P2X7 receptor as the regulator of T-cell function in intestinal barrier disruption
Zhi-Feng Jiang, Wei Wu, Han-Bing Hu, Zheng-Yang Li, Ming Zhong, Lin Zhang
Abstract The intestinal mucosa is a highly compartmentalized structure that forms a direct barrier between the host intestine and the environment, and its dysfunction could result in a serious disease. As T cells, which are important components of the mucosal immune system, interact with gut microbiota and maintain intestinal homeostasis, they may be involved in the process of intestinal barrier dysfunction.P2X7 receptor (P2X7R), a member of the P2X receptors family, mediates the effects of extracellular adenosine triphosphate and is expressed by most innate or adaptive immune cells, including T cells. Current evidence has demonstrated that P2X7R is involved in inflammation and mediates the survival and differentiation of T lymphocytes, indicating its potential role in the regulation of T cell function.In this review, we summarize the available research about the regulatory role and mechanism of P2X7R on the intestinal mucosa-derived T cells in the setting of intestinal barrier dysfunction.
Key Words: Intestinal barrier dysfunction; P2X7 receptor; T lymphocyte
INTRODUCTION
The intestinal tract is one of the largest interfaces in the human body that directly contacts the external environment[1]. The intestine, a highly specialized and complex organ, plays an important role in absorbing useful substances and presenting potentially harmful substances[2]. The intestinal barrier also maintains the homeostasis of the inner environment and develops the intestinal immune system[3]. The intestinal barrier is composed of several parts, including the microbiological barrier, the chemical barrier, the physical barrier and the immune barrier[4]. Dysfunction of the intestinal barrier increases intestinal permeability and is related to the pathophysiology of several serious diseases[5]. The intestinal tract is exposed to various commensal bacteria, dietary antigens and pathogens that are related to immune tolerance and defense, showing the importance of the immune system in the intestine[6]. The immune barrier mainly includes the lamina propria lymphocytes, dendritic cells (DCs), mast cells, macrophages and lymphocytes — mainly CD8+T cells — located among epithelial cells[7].Considering the involvement of T cells in the oral tolerance and immune defense against pathogens in the intestine, it is not surprising that they have an essential role in the pathology of intestinal barrier dysfunction[8,9].
The purinergic signaling pathway is highly conserved and plays a critical role in immune regulatory response[10]. This signaling pathway is mainly mediated by adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide (NAD+). Purinergic receptors are a group of transmembrane proteins widely expressed in immune cells[11]. According to their different structural properties, these receptors can be divided into the following three families: P2X receptor, P2Y receptor and P1 receptor[12]. Among them, P2X receptors, a class of ligand-gated, cationic-selective channels, are mainly activated by extracellular ATP (eATP)[13]. P2X7 receptors (P2X7R) have a low affinity for ATP and need to be triggered by high concentrations of ATP[14]. When the concentration of ATP is low, P2X7R can act as ion channels for Na+, K+or Ca2+. However, P2X7R can form nonselective and large-conductance pores in settings with high concentrations of ATP, thereby inducing cell apoptosis[14]. Under normal conditions,the cell membrane is impermeable to ATP and other related substances, and the maintenance of low eATP concentration is achieved by the strong degrading activity of ATPases[15,16]. The leakage of intracellular ATP due to the destruction of cell membrane could result in significant elevation of eATP concentration, thereby inducing the activation of the immune system[17-19]. Indeed, an ultrahigh concentration of eATP can be observed at the inflammatory sites[20]. In the process of acute inflammation, ATP activates P2X7R on the Treg cells, inhibiting their activity and viability[21]. P2X receptor channels on effector T cells are stimulated by eATP, facilitating the activation of nuclear factor of activated T cells and the production of IL-2, which could increase the activation of effector T cells[22,23]. The receptor can also be activated by NAD+released from damaged cells or activated T cells[24,25].This NAD+-dependent process is associated with ecto-ADP-ribosyltransferase ARTC2.2, which is activated by NAD+and induces the ADP-ribosylation of P2X7. In the presence of low micromolar concentrations of extracellular NAD+, this process finally leads to cell death because of the activated P2X7R, a phenomenon known as NAD+-induced cell death (NICD)[26,27]. In contrast, NAD+may be degraded into hydrolysate in the case of high levels of ATP, which would block the NAD+-dependent process[28,29] (Figure 1). Intestinal barrier dysfunction is usually accompanied by inflammation and the death of epithelial cells, which may lead to an elevated concentration of eATP and the intestinal immune response[30]. Meanwhile, available studies have demonstrated that P2X7R can be an important regulatory factor in the activation and differentiation of T cells[31], suggesting that P2X7R may play a key role in intestinal barrier disruption by regulating T cells.
