超分子相互作用主导的纳米药物成核
许文哲,张皓
(吉林大学化学学院,超分子结构与材料国家重点实验室,长春 130012)
1 Introduction
The past two decades have witnessed the remarkable achievements of nanodrugs both in fundamental researches and clinical applications because of the advantages of flexible design,facile preparation,controllable functionality and feasible modification beyond molecular drugs[1].According to the statement of U.S.Food and Drug Administration(FDA),nanodrugs refer to the nanometer-sized systems composed of active pharmaceutical ingredients(APIs),which exhibit distinct functionalities and efficacy compared to larger-scale counterparts[2].In general,the reported nanodrugs can be divided into carrier-free nanodrugs(CFNs)and nano-drug delivery systems(NDDSs)with a variety of carriers,including nanosuspension,liposomes,nanocapsules,nanomicelles and nanoparticles(NPs)[3—5].On the basis of the encouraged results from prudent clinical trials,some nanodrugs have already been approved by FDA,such as Doxil@,Abraxane@,Rapamune@and so on,indicating a bright future of nanodrugs[6,7].Even so,suboptimal outcome for further applications of the majority of nanodrugs remains insurmountable,resulting from the addition of excipients such as carriers and surfactants[8].To be specific,the dilemmas caused by excipients,for instance,the unpredicted biosafety issues,low drug loading efficiency,as well as poor biodegradation and metabolism impose a major challenge for the clinical applications of nanodrugs[9,10].
To solve the aforementioned problems,the CFNs without carriers but preserving the merits of nanodrugs have attracted considerable attention in the recent investigations[8—10].Owing to the flexibility for preparing CFNs,both natural drugs and modified drugs are potential candidates as the APIs[11,12].The CFNs made of only API ensure the maximum drug contents and relatively high bioactivity[9].In order to integrate APIs into CFNs rationally and straightforward,several preparation strategies have been exploited,including high-pressure homogenization method,template-assisted method,medium milling method and nanoprecipitation[13—16].Among these strategies,nanoprecipitation,based on the spontaneous precipitation of drug molecules from solvent/antisolvent-induced supersaturated system in a controlled manner,is superior to other methods due to its convenience and better maneuverability,thus becoming one of the most successful methods for preparing CFNs[16].
As schemed in Fig.1,nanoprecipitation can be described as a process that hydrophobic drug molecules predissolved in a water-miscible organic solvent are immersed in water or aqueous buffer solutions to generate a supersaturated system,and followed by solvent diffusion and local supersaturation to induce the nucleation and subsequent growth of drug NPs[17].Intriguingly,nanoprecipitation also permits to prepare surfactant-free nanodrugs by reasonably selecting the species of drugs,solvents and antisolvents,presenting the phenomenon namely“Ouzo effect”[17].Until now,this phenomenon has been summarized in detail by many review articles,but almost all of these overviews focused on single-drug molecules[16—18].While the surfactant-free nanodrugs composed of multidrugs are reviewed in limited reports.With respect to the urgent needs of next-generation nanodrugs,characterized by bio-safety,multi-functionality and in particular combination and cascade therapies,a systematical summary of the recent progresses of carrier-free and surfactant-free nanodrugs from multidrug systems are greatly welcome.

Fig.1 Schematic illustration of nanoprecipitation method and nucleation-growth process of CFNs
Meanwhile,the structures of drug molecules usually possess aromatic rings,carboxyl/hydroxyl/amino groups and other hydrophobic alkyl units[19].This characteristics on the one aspect leads to the good solubility of drugs in organic solvents,which is the prerequisite for nanoprecipitation,and on the other aspect provides a variety of intermolecular weak interactions for performing self-assembly and/or co-assembly of drugs during nanoprecipitation[20].Tremendous researches have demonstrated that the main driving force of self-assembled nanodrugs is supramolecular interactions,includingπ-πstacking,hydrophobic interaction,hydrogenbonding,electrostatic interaction,coordination interaction and so forth[12,20—22].Despite these evidences,the key role of supramolecular interactions in the process of nanoprecipitation is always ignored,not to mention a detailed overview.Consequently,it is imperative to perfect the theory of nanoprecipitation by considering the important contribution of supramolecular interactions,which is undoubtedly instructive for designing novel CFNs.
