Roles of constitutively secreted extracellular chaperones in neuronal cell repair and regeneration
Sandeep Satapathy , Mark R.Wilson
Abstract Protein quality control involves many processes that jointly act to regulate the expression, localization,turnover, and degradation of proteins, and has been highlighted in recent studies as critical to the differentiation of stem cells during regeneration.The roles of constitutively secreted extracellular chaperones in neuronal injury and disease are poorly understood.Extracellular chaperones are multifunctional proteins expressed by many cell types, including those of the nervous system, known to facilitate protein quality control processes.These molecules exert pleiotropic effects and have been implicated as playing important protective roles in a variety of stress conditions, including tissue damage, infections, and local tissue inflammation.This article aims to provide a critical review of what is currently known about the functions of extracellular chaperones in neuronal repair and regeneration and highlight future directions for this important research area.We review what is known of four constitutively secreted extracellular chaperones directly implicated in processes of neuronal damage and repair, including transthyretin, clusterin, α2-macroglobulin, and neuroserpin,and propose that investigation into the effects of these and other extracellular chaperones on neuronal repair and regeneration has the potential to yield valuable new therapies.
Key Words: cell viability; clusterin; extracellular chaperones; inflammation; neuroserpin; protein misfolding; transthyretin; α2-macroglobulin
Introduction
Local tissue inflammation and mechanical injury to neurons have been associated with the aggregation of misfolded proteins and subsequent cell death (Gidalevitz et al., 2011).Relative to mature differentiated cells,regenerating cells have higher rates of protein synthesis (Noormohammadi et al., 2018), and recent studies have highlighted the critical need for effective protein quality control in stem cells during regeneration (Yan et al., 2020).Proteomic studies of human mesenchymal stromal stem cells have also shown that during regeneration, the secretion of a number of constitutively secreted chaperones (e.g.α2-macroglobulin, A2M) is selectively enhanced (Kehl et al.,2019).Constitutively secreted extracellular chaperones (ECs) are an integral part of the systems that act to maintain protein homeostasis (proteostasis)(Yerbury et al., 2007; Wyatt et al., 2013) and are almost certain to influence the ability of an organism to repair and regenerate cells and tissues.An effective protein quality control system ensures the timely recognition,refolding, or clearance of misfolded proteins to enhance the survival and proper functioning of living organisms (den Brave et al., 2021).
Regenerating neurons need to communicate with other neurons and surrounding cells to generate an effective response to a physiological or pathological stimulus, in which both intracellular and extracellular signaling mechanisms play an important role (Liu et al., 2021).Our understanding of the roles of ECs in neuronal regeneration and repair is currently limited.ECs are multifunctional proteins expressed by many cell types in the body(including neurons and astrocytes), known to facilitate extracellular protein quality control processes.We propose that future studies of ECs in the context of neuronal damage and disease have significant potential to lead to the development of valuable new therapies.In this article, we have focussed on four ECs (transthyretin, clusterin, α2-macroglobulin, and neuroserpin)because these proteins are constitutively present in cerebrospinal fluid (CSF)and have been directly implicated in neurodegenerative disease and diseases associated with neuronal damage and repair (Satapathy and Wilson, 2022).We aim to provide a critical review of what is currently known about the functions of ECs in neuronal repair and regeneration and highlight outstanding questions and future directions for this important research area.
Database Search Strategy
The manuscript used peer-reviewed articles chosen from PubMed, PubMed Central, Google Scholar and Web of Science (Clarivate) identified using individual or combinations of the following keywords: Protein misfolding,extracellular chaperones, transthyretin, clusterin, α2-macroglobulin,neuroserpin, inflammation, cell viability.The date of the last database search is between February 20 and March 30, 2022.
Current Knowledge of the Role of Extracellular Chaperones in Neuronal Repair and Regeneration
A major role of chaperones is to protect organisms from the consequences of inappropriate protein aggregation and toxicity.As a result of age or ongoing chemical or physical stresses, proteins can misfold to form aggregates that are either amorphous or amyloid (fibrillar) in structure, some of which are cytotoxic (Gidalevitz et al., 2011; Hidalgo San Jose et al., 2020).ECs have an ATP-independent action and are best-known for their abilities to (a) inhibit the aggregation of misfolded or damaged proteins, (b) maintain aggregating proteins in a soluble state, and (c) form stable complexes with aggregating extracellular proteins to facilitate their clearance from the extracellular space and subsequent safe disposal by intracellular degradation (Wyatt et al., 2011).ECs are abundant in human body fluids such as plasma and CSF (Prikrylova Vranova et al., 2016), saliva (Pallardo-Fernández et al., 2020), urine (Musiał et al., 2020), and semen (Saleh et al., 2020).In addition, these multifunctional proteins have roles that include suppressing inflammation, inhibiting apoptosis, promoting cell proliferation and survival, modulating ECM composition and organization, and acting as immune modulators (Satapathy and Wilson, 2022).Many of the biological functions of ECs outlined above have been proposed to play critical roles in the regeneration of mature cells,including neuronal cells (Guerin et al., 2021).Further investigation into the effects of ECs present in CSF on neuronal repair and regeneration has the potential to lead to the development of new therapies.