Therefore, in this review, we summarized the recent advances regarding the intestinal barrier, the role of P2X7R and T-cells in the pathophysiology of intestinal barrier disruption, and the role of T cellderived P2X7R in the pathophysiology of intestinal barrier dysfunction.
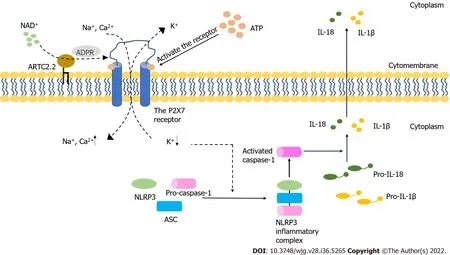
Figure 1 The activation of the P2X7 receptor and NLRP3 inflammasome. The P2X7 receptor is activated by extracellular ATP and NAD+ and serves as an ion channel. It can also form non-selective macropores. The activated P2X7 receptor induces the decreasing of intracellular K+, which initiates NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome activation. The activated process of pro-interleukin (IL)-1β and pro-IL-18 are triggered by the active caspase-1 that results from the formation of NLRP3 inflammasome. Mature inflammatory cytokines are released into extracellular space from cells, which finally results in the cell death. ADPR: ADP-ribose; ARTC2.2: ADP-ribosyltransferase 2.2; ASC: Apoptosis-associated speck-like protein containing a CARD; NLRP3: NODlike receptor family pyrin domain containing 3; IL-18: Interleukin-18; IL-1β: Interleukin-1β.
THE OVERVIEW OF THE INTESTINAL BARRIER
The intestinal barrier, one of the most important biological barriers in the body, is composed of various extracellular and cellular components. It works as a semipermeable membrane that allows nutrients to pass through while limiting the transport of pathogens and noxious substances. This dual function is regulated by the interaction among the four components of the intestinal barrier (the microbiological barrier, the chemical barrier, the physical barrier and the immunological barrier)[2].
There are over 1014microorganisms and about 10000 bacterial species in the human intestine[32]. The microbiological barrier is formed by symbiotic microorganisms in the outermost position of the mucus layer, which effectively prevents harmful substances from entering intestinal epithelial cells[31,33,34].The chemical barrier, also known as the inner mucus layer, is composed of macromolecules, including proteins, enzymes, peptides and immunoglobulins[35,36]. Mucin2 secreted by goblet cells is the main mucus protein and it serves as a protective barrier[37]. In intestinal crypts, pluripotent stem cells can differentiate into five different cell types, including enterocytes, goblet cells, Paneth cells, enteroendocrine cells and microfold cells[38]. The physical barrier beneath the mucus layer is composed of intestinal epithelial cells which are critical to the physical features of the intestinal barrier[30].
Beneath the intestinal epithelium, the immunological barrier consists of various immune cells,including T lymphocytes, B lymphocytes, dendritic cells, macrophages and plasma cells. This barrier is involved in innate and adaptive immune responsesviaantigen presentation and the secretion of inflammatory mediators and antibodies[39,40]. In addition to immune cells, substances secreted from these cells are also important in the construction of the intestinal immunological barrier. Secretory IgA,another constituent of the immunological barrier, is mainly found at the intestinal mucosal surface, and it provides antipathogen protection by interacting with bacteria[41].
There are several interactions among different components of the intestinal barrier. The physical barrier and the inner mucus layer separate the microbiological barrier and the intestinal immunological barrier, preventing unnecessary conflict and maintaining intestinal homeostasis[42]. The intestinal microbiota induces the functional maturation of innate and adaptive immunity, and instructs immune response through microbiota-derived metabolites and components (such as lipopolysaccharides and peptidoglycans)[43,44]. The metabolites maintain intestinal homeostasis and regulate inflammation through immune responses, while the components of the microbiota direct immune responses by activating the intestinal TLR pathway[45-47]. For example, the expression of IL17, an inflammatory cytokine produced by γδ T cells, can be inhibited by propionate, a metabolite of intestinal bacteria[48].Conversely, intestinal immune cells precisely regulate the microbial community both directly and indirectly, thus establishing a sustainable balance between the immune cells and intestinal microbiota[49-51].