This review article focuses on the influence of supramolecular interactions on the nucleation process of CFNs.Firstly,according to the classical nucleation theory(CNT)of nanoprecipitation,the dominant contribution of supramolecular interactions in CFN nucleation is proposed.Secondly,the progresses of single-drug self-assembled CFNs both from natural drugs and modified drugs are introduced.Thirdly,the CFNs composed of multidrugs are highlighted to elucidate the advantages of multidrug co-assembly strategy.Finally,supramolecular interactions-mediated nucleation is expanded to the CFNs constructed through metal ions-involved coordination.This overview systematically indicates that the supramolecular interactions are dominant in the nucleation of CFNs,which provides a crucial guidance for the development of nanoprecipitation technique as well as the design and preparation of next-generation CFNs.The abbreviations and full names of the drugs in this review are listed in Table 1.

Table 1 Abbreviations and full names of the drugs in this review
2 A Modified Nucleation Theory of Nanoprecipitation
According to the classical theory of nanoprecipitation,the formation of nanodrugs always involves twoseparate stages,namely nucleation and growth,like the synthesis of all other nanometer-sized particlesviacolloidally chemical pathways(Fig.1)[16].However,different from the rapid nucleation of inorganic NPs,the relatively slow condensation and coagulation of drug molecules from the solvent/antisolvent-induced supersaturated system makes the nucleation stage dominant during nanoprecipitation[18].Consequently,the nucleation process has attracted significant attentions in the past theoretical studies of nanoprecipitation[23,24].It is generally accepted that chemical instability of the system is the precondition for nanoprecipitation.Hence,the CNT can be expanded to nanoprecipitation[25].The CNT elucidates that once the Gibbs free energy of system crosses the critical free energy barrier of nucleation,nucleation can occur persistently and the resultant nuclei are stable.The critical free energy of nucleationΔGcrit-classicalfollows the equation shown below[16],which was originally derived from bulk crystallization:

whereγ(J/m2)is the surface tension,v(L/mol)is the molar volume of drug molecules,kis Boltzmann’s constant,andT(K)is the temperature.It should be mentioned thatΔGcrit-classicalhighly depends on the supersaturation ratioS,which is defined as the ratio of the actual molecular concentration and the solubility of the molecules in the system[16].Based on this notion,rational regulations ofS,such as varying the types of solvents and antisolvents,altering their volume ratios,and adjusting molecular concentrations are general and efficient methods to manipulate nucleation process,especially for single-drug nanoprecipitation[26—28].However,owing to the limitation of macroscopic nucleation,the supramolecular interactions between drug molecules involved in the formation of nanometer-sized nuclei are previously out of consideration when CNT is applied for nanoprecipitation.Nevertheless,these intermolecular weak interactions are of vital significance both for manipulating the nucleation and preserving the structural stability of as-prepared nanodrugs[20].In addition,besides the quasi-spherical morphology,the as-prepared nanodrugs may also in the form of rods,ribbons or sheets,which cannot be well explained following the CNT of nanoprecipitation[29,30].These exceptions imply that after or during the formation of nanodrug nuclei,the supramolecular interactions are capable to direct the oriented self-assembly of drug molecules,thus greatly determining the microscopic structures and macroscopic morphologies of the as-prepared nanodrugs.
With respect to nucleation,supramolecular interactions are proposed in the CNT on the basis of the foregoing speculations,which is considered to modify the nucleation theory of nanoprecipitation.Considering the distinct origin ofΔGcrit-new,defined as supramolecular interactions-involved critical free energy of nucleation,we divide it into two main categories,ΔGsiandΔGss,which are defined as the free energy variation induced by supramolecular interactions and supersaturation,respectively.

