs
.SE (Raoof et al., 2017).Upregulation of miR-886-3p and downregulation of miR-19b-3p were found in CSF of TLE vs.other neurological diseases (Raoof et al., 2017).
Table 1 |Alterations of miRNA expression in CSF, blood plasma, blood serum, and brain tissues/cells in adults and children with TLE
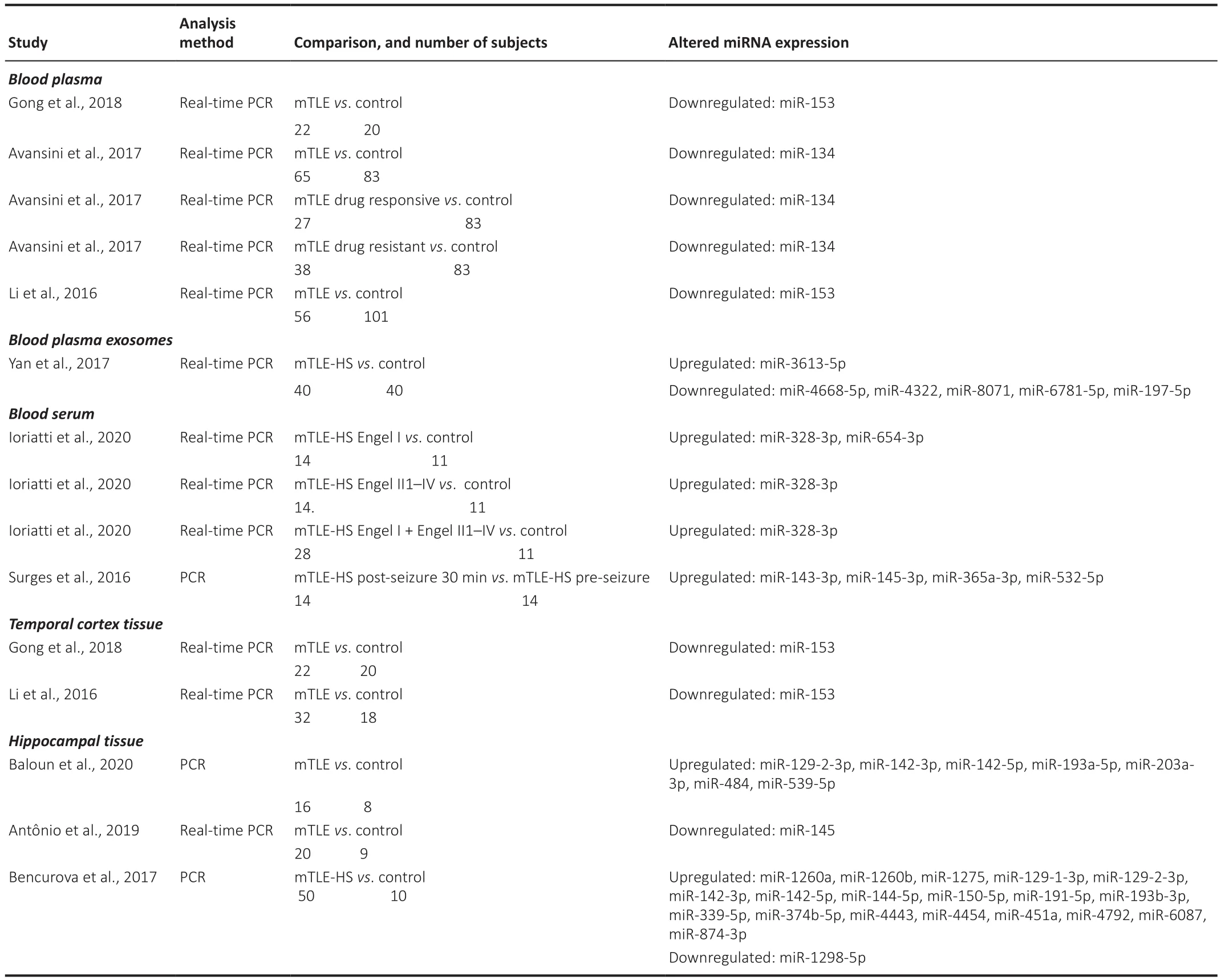
Table 2 |Alterations of miRNA expression in blood plasma, blood plasma exosomes, blood serum, and brain tissues in adults with mTLE
It was noteworthy that several of the studies reviewed had included a substantial number of patients with hippocampal sclerosis in the TLE cohort.For instance, there were 9/30 (30%) in the study by Xiao et al.(2018), and patients with hippocampal sclerosis were included in the studies of Raoof et al.(2018) and Zucchini et al.(2014).Wu et al.(2021) reported that 11/65 (17%)TLE children had developed mesial sclerosis.Similarly, Li et al.(2020) found that 11/63 (17%) TLE children had developed mesial sclerosis.In addition,given that mTLE is the most common form of TLE, it is likely there might be some overlap in the miRNA findings in the studies for adult TLE patients andthose for adult mTLE patients, with certain individual miRNAs having altered expression both in studies with adult TLE patients and those with adult mTLE patients.MiR-654-3p was upregulated in the blood plasma of TLE pre-seizurevs
.control (Raoof et al., 2018) and upregulated in blood serum of mTLE-HS Engel Ivs
.control (Ioriatti et al., 2020).MiR-142-5p was upregulated in the blood plasma of TLE post-seizurevs.
TLE pre-seizure (Brennan et al., 2020)and upregulated in blood serum of TLEvs
.control and TLE drug resistantvs.
TLE drug responsive (De Benedittis et al, 2021), as well as being upregulated in hippocampal tissue of mTLEvs.
control (Bencurova et al., 2017; Baloun et al., 2020).Similarly, miR-339-5p was upregulated in blood plasma in TLE pre-seizurevs
.control (Raooof et al., 2018) and upregulated in hippocampaltissue of mTLE-HSvs
.control (Bencurova et al., 2017).There was a possible inconsistency in the reported findings for blood plasma miR-134 which was upregulated in TLE severevs
.control and no difference for TLE moderate or TLE mildvs.
control (Wang et al., 2017) but was downregulated in mTLEvs.