The intestinal barrier should be considered a highly dynamic and complex structure that responds to internal and external stimuli[52,53]. Dysfunction of the intestinal barrier often occurs when the damage of the intestinal mucosa is severe and the components of the intestinal barrier change[54]. Under pathological conditions such as stress[55] and ischemia or hypoxia[56], the intestinal barrier is destroyed and the permeability of the intestine increases, thereby inducing bacterial translocation, electrolyte disorders and inflammatory response[57] (Figure 2). With the increasing permeability of the intestine,locally produced ATP is released into the intestinal microenvironment, followed by the activation of immune cellsviaATP receptors, including P2X7 purinoceptor. When the intestinal immune system is activated, the inflammatory effects may not be regulated, which may lead to the irreversible destruction of the intestinal barrier[58]. More recently, it has been reported that increased ATP concentrations promote T-cell responses by enhancing the expression of the CD86 costimulatory molecule on antigenpresenting cells, an effect mediated through P2X7 purinergic receptor. Thus, the immune system may be the key player in barrier dysfunction and T cells may be involved in adaptive immune responses.
THE ROLE OF T CELLS IN THE INTEGRITY OF THE INTESTINAL BARRIER
T lymphocytes, which are adaptive immune cells, respond to specific antigens and remain the bacterial diversity by complex mechanisms in the homeostatic condition[2]. In the intestine, invariant NKT(iNKT) cells could either enhance or inhibit the immune response, and they might directly or indirectly regulate the microbiota in the intestine[59-62]. CD8+T cells are the main intraepithelial lymphocytes that monitor and respond to pathogens[63]. CD4+T cells and T-helper (Th) cells are mainly located in the intestinal lamina propria, and Th1 and Th17 cells can be found in the intestine[64]. Activated intestinal effector T cells could mount immune responses and influence the gut microbiota, and the excess of these cells might induce advanced inflammatory responses and acute or chronic inflammatory diseases[65-67]. In the intestinal adaptive immune response, dendritic cells ingest antigens and activate T cells, and Th cells are induced to differentiate into three different types of Th cells[68]. When lymphocytes respond to different stimuli, they can be divided into different groups based on their cytokine profile,such as Th1, Th2, or Treg cells, which are regulated by P2X7 purinergic receptor, mechanistically[69].Activated T cells can modulate immune responses by secreting inflammatory cytokines or by interacting with other cells. The function of Th1 cells is to activate and proliferate cytotoxic T cells, thereby inducing the damage of infected intestinal epithelial cells[70]. Th2 cells can release inflammatory cytokines (IL-4,IL-5, and IL-13) and activate B cells to attack the infected cells[71-73]. Transforming growth factor β is capable of suppressing immunoglobulins M and G and promoting their switch to immunoglobulin A.This cytokine is secreted by T cells in Peyer's patches, suggesting the role of T cells in oral tolerance[74].When intestinal permeability is damaged, antigens can pass through the intestinal epithelial cells and be taken up by macrophages or dendritic cells. Then, the antigens are presented to T cells in the lamina propria by these antigen-presenting cells, which stimulates T cells and induces their proliferation[75,76]. Some antigens may be taken up by intestinal epithelial cellsviaendocytosis and then be presented to T cells after intracellular processing. This process is based on the classical and nonclassical histocompatibility molecules[77,78]. T cells use both their receptor and a costimulatory signal to recognize antigens[79].
Intestinal barrier disruption is usually accompanied by intestinal inflammation and pathogen invasion. T cells, the key components of adaptive immunity, can effectively limit the invading bacteria and regulate the inflammatory response together with the innate immune system and cytokines[80]. For example, the T helper cell type (Th)1 immune response is necessary in antipathogen protection and is involved in intestinal inflammation[81,82]. In humans, Th17 cells mainly reside in the intestine, where their polarization occurs. Because of the plasticity of Th17 cells, polarized cells have antipathogenic functions and maintain the intestinal epithelial integrity under normal physiological conditions, but they may turn into proinflammatory cells when exposed to IL-23[83]. Th17 cells can mediate inflammation by secreting a proinflammatory cytokine, IL-17A[70]. Peripheral Th17 cells are produced and migrate to the intestine in the case of oral inflammation, which may cause intestinal inflammation[84].In addition to suppressing the proliferation of Th cells, Treg cells can protect against bacteria and dietary antigens and can produce anti-inflammatory cytokines to exert their anti-inflammatory function,thereby maintaining the homeostasis of the intestinal epithelium[85-87]. With the development of intestinal inflammation, the balance between Th17 cells and Treg cells may be broken up, biasing the function of Th17 cells[88]. In a recent study, it has been found that tissue-resident memory T cells are important in the development of intestinal inflammation, but the role of these cells in this process is not clear[89]. In summary, T cells are very likely to become effective regulatory targets in the intestinal barrier, and T cell-associated therapy may be used in clinical settings in the future.