According to Gibbs-Helmholtz equation,ΔGsifollows equation below:

whereΔHsiis the change of enthalpy induced by the supramolecular interactions,andΔSsiis the change of entropy.OnceΔGsiis less than zero,the self-assembly of drugs occurs spontaneously.In order to promote this spontaneous process,decreasingΔHsior increasingΔSsican minimize theΔGsi.Among a variety of supramolecular interactions,hydrophobic interaction andπ-πstacking relate to entropy gain,whilst hydrogen bonding causes enthalpic loss[31].Benefiting from the tremendous researches on supramolecular interactionsmediated self-assembly,these convincing results can be extended to the nucleation process of nanoprecipitation directly[12,20].Meanwhile,because of the supersaturation-dependentΔGcrit-classical,we assume thatΔGssis the same as classicalΔGcrit-classical.Thus,substituting Eq.(1)and Eq.(3)into Eq.(2)yields the final formation ofΔGcrit-new:
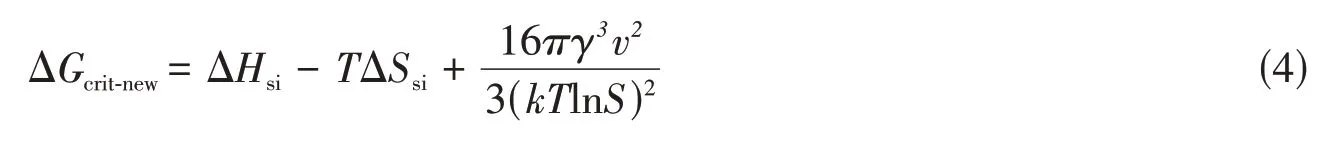
Eq.(4)indicates that the nucleation is a thermodynamics-controlled process significantly determined by supramolecular interactions.With regard to single-drug self-assembled CFNs,the monotonous molecular structures and the accompanied supramolecular interactions make little contribution toΔGsi.To introduce stronger supramolecular interactions and therefore reduce theΔGcrit-new,drug molecules have been modified with functional moieties or other drugs.When it comes to multidrug co-assembled CFNs,diversified structures and synergetic supramolecular interactions are involved in one system,which decreasesΔHsior increases ΔSsiobviously,thus reducingΔGcrit-newand facilitating nucleation significantly.In all,this modified nucleation theory indicates that supramolecular interactions-mediated nanodrug nucleation provides a potential method to regulate the process of CFN preparation.
3 Single-drug Self-assembled CFNs
When single-drug molecules assemble into CFNs,the“Ouzo effect”is crucial.Only the composition of single-drug system is exactly located in the Ouzo region,nanoprecipitation can occur spontaneously and produce stable CFNs[17].Although the supramolecular interactions in single-drug self-assembled CFNs are simple,such model systems provide basic perception and knowledge for in-depth understanding of the influence of drug molecular structures on nucleation process.Actually,the types and priority of supramolecular interactions are completely determined by the structural properties of drug molecules.The previous reports have demonstrated that the reasonable selection and/or deliberate modification of drugs is capable to introduce and tune the supramolecular interactions,permitting the state-of-the-art preparation of CFNs[20].According to the source,the drugs for preparing CFNs can be divided into natural drugs and modified drugs.On the basis of these two categories,the following overview is carried on.
3.1 Natural Drug-based CFNs
Natural drug-based nanodrugs have been studied extensively[32],but the reports about carrier-and surfactant-free ones are limited because of the uncontrollable inter-drug interactions.Here,the limited examples are further divided into two categories according to the possible supramolecular driving forces during nucleation,namely hydrophobic interaction in Section 3.1.1 andπ-πstacking in Section 3.1.2.
3.1.1 Hydrophobic Interaction-mediated Nucleation Nucleation UA is a kind of pentacyclic triterpene carboxylic acid consisting of five fatty rings with poor water-solubility[33].Inspired by the hydrophobic interaction,UA-based CFNs were prepared by ultrasound-assisted nanoprecipitation[Fig.2(A)and(B)][34].According to Eq.(4),the nucleation was improved with the participation of hydrophobic interaction due tothe contribution in the decline ofΔGcrit-new.In addition,the introduction of exogenous energy such as ultrasonic irradiation is another promotion factor for the successful preparation of UA CFNs,which facilitates local supersaturation by accelerating solvent diffusion and subsequent energy fluctuation.Note that besides hydrophobic interaction,other supramolecular interactions,such as hydrogen bonding,may also contribute to the nucleation because of the complex structure of UA.The assistance of other supramolecular interactions breaks the limitation of hydrophobic interaction in oriented self-assembly,leading to the ordered arrangement of UA in CFNs.