Transthyretin
Transthyretin (TTR) is an amyloid-specific EC (West et al., 2021) present at~15.5 μg/mL in the CSF of healthy human adults (Maetzler et al., 2012).TTR in complex with retinol-binding protein (RBP) transports retinoic acid (RA,a growth factor) to sites of neuronal growth, thereby promoting neuronal regeneration (Vancamp et al., 2019; Eira et al., 2021), differentiation,and patterning under physiological (Wilson et al., 2004) and pathological conditions (Ikeda et al., 2005).RA carried by the TTR:RBP complex also induces the differentiation of neural stem cells into neurons and glial cells(Nonaka et al., 2004).
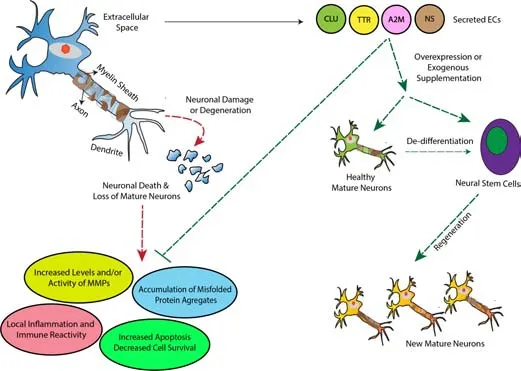
Figure 1|Proposed model for the roles of ECs in the repair and regeneration of neurons.
TTR has also been suggested to play a cytoprotective role in neurodegeneration and acute neuronal injury cases.A decrease in the level of TTR in the CSF has been associated with Alzheimer’s disease (AD; Gião et al., 2021).TTR has been shown to (a) inhibit amyloid (but not amorphous)protein aggregation bothin vitro
(West et al., 2021) andin vivo
(Schwarzman et al., 1994), (b) proteolytically cleave the amyloid beta peptidein vitro
(Costa et al., 2008), and (c) rescue a mouse model of AD from cognitive and motor impairment (Buxbaum et al., 2008).TTR has been proposed to facilitate the safe disposal of aggregating proteins from the neuronal extracellular space(Buxbaum et al., 2008; Santos et al., 2010).Similarly, increased CSF TTR levels have been shown to confer cytoprotection against cerebral ischemia (Santos et al., 2010), promote axonal and neurite growth, and facilitate cytoskeletal reorganization following sciatic nerve injury (Eira et al., 2021).Collectively,this preliminary evidence suggests that TTR has the potential to act as a therapeutic agent to reduce inflammation, and promote the survival, repair,and regeneration of neurons.Clusterin
Clusterin (CLU) is the best-studied EC found at ~2 μg/mL in the CSF of healthy human adults (Polihronis et al., 1993).It has been shown to promote thein vitro
andin vivo
proliferation and regeneration of damaged (a) epithelial cells of the cornea (Okada et al., 2011) and intestine (Liu and Chen, 2020),(b) renal cells (Gobe et al., 1995), and (c) hair and cochlear cells (Zhao et al.,2021).CLU inhibits the aggregation of proteins that form either amyloid or amorphous aggregatesin vitro
(West et al., 2021), and has been implicated as performing a cytoprotective role in neurodegenerative diseases and neuronal injuries.For example, increased levels of CLU are detected in the CSF of AD (Nilselid et al., 2006) and Parkinson’s disease (PD) patients, and in the conditioned medium of human neuronal (SHSY5Y) (Gregory et al., 2017) and rat primary hippocampal and glial cells (Cascella et al., 2013) that have been exposed to chemical or proteotoxic stress.Rare non-synonymous mutations in the CLU gene are associated with the progression and severity of AD (Bettens et al., 2015) and PD (Ma et al., 2011), and CLU is often found co-localized with amyloid fibrils or plaques surrounding both neurons and glial cells in these diseases (Thambisetty et al., 2010).Furthermore, in mice with transected hypoglossal nerves (a model system for paralysis), reduced levels of CLU in the plasma inhibited the regeneration of sensory neurons (Wicher et al., 2008;Wright et al., 2014).The cytoprotective role of CLU is further supported by its ability to inhibit(a) local tissue inflammation (Pucci et al., 2019; De Miguel et al., 2021), (b)oxidative stress (Tarquini et al., 2020), and (c) cell death via apoptosis (Cunin et al., 2016).CLU can also influence the composition of the extracellular matrix by inhibiting matrix metalloproteinases MMP9 (Jeong et al., 2012) and MMP25 (Matsuda et al., 2003), and preserving neuronal function at synapses(Chen et al., 2021).All of these findings point toward the potential use of CLU as a therapeutic agent to promote the repair and regeneration of injured neurons.