Figure 2 Intestinal barrier components and the intestinal barrier dysfunction. The normal intestinal barrier is formed by many layers which includes cytokines, bacteria, cells and secretory IgA. The intestinal barrier dysfunction results in the increasing of the intestinal permeability, which subsequently causes the inflammatory response and bacteria translocation.
THE ROLE OF P2X7R IN THE INTESTINAL BARRIER DYSFUNCTION
Among the members of P2X receptor family, P2X7R (encoded by p2rx7) is the largest (with 595 amino acids in humans). It has special structural and signaling features because of its long intracellular carboxy-terminal, which helps prevent receptor desensitization[90,91]. The monomeric structure of P2X7R has two intracellular domains (C-terminal and N-terminal) and an extracellular ATP-binding domain that separates two transmembrane domains[92]. There were over 1500 single nucleotide polymorphisms (SNPs) reported in NCBI database, and most of them were missense, intronic or nonsynonymous[93]. In highly polymorphic human P2RX7, SNPs play a critical role in the biological process and function of P2X7R. About 10 loss of function SNPs and 3 gain of function SNPs have been identified[12]. For example, the activity of human P2X7R was reduced when Ala replaced Glu 496[94].When Asn replaced lle-568, the expression of P2X7R was decreased to approximately 50% of normal,and P2X7R became nonfunctional[95]. The mutation of R307Q located in the ATP-binding pocket impaired the binding of ATP to P2X7R[96]. Genetic variants in P2X7R may be involved in the inflammatory response[97,98]. P2X7R function related SNPs played a regulatory role in inflammatory diseases[32]. Unlike other P2X receptors, the complete activation of P2X7R requires a higher concentration of ATP (range from about 0.1 to 2.5 mmol/L)[99]. When activated by ATP, P2X7R not only mediates the uptake of cations and macromolecules, but also leads to the activation of intracellular signaling pathways[100-102]. It has been demonstrated that the formation of macropores requires pannexin-1 channels, and pannexin-1 antagonists can decrease the formation of these pores[103]. However, recent data have suggested that the formation of macropores may be intrinsic to P2X7R without accessory molecules[104-106]. Moreover, P2X7R is associated with the activation of the signaling pathway and transcription factors, including MAP kinases, the cyclic AMP response element[107,108]. P2X7R is widely expressed in immune cells, which suggests its importance in the regulation of both the innate and adaptive immunity, especially in the regulation of inflammation[109,110].
ATP is the most important energy molecule and a common extracellular signaling nucleotide that participates in the regulation of cellular proliferation, differentiation and death[111-113]. In a healthy body, eATP is maintained in a low concentration thanks to ATPases in extracellular spaces. ATP can leak from damaged or distressed cells, and can also be released by nonlytic regulated mechanisms,which increase the concentration of eATP16[114-117]. It has been proven that the concentration of eATP is higher in different inflammatory conditions than in normal conditions[118,119].
The intestinal barrier dysfunction induces the inflammatory response and epithelial cell death[120,121]. In the acute-inflammatory tissue, high amounts of IL-6 are released, thereby inducing the synthesis and release of ATP from Treg cells exposed to IL-6[122]. The concentration of eATP may increase after intestinal barrier dysfunction, which may activate P2X7R. T follicular helper cells enhance germinal center reactions by deleting P2X7, resisting ATP-mediated immune cell death[123]. When the concentration of eATP produced by the intestinal microbiota is high, commensal-specific IgA responses initiated by intestinal lymphoid tissues are inhibited, which influences the composition of intestinal microbiota[124,125]. Moreover, Perruzzaet al[126] showed that the blockade of P2X7R could decrease proinflammatory cytokines and protect the intestinal barrier function by inhibiting the activation of macrophages. Nucleotide-binding domain, leucine-rich-repeat receptor, pyrin domain-containing NLR family pyrin domain containing 3 (NLRP3) is a multiprotein complex that participates in the occurrence and development of many inflammatory diseases[127]. The inhibition of NLRP3 can reduce intestinal inflammation and enhance the barrier function[128]. Both NLRP3 and P2X7R are expressed in different immune cells, including T cells, B cells and monocytes[99]. Several signaling pathways induced by activated P2X7R may lead to a decrease in intracellular K+, an increase in Ca2+and the production of reactive oxygen species, which are key steps in NLRP3 activation[129-132] (Figure 1).