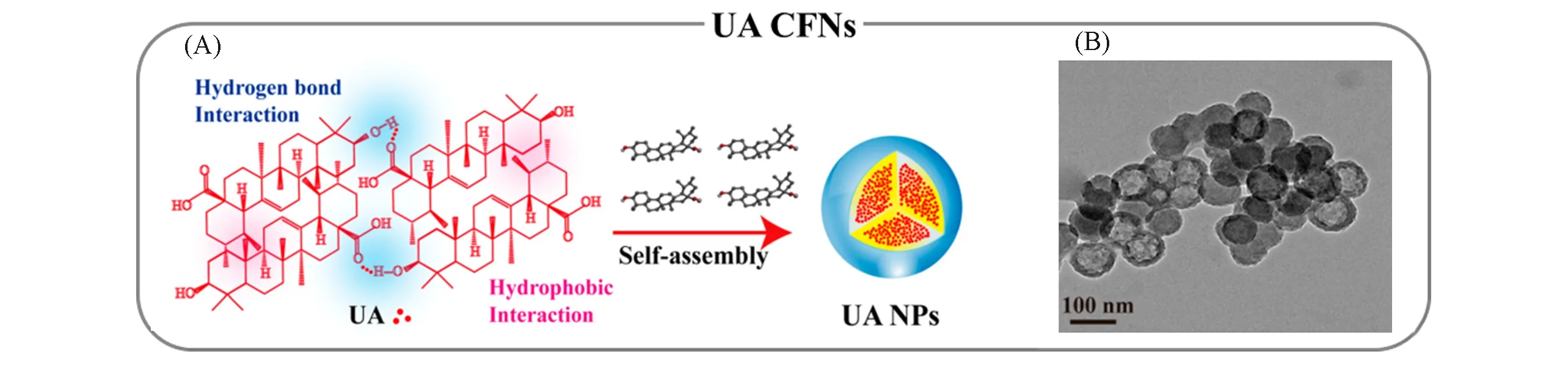
Fig.2 Representative natural drug-based self-assembled CFNs through predominant supramolecular interactions[34]
3.1.2π-πStacking-mediated Nucleation In comparison to UA,π-πstacking is more favorite for HCPT and Cur due to the robust aromatic rings with higherπelectron densities.The quasi-spherical HCPT NDs were prepared by nanoprecipitation at the temperature higher than room temperature with vigorous stirring[35].Like the promotion of ultrasonic irradiation mentioned in Section 3.1.1,the high temperature and vigorous stirring make solvent diffusion dynamic,since the nucleation process is also related to kinetics besides thermodynamic regulation.In contrast,Cur CFNs can be prepared by nanoprecipitation without exogenous energy promotion[36].Relying on the strongπ-πstacking and correct supersaturation,the nuclei of Cur CFNs formed spontaneously,producing uniform NPs with a diameter of 80—90 nm.In addition,the fluorescence quenching of Cur CFNs after self-assembly is also attributed to the energy transfer induced byπ-πstacking[36].
3.2 Modified Drugs with Strengthened Supramolecular Interactions
To date,the progresses in the CFN preparation from natural drugs meet the bottleneck of nonuniversality,which is ascribed to the limited supramolecular interactions for the further regulation in single-drug systems.As a result,the nucleation process is overdependent on the supersaturation of drugs with the lack of additional control means.Aiming to introduce efficient supramolecular interactions,the modification of drugs with various functional groups is a conventional method[20].In addition,given the balance of each supramolecular interaction,both hydrophobic drugs and hydrophilic drugs can be employed flexibly to conjugate with other drugs in hope of promoting nucleation.
3.2.1 Modifying Drugs with Complementary Hydrophobic Drugs The relatively good water-solubility of some small molecule drugs restrains themselves from supersaturation in water and suppresses nucleation.According to Eq.(4),reducing drug water-solubility can not only promote the supersaturation by increasingS,but also introduce additional hydrophobic interaction between drug molecules by increasingΔSsi,which is beneficial to nucleation.Taking the merit of hydrophobic drugs into account,the aforementioned purpose can be realized by modifying drugs with complementary hydrophobic drugs.CPT is an analogue of HCPT with similar hydrophobicity and bioactivity.To strengthen the hydrophobicity of Ir,CPT was conjugated through a redox responsive linker,namely disulfide bond,to form CPT-SS-Ir conjugates for tuning the nucleation process of CFNs[37].Following the same strategy,gemcitabine-based CFNs were successfully prepared by conjugating with CPT,indicating the universality of modifying drugs with complementary hydrophobic drugs for promoting nucleationviahydrophobic interaction[38].Based on this concept,polyunsaturated fatty acids with long alkyl chain were also demonstrated as candidates to promote nucleation by enhancing the hydrophobicity of drug molecules[39].