control (Avansini et al., 2017).This would need to be further investigated.With regard to possible non-invasive biomarkers of TLE/mTLE in adults,potential candidates are miR-129-2-3p, miR-142-5p, miR-145-3p, miR-153,miR-199a-3p miR-339-5p, and miR-654-3p.Changes were also found for some of these miRNAs in the temporal lobe and hippocampal tissue, increasing the importance that they might have in epilepsy.Upregulation of miR-129-2-3p occurred in temporal lobe tissue in adults with TLE vs.control and in hippocampal tissue of adults with mTLEvs
.control.Upregulation of miR-142-5p and miR-339-5p and downregulation of miR-145-3p were found in hippocampal tissue of adults with mTLEvs.
control.Moreover, downregulation of miR-153 occurred in temporal cortex tissue of mTLEvs.
control.Many of the TLE patients in the articles reviewed had been receiving mono or poly drug therapy.In one study, patients did not receive AEDs for 2 weeks prior to miRNA profiling (Wang et al., 2017).In the patients with newonset severe epilepsy, the blood plasma expression level of miR-134 was significantly higher than in healthy controls, and administration of valproate acid was found to decrease the plasma level of miR-134 in these patients so that it was no longer significantly greater than controls (Wang et al., 2017).
The limited overlap in findings of the reviewed studies could be due to several factors.The mean age of the adult TLE patients varied from 28.5 to 55.8 years and for adult mTLE patients from 24.9 to 43.1 years.The mean duration of epilepsy for adult TLE varied from new onset to 36.8 years and for adult mTLE patients from 11.5 to 27.4 years.Also as mentioned above, the use AEDs by adult TLE and mTLE patients varied.Where reported, the proportions of drug resistant and drug responsive TLE patients varied between different studies,e.g.in the study by Avansini et al.(2917), there were 27 drug sensitive and 38 drug resistant mTLE patients, while that of De Benedittis et al.(2021)comprised 17 drug sensitive and 10 drug resistant TLE patients.The levels of miRNA expression need to be measured separately in drug resistant and drug responsive patients as was done by Avansini et al.(2017) and De Benedittis et al.(2021).These are all important factors and together with other variables such as comorbid disease and the nature of the precipitating injury could influence the levels of miRNA expression (Tables 3 and 4).Furthermore, in some studies, there was a significant difference in mean age between mTLE patients and control subjects (e.g.Bencurova et al., 2017).