THE ROLE OF T CELL-DERIVED P2X7R IN THE INTESTINAL BARRIER DYSFUNCTION
It has been reported that activated P2X7R can affect several of the biological processes of T cells,including activation, differentiation and death[133]. After recognizing antigens, T cells rapidly release ATP through pannexin channels due to the T cell receptor signaling and co-stimulatory molecules[134,135]. Because of the highly expressed P2X7 in iNKT cells, they were susceptible to P2X7-mediated cell death and regulated by vitamin A, finally influencing the intestinal homeostasis[136]. ATP released from T cells can activate P2X receptor which increases the expression of thep2rx7gene[14,135]. Yipet al[135] found that the silencing of P2X7R blocked Ca2+influx and inhibited T cell activation in human CD4+T cells. These findings suggest that activated P2X7R is essential for the activation of T cells. Lselectin (CD62L) is related to the migration of T cells[137,138]. Low expression of L-selectin is necessary for activated or differentiated T cells to egress from the lymph node[139]. P2X7R activated by ATP can trigger CD62L shedding in human naïve T cells[140]. In a lymph node, activated P2X7R also affects the motility of T cells by inducing their calcium waves[141]. When intracellular ATP and NAD+nucleotides are released from cells, they can trigger the activation of P2X7R and induce apoptosis or necrosis[142].In the case of low micromolar concentration of extracellular NAD+, ADP ribosylation of P2X7R induces cell death because of persistent P2X7R activation[27]. Under the condition of activated P2X7R, there are two independent ways to induce T cell death: one of them depends on the phosphorylation of ERK1/2,and the other is associated with the nonselective pore[143,144]. In addition, CD62L shedding triggered by the activated P2X7R may induce cell death by apoptosis[145,146]. Compared with native T cells,activated T cells are less sensitive to NICD induced by P2X7R[147]. The expression of P2X7R is different in different populations of T cells. For example, Tregs and follicular helper T cells exhibit high expression of the P2X7 receptor, suggesting that they are more susceptible to cell death than other populations of T cells[148,149]. eATP and P2X7R influence the differentiation of T cells and play a significant role in the metabolism, generation, and memory function of CD8+T cells[150]. It has been shown that the AMP-activated protein kinase signaling pathway may promote constant efflux of intracellular ATP in memory CD8+T cells, and is involved in the differentiation and maintenance of memory T cells induced by P2X7R[151,152]. In an inflammatory environment, activated P2X7R drives the differentiation from T cells to Th17 cells[153], and the receptor reduces the differentiation of Tr1 cells with a high expression of IL-10 without Foxp3[21,155]. Activated P2X7R can also regulate the plasticity of Th17 cells and induce Th17 cells to differentiate[156]. In addition to acting directly on T cells, P2X7R can regulate the differentiation of T cells by affecting the physiological functions of dendritic cells[157,158]. Although ATP may not only reduce the DCs-induced Th1 cell differentiation but can also influence the interaction of DCs with T cells, there is little research on the role of P2X7R during this process[159,160]. Moreover, activated P2X7R regulates the cytokine secretion and polarization of Th17 cells by influencing dendritic cells[161,162] (Figure 3). Myeloid derived suppressor cells were considered as the regulator of immunosuppressionviaaffecting the amounts, functions, or phenotypes of T cells, and the ATP/P2X7R signaling axis may be involved in this process[163].
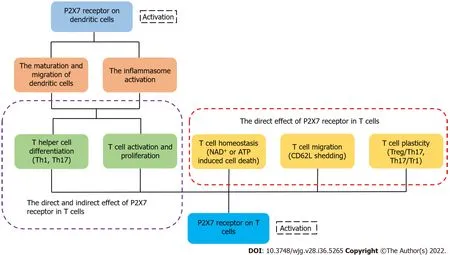
Figure 3 P2X7 receptor on dendritic cells and T cells influences the fate of T cells indirectly or directly, respectively. The activation of P2X7 receptor on dendritic cells induces the maturation and migration of cells and promotes the inflammasome activation, affecting the activation and differentiation of T cells finally. The activation of P2X7 receptor in T cells can directly regulate the activation, differentiation, migration and homeostasis of T cells[33].