Apart from hydrophobic interaction,it is found thatπ-πstacking is another nonnegligible supramolecular interaction for promoting nucleation by increasingΔSsi.In addition,the strengthenedπ-πstack of aromatic moieties after drug-drug conjugation increases the planarity of drugs,which may further direct the arrangement of drug molecules orderly.In the system of Cb-Ir conjugates,πelectron densities were enhanced due to the introduction of additional aromatic moieties[Fig.3(A)][40].The proton shift of the pyridone moiety was observed in1H nuclear magnetic resonance spectroscopy,which was caused by the strongπ-πstacking afterconjugation.Hence,the formation of Cb-Ir nuclei is greatly promoted byπ-πstacking.Another twin-drug system of floxuridine-bendamustine with several heterocyclic rings was also investigated[41].The ultraviolet-visible spectrum showed a slight redshift from 324 to 327 nm after drug conjugation,presumably induced by greater electron delocalization and lower transition energy of conjugated systems,indicating thatπ-πstacking can play a key role in the nucleation of CFNs[41].In addition,it is speculated that the linkers cannot extinguishπ-πstacking in drug-drug conjugation,because theπgroups distribute separately at both ends of the modified drugs,for instance theπ-πstacking-driven nucleation of curcumin-polyethylene glycol-erlotinib[42].
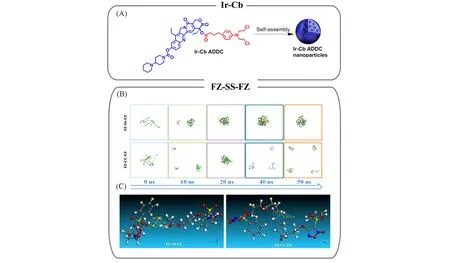
Fig.3 CFNs from drugs conjugated with complementary hydrophobic drugs and representative mechanism
Note that hydrophobic interaction andπ-πstacking usually operate together.A complete division of the priority and the role of them remain challenging.Theoretical simulations,such as Molecular dynamics(MD)and Materials Studio,seem to be potential solutions.As for hydrophobic FZ-based homodimeric prodrugs with disulfide bond(FZ-SS-FZ),MD simulation results revealed that FZ-SS-FZ molecules first aggregated to form nuclei through hydrophobic interaction and then were stabilized byπ-πstacking[Fig.3(B)][43].To further confirm the influence of linkers,FZ-CC-FZ molecules with—C—C—bond instead of—S—S—bond were prepared as a contrast.Both experiment and MD simulation results showed that the disulfide bond provided sufficient structural flexibility for FZ-SS-FZ molecules to balance multiple supramolecular interactions,thus achieving stable nucleation[Fig.3(C)][43].In this context,drug molecules,linkers and modified moieties are all of significance for nucleation manipulationviasupramolecular interactions.
3.2.2 Modifying Drugs with Complementary Hydrophilic Drugs Actually,most small molecule drugs exhibit poor solubility in aqueous media,and their strong hydrophobicity is the main reason why they arelimited for clinical applications[44].Besides,the insufficient hydrophilic-hydrophobic balance also hinders drug nucleationviasupramolecular interactions.Hydrophilic moieties usually possess charges.The conjugation of drugs with them is capable to induce electrostatic interaction for decreasingΔHsiand reduce hydrophobic interaction for decreasingΔSsi.In the light of Eq.(4),these two effects are apparently contradictory for ΔGcrit-newchange.Nevertheless,the influence of hydrophilic moieties onΔHsidecrease is more obvious than that onΔSsidecrease,because the strength of electrostatic interaction is 50—300 kJ/mol[45],which is the strongest among a variety of non-covalent bonds.So,the introduction of electrostatic interaction can in general promote nucleation.FA is a water-soluble targeting ligand,which can bind with FA receptor on tumor cell surface[46].When conjugated with other drugs,the charged carboxyl/amino groups of FA can induce electrostatic interaction and enhance hydrophilicity.In a proof-of-concept,DOX-FA and CPT-FA CFNs were selected for instance[47,48],where the electrostatic interaction between DOX-FA or CPT-FA molecules decreases theΔHsiand therefore reduces theΔGcrit-new,thus promoting nucleation[Fig.4(A)and(B)].In addition,the enhanced water solubility suppresses the hydrophobic interaction and therefore prevents the aggregation of disordered drug bulk from the system.This strategy can be further applied to the drug modification with short polymeric chains,such as ethylene glycol(OEG)oligomer-conjugated CPT,where OEG is composed of only eight repeating units and charged hydroxyl groups[49].Following the foregoing strategy,CFNs are also prepared from the drugs with more than two moieties.The successful assembly of PTX conjugated with dye and platinum compounds is a convincing example[50].