α2-Macroglobulin
α2-Macroglobulin (A2M) is a broad-range protease-inhibitor and well-studied EC normally present at ~1.5 μg/mL in CSF (Suzuki et al., 2019).A2M inhibits proteases associated with local tissue inflammation and is known to promote the regeneration of (a) stem cells including hematopoietic and lymphopoietic cells in an irradiated mouse model, and (b) mature cells such as retinal epithelial cells (Jaldín-Fincati et al., 2019) and skin epithelial cells, to promote wound healing (Bakhtyar et al., 2018).Similar to CLU, increased levels of A2M have been reported in the CSF of AD (Varma et al., 2017) and PD (Gupta et al.,2019) patients, and correlate with the increased detection of neuronal injury markers such as tau and phosphorylated tau proteins (Varma et al., 2017).
Furthermore, A2M transports inflammatory cytokines (e.g.tumor necrosis factor α and interleukins-6 and -1β; Marino-Puertas et al., 2019), growth factors (e.g.transforming growth factor β; LaMarre et al., 1991) and neurotrophin (a secreted protein that acts as a growth factor and promotes neuronal cell survival and function; Wolf and Gonias, 1994) to sites of tissue inflammation.Therefore, A2M has been suggested to play a key role in suppressing inflammation and promoting neuronal repair and regeneration in neurons subject to mechanical injury (Garcia-Fernandez et al., 2021) or exposure to misfolded protein aggregates (Guan et al., 2021).This trafficking of regulatory molecules may involve the interaction of A2M with the cell surface receptor LRP1 (low-density lipoprotein receptor-related protein-1)(Galliano et al., 2008).A2M also influences extracellular matrix remodeling by inhibiting the protease activity of MMP-2 (Kim et al., 2017) and MMP-9 (Serifova et al., 2020).The A2M-MMP interaction has been suggested to inhibit inflammation and promote the survival of neurons in humans treated with methotrexate (a model for blood-brain barrier damage) (Cucullo et al., 2003).Taken together, the existing evidence supports a role for A2M in enhancing neuronal survival, repair, and regeneration following mechanical injury or neurodegeneration.
Neuroserpin
Neuroserpin (NS) is a constitutively expressed neuronal protein found at relatively low abundance in normal CSF (~7 ng/mL) (Nielsen et al., 2007).NS is a serine protease inhibitor that inhibits the activity of tissue-type plasminogen activator (tPA) (Hastings et al., 1997).Like TTR, NS acts as an amyloid-specific EC (West et al., 2021).Several studies have suggested that NS protects regenerating neurons from injury and neurodegeneration.For example, in a mouse model of cerebral hypoxia, a decrease in the CSF NS level has been suggested to result in an increased expression of tPA in brain neurons, ultimately resulting in neuronal cell death (Tsirka et al., 1995;D’Acunto et al., 2021).Furthermore, in a rat model of stroke, the level of NS protein in brain neurons was significantly increased as early as 6 hours poststroke and remained high for 1 week after the stroke (Yepes et al., 2000).In the same study, direct injection of NS protein into the brain resulted in a ~64% reduction in the stroke volume when compared with rats injected with a placebo (Yepes et al., 2000).These observations suggest that the cytoprotective potential of NS makes it an attractive candidate for exploration as a therapeutic agent to promote neuronal repair and regeneration.
Concluding Hypothesis and Future Directions
ECs are (i) abundantly found in CSF, and their levels increase following neuronal injury or neurodegenerative stress, and (ii) known to regulate multiple biological processes including those that are important for the repair and regeneration of cells, such as cell proliferation, apoptosis, inflammation,and interactions with the ECM.Based on these observations we propose that ECs are key players in neuronal repair and regeneration and that future studies to better characterize their effects in this specific context has the potential to lead to the development of valuable new therapies for neuronal damage and diseases.We suggest that a focus in future studies on the following outstanding questions in the field would bring us closer to being able to harness the therapeutic potential of ECs to treat neuronal damage and disease:
1.In neuronal culture systems, what are the effects on receptor expression and neuronal cell viability of (i) exogenous supplementation of ECs, and (ii)silencing of the expression of one or multiple ECs using CRISPR-mediated gene editing (Bock et al., 2022)?
2.Is the level of expression of cell surface receptors known to be important in neuronal growth and regeneration (e.g.LRP1, integrins, and neurotrophic receptors) affected by the expression of ECs in injured and healthy neurons?
3.Does the level of expression of ECs affect (a) the expression of other intracellular or secreted proteins known to be important for cell repair and regeneration and/or (b) the differentiation of neural stem cells?
Author contributions:
SS and MRW contributed equally to the design and the writing of the manuscript and data search and analysis, and approved the final version of the manuscript.
Conflicts of interest:
The authors declare that there are no competing interests associated with the manuscript.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Potential physiological and pathological roles for axonal ryanodine receptors
- Multi-targeted anti-inflammatory drugs for the treatment of neurological disorders
- Melatonin, tunneling nanotubes, mesenchymal cells,and tissue regeneration
- MicroRNAs as potential biomarkers in temporal lobe epilepsy and mesial temporal lobe epilepsy
- Notice of Retraction
- Emerging roles of GPR109A in regulation of neuroinflammation in neurological diseases and pain