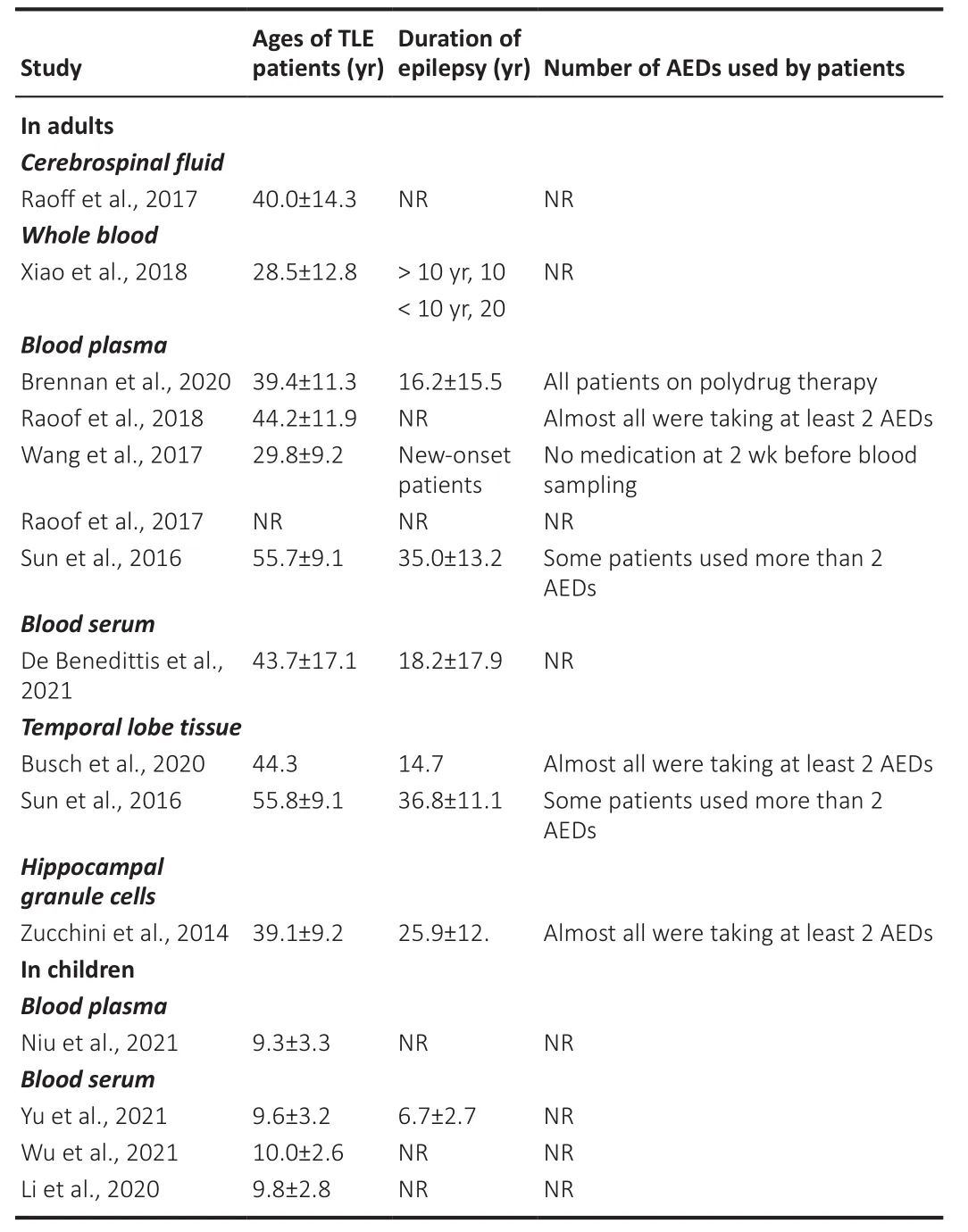
Table 3 |Ages, duration of epilepsy, and use of AEDs in TLE patients in studies of miRNAs in cerebrospinal fluid, whole blood, blood plasma, blood serum, and brain tissue samples
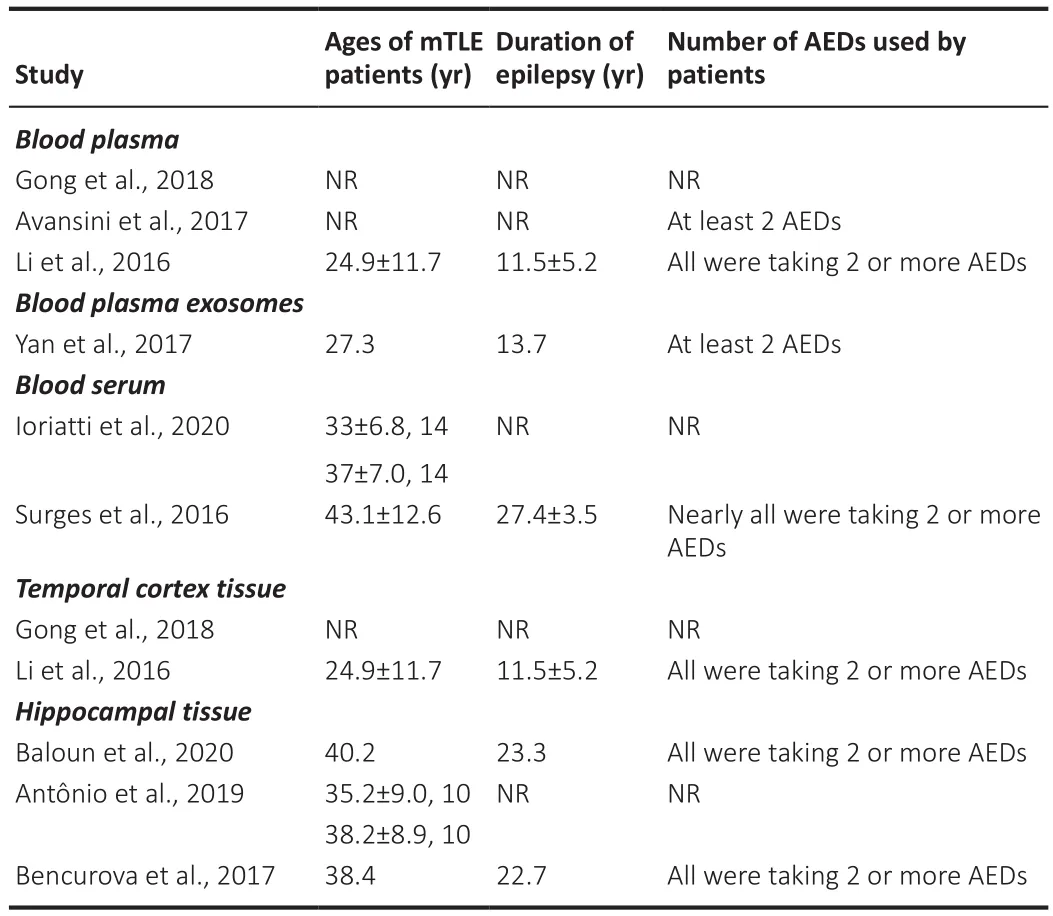
Table 4 |Ages, duration of epilepsy, and use of AEDs in adults with mTLE in studies of miRNAs in blood plasma, blood plasma exosomes, blood serum, and brain tissue samples
In a recent study involving a median 5-year follow-up period (range 2-13 years), it was reported that 26/112 (23.2%) TLE patients achieved seizure freedom after treatment with AEDs alone, while 86/122 (76.8%) were diagnosed as drug resistant (Yang et al., 2020).Possible treatment of TLE patients, especially those that are drug resistant, could be by the use of miRNA agomirs (mimics) or antagomirs (inhibitors).Hippocampal cells treated in magnesium-free medium were used to examine the effects of overexpression of specific miRNAs on cell viability and the influence on cell apoptosis induced by TLE (Li et al., 2020).In addition, the miRNA profile in rats with adult-onset TLE closely resembled the profile in mTLE-HS patients (Baloun et al., 2020).The rat model of TLE involved inducing SE in male Wistar albino rats aged P12 (postnatal day 12), P25, or P60 by intraperitoneal injection with 127 mg/kg LiCl 24 hours prior to pilocarpine/saline injection (Kubová et al., 2004).Video-EEG monitoring 3 months after SE demonstrated that 25%of rats in the P12 group and 50% in the P25 group developed spontaneous seizures (Kubová et al., 2004).The animals developing SE could subsequently be treated with specific miRNA agomirs and