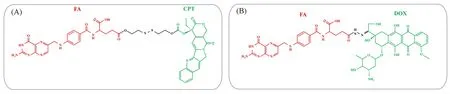
Fig.4 Constitutional formulas of FA-based prodrugs
4 Multidrug co-Assembled CFNs
Single-drug self-assembled CFNs from modified drugs still suffer from the problems of complex preparation,high cost and low efficacy.While the preparation of CFNs from multiple drug mixtures,namely multidrug co-assembled CFNs,is capable of overcoming these problems with minimal cost.One merit of multidrug co-assembly is the potential to introduce complementary supramolecular interactions for manipulating the nucleation process simply by virtue of correct drug combination.Therefore,the retrospective exhibition of multidrug co-assembled CFNs is significantly important for designing and preparing“All-in-One”CFNs by nanoprecipitation.According to non-covalent and covalent supramolecular interactions,the discussion of multidrug co-assembled CFNs is focused onπ-system drugs and metal ions-involved coordinated drugs.
4.1 co-Assembly of π-System Drugs
As mentioned above,non-covalent supramolecular interactions,for instanceπ-πstacking and hydrophobic interaction,can promote nucleation by reducingΔGcrit-new.With respect to multidrug CFNs,non-covalent bonds-driven nucleation can bring additional advantages,such as size and morphology control,improved stability,controlled release,function integration,enrichment of CFN types and so forth.In this subsection,these advantages are highlighted in detail.
4.1.1 Size and Morphology Control It is well known that the size and morphology of nanodrugs greatlydetermine cellular uptake,physiological stability and ADME profile(Absorption,Distribution,Metabolism,and Excretion).For instance,spherical nanodrugs with smaller sizes can be internalized through multiple endocytotic mechanisms and lead to high cellular uptake,while the cellular uptake of rod-like and branched nanodrugs is prior to the spherical counterparts[51].Since the planarity ofπ-πstacking usually direct the arrangement of drug molecules with orientation,a morphology control of the as-prepared CFNs during nucleation is further allowed.HCPT-based multidrug co-assembled CFNs are featured with adjustable size and morphology due to the combination of the strongπ-πstacking of HCPT with the complementary supramolecular interactions from additional drugs.In this context,HCPT self-assembled CFNs originally possess micronsized structures and irregular morphology[52,53],which are out of biomedical applications.While the co-assembly of HCPT with DOX leads to a morphology transformation of CFNs from rods to squares and finally spheres[Fig.5(A)][54].Given the protonation or deprotonation of the hydrophilic groups of DOX,the complementary electrostatic interaction of DOX disturbed the oriented arrangement of HCPT molecules driven byπ-πstacking,thus suppressing the formation of HCPT rods[54].Such morphology variation is also promoted by increasing DOX-to-HCPT molar ratio,further verifying the weakening ofπ-πstacking between HCPT by DOX[Fig.5(B)].Coincidentally,the co-assembly of HCPT with MTX exhibits similar behavior[53].The deprotonation of MTX carboxyls provides additional electrostatic repulsion,leading to the morphology transformation of MTX-HCPT CFNs from needles to rods and spheres.