DISCUSSION
The intestinal barrier dysfunction is a complex and severe pathological condition, which induces the inflammatory response and bacterial invasion. Sepsis is a serious systemic inflammatory disease with high morbidity and mortality in the intensive care unit because it can cause multiple organ failure in patients[164]. Given that the progression and pathogenesis of sepsis have been attributed to intestinal barrier dysfunction, further research on the immune and inflammatory factors of the intestinal barrier dysfunction is necessary[165,166].
According to the above description, T cells are involved in oral tolerance and immune response to antigens in the intestine, and they are the most common lymphocytes that reside in the intestine[167].Moreover, infiltration by inflammatory T cells is a significant pathological characteristic of intestinal inflammation[168]. Thus, an appropriate number and population of T cells may mitigate the damage of intestinal barrier dysfunction.
P2X7R is widely expressed in T cells and serves as a regulatory factor of their biological processes.Heisset al[169] found that intestinal CD8+T cells express a high concentration of P2X7R and are highly sensitive to extracellular nucleotides, indicating that P2X7R can regulate intestinal T cell responses.Inflammatory effector T cells can be depleted and intestinal inflammation can be relieved after treatment with NAD+[170]. P2X7R has been shown to be the trigger for the activation of NLRP3,indicating that this receptor regulates the release of inflammatory cytokines (IL-18, IL-1β) and the initiation of an inflammatory response[171-174]. Therefore, P2X7R may influence inflammationviaT cells which is indirect. The selectively P2X7 antagonist was proven to significantly inhibit the innate immune cells and upregulate the immunosuppressive-associated T cells, indicating that this antagonist may be a kind of potential treatment[175]. The effect of P2X7-blockade drug has also been demonstrated in the mouse models with advanced tuberculosis[176]. In addition to the above intracellular signaling pathways (MAPK pathway), previous studies verified that P2X7R also regulated MyD88/NF-κB and PI3K/Akt/mTOR signaling pathways in innate and adaptive immune responses, which suggested that the key proteins in these pathways can be considered as novel therapeutic targets[177].
CONCLUSION
In summary, T cells, the key participant in the intestinal barrier dysfunction, are regulated by P2X7R.The roles and mechanisms of P2X7R are associated with T lymphocytes in the intestinal barrier dysfunction and may be a potential research direction, although there have been few studies on this topic (Figure 4). Furthermore, different specific molecules that inhibit the expression of P2X7R may be potential therapeutic drugs in the future.

Figure 4 The schematic diagram of the hypothesis of the P2X7 receptor as the regulator of T-cell function in intestinal barrier disruption.
FOOTNOTES
Author contributions:Jiang ZF and Wu W contributed equally to this work; Zhong M and Zhang L were cocorresponding authors; Jiang ZF and Wu W wrote the manuscript; Zhang L and Zhong M conceived the topic and reviewed the manuscript; Jiang ZF and Zhang L revised the manuscript; Hu HB and Li ZY reviewed the manuscript;all authors contributed to the article and approved the submitted version.
Supported byThe National Natural Science Foundation of China, No. 81801943; Shanghai Pujiang Program, No.21PJD009; and The Research Grant for Public Health Key Discipline of Shanghai Municipality, China, No. GWV-10.1-XK26.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Zhi-Feng Jiang 0000-0003-0714-3453; Lin Zhang 0000-0002-0950-7145.
S-Editor:Gong ZM
L-Editor:Filipodia
P-Editor:Gong ZM
 World Journal of Gastroenterology2022年36期
World Journal of Gastroenterology2022年36期
- World Journal of Gastroenterology的其它文章
- Histopathological assessment of the microscopic activity in inflammatory bowel diseases: What are we looking for?
- Machine learning-based gray-level co-occurrence matrix signature for predicting lymph node metastasis in undifferentiated-type early gastric cancer
- Atherogenic index of plasma combined with waist circumference and body mass index to predict metabolic-associated fatty liver disease
- Deciphering the role of transforming growth factor-beta 1 as a diagnostic-prognostic-therapeutic candidate against hepatocellular carcinoma
- Liver-specific drug delivery platforms: Applications for the treatment of alcohol-associated liver disease
- Esophageal magnetic compression anastomosis in dogs