antagomirs and the effects on epileptic behavior examined.SE has also been induced in male C57BL6/J mice(4-6 weeks of age) by intraperitoneal injection of 30 mg/kg kanic acid (Zhu et al., 2019).SE was defined as continuous tonic-clonic seizures following several discontinuous convulsive seizures.The seizure intensity was assessed on the Racine scale: Stage 1, mouth and facial movements; Stage 2, head nodding;Stage 3, forelimb clonus; Stage 4, seizures characterized by rearing; and Stage 5, seizures characterized by rearing and falling (Racine, 1972).The onset of SE was defined when stage 5 seizures continued for at least 5 minutes (Chen and Wasterlain, 2006; Trinka and Kälviäinen, 2017; Jain et al., 2019).Intracerebral delivery of miR-23a agomir or antagomir was performed in kanic acid-induced TLE mice and effects on spatial learning and memory were assessed (Zhu et al., 2019).Interestingly, a non-human primate model of TLE was describedin which SE was induced in adult marmosets by intraperitoneal injection of pilocarpine (250 mg/kg).To manage the severity of SE, animals received an intraperitoneal injection of diazepam 5 minutes after the onset of SE (1.25 mg/kg or 2.5 mg/kg) (Perez-Mendes et al., 2011).Intrahippocampal injections of kainic acid to cynomolgus monkeys developed a model of TLE (Chen et al., 2019) and to macaques, which developed a model of mTLE (Chen et al.,2013).An infant model of TLE was developed by eliciting SE in immature pigtailed macaques (5.5- to 7.0-month-old) by unilateral microinfusion of bicuculline methiodide into the entorhinal cortex (Wenzel et al., 2005).A search of the NIH Clinical Trials registry (https://www.clinicaltrials.gov/)showed that no clinical trials are presently recruiting or in progress to test miRNA agomirs or antagomirs in patients with temporal lobe epilepsy.
In conclusion, a large number of miRNAs were found to be dysregulated in CSF, blood plasma, blood serum, and brain tissue samples collected from patients diagnosed with TLE and mTLE, and with some overlap in findings for TLE and mTLE patients.However, the main difficulty with interpreting the study findings is determining to what extent the taking of AEDs by the TLE and mTLE patients had influenced the levels of miRNA expression in the body fluids and brain tissues examined.Further studies using the rat and mouse models of TLE could provide useful information, especially when performed with immature (childhood) and adult animals.Most of the articles reviewed were performed with samples collected from adult patients, and with relatively few from children.This needs to be addressed in future studies.
Author contributions:
Both authors contributed equally to this manuscript and approved the final manuscript.
Conflicts of interest:
The authors declare no conflicts of interest.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Melatonin, tunneling nanotubes, mesenchymal cells,and tissue regeneration
- Multi-targeted anti-inflammatory drugs for the treatment of neurological disorders
- Emerging roles of GPR109A in regulation of neuroinflammation in neurological diseases and pain
- Potential physiological and pathological roles for axonal ryanodine receptors
- Notice of Retraction
- Roles of constitutively secreted extracellular chaperones in neuronal cell repair and regeneration