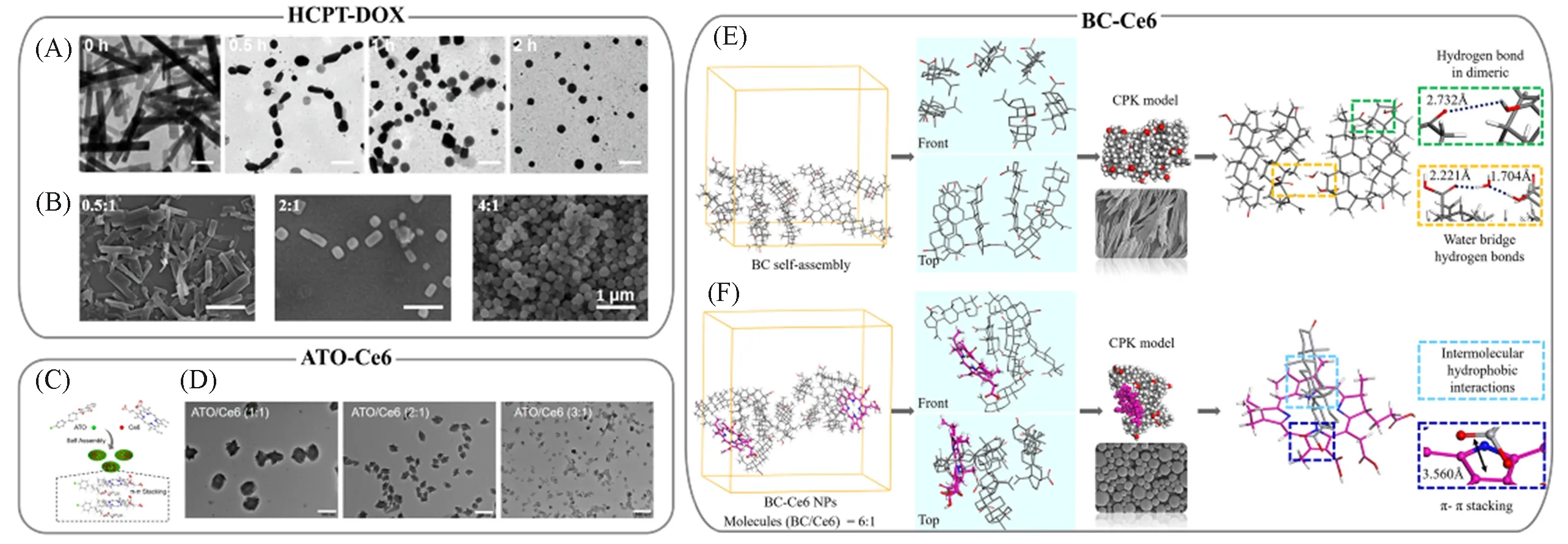
Fig.5 Representative π-system multidrug co-assembled CFNs with controllable size and morphology
Note that for the multidrugs both with strong ability ofπ-πstacking,the morphology manipulation is failed in the nucleation process.For instance,rod-like CFNs rather than spherical particles are obtained throughout as dissolving HCPT and Ce6 in organic solvent and subsequently dropped into water[55].In this scenario,both the HCPT and Ce6 molecules are featured withπ-electronic planar structures.So,theπ-πstacking between them is always dominant,favoring the oriented arrangement of drug molecules along specific direction and therefore the formation of one-dimensional(1D)CFNs.This consideration is further supported by the co-assembled CFNs of ATO and Ce6.ATO and Ce6 were both mixed in dimethyl sulfoxide and then added into water,generating quasi-spherical NPs[56].Although both ATO and Ce6 possessπ-electronic structures,the planarity of ATO is worse than Ce6.And the molecular volume of ATO is also smaller than Ce6[Fig.5(C)].So,as inserting ATO into Ce6 assemblies during nucleation,theπ-πstacking between Ce6 molecules is weakened,which is adverse to the oriented arrangement of drug molecules and produces quasispherical CFNs[Fig.5(D)].
In order to reveal the role ofπ-πstacking in morphology evolution systemically,a group of terpenoid molecules were co-assembled with Ce6 separately[29].Both the experiment results and theoretical simulations indicate that these terpenoid molecules with similar structure is capable to adjust CFNs morphology,including fibers,rods,nets and spheres.Representatively,the self-assembly of BC tends to from 1D fibers,mainly driven by the longitudinal intermolecular hydrogen bonding.However,for the Ce6-BC spherical CFNs,the main driving forces during nucleation includeπ-πstacking and hydrophobic interaction between aromatic rings of Ce6 and pentacyclic triterpene of BC,which disturb the longitudinal hydrogen bonding between BC molecules[Fig.5(E)and(F)].It is believed that the difference of pendant groups in terpenoid molecules impacts theπelectron densities.If such effect is adverse to reduceΔGcrit-newto meet the requirement of nucleation,the spontaneous assembly becomes difficult.
4.1.2 Improved Stability Excellent colloidal and physiological stability are the prerequisite for drug storage and biomedical applications.As shown in Table 2,thezetapotential and long-term stability of a variety of multidrug co-assembled CFNs are summarized and compared[57—64].During blood circulation,many liposoluble drugs are unstable and may be cleaned by blood rapidly.While the co-assembly of drug molecules into nanodrugs allows to improve stability by introducing drug-drug interactions.In addition,it has been reported that a negativezetapotential of NPs is helpful for avoiding adverse protein adsorption and NP aggregation in normal tissues,thus increasing the blood circulation half-life of NPs.Because negatively charged groups can be introduced into CFNs in the nucleation process flexibly by correct drug combination,the stability of multidrug co-assembled CFNs is significantly improved(Table 2).
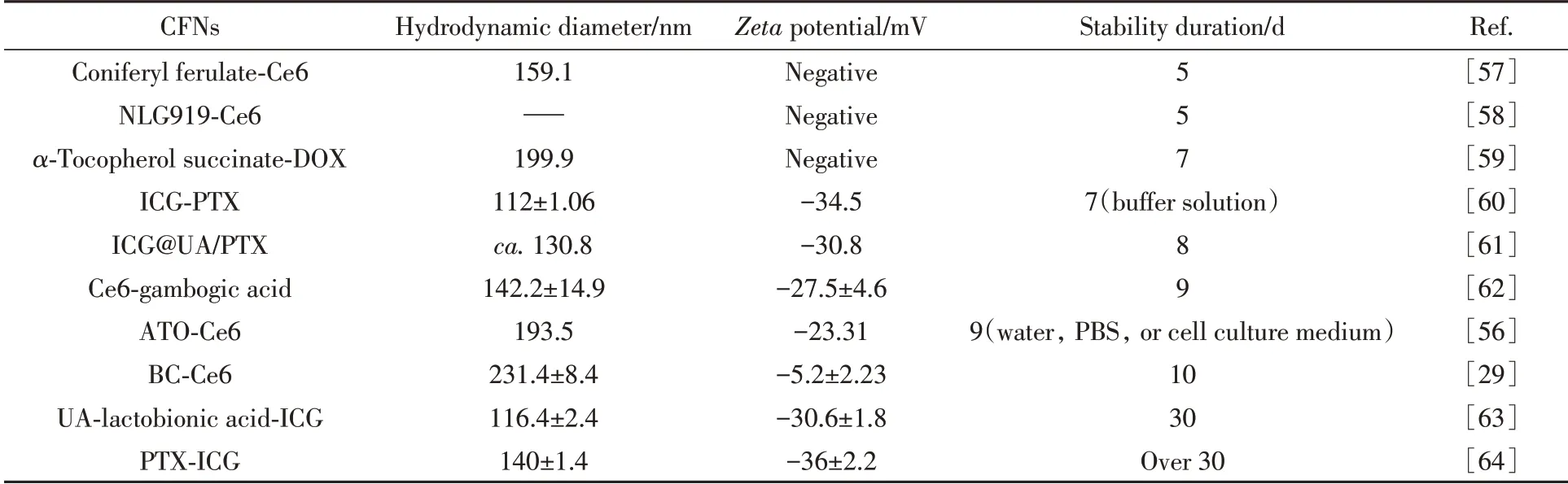
Table 2 Summary of hydrodynamic diameter,zeta potential,and stability duration of CFNs
4.1.3 Controlled Release To minimize the side effects,the disassembly of nanodrugs should be prevented in normal tissues but promoted around the lesion location.Thus,supramolecular interactions-mediated nanodrug nucleation is considered as a potential candidate for constructing multidrug co-assembled CFNs with controlled release behavior.Among many of the FDA-approved chemotherapeutic drugs,DOX is one of the most widely used drugs[6].Accordingly,DOX-based nanodrugs with enhanced tumor microenvironment responsiveness have attracted great attentions.During the nucleation of co-assembled CFNs,DOX can interact with neighboring DOX and/or other chemotherapeutic drugsviaelectrostatic interaction.As immersed in acidic environment,the electrostatic interaction will be broken due to the protonation of the amino groups of DOX,leading to the disassembly of CFNs and the release of bioactive DOX and other drugs.Taking DOX-digoxin as the example,the cumulative release of DOX was up to 80%at pH of 5.0,while only approximately 50%DOX release was observed at pH of 7.4[65].Note that tumor microenvironment is featured with acidic and redoxcondition,namely low pH,high glutathione(GSH)concentration and elevated H2O2level,which is perfect for performing hydrogen bonds-involved disassembly of CFNs[66].Therefore,DOX-HCPT,DOX-UA and DOXzinc phthalocyanine CFNs have been designed for tumor therapy with controlled release of corresponding drugs[67—69].
4.1.4 Function Integration Although chemotherapy is widely applied in clinical tumor treatments,the therapy on the basis of single chemotherapeutic drugs still meets the challenges of low efficacy,high side effects,drug resistance,as well as tumor metastasis and recurrence.Combination therapy and synergistic therapy using correct drug combinations are proved efficiently to solve the aforementioned problems both by fundamental researches and clinical practices.Moreover,the co-assembly of chemotherapeutic drugs and other nano-building blocks with specific therapeutic efficacy is capable to further strengthen the conventional chemotherapeutic strategy,for instance,the chemotherapy enhancementviaphotothermal therapy(PTT)and photodynamic therapy(PDT).In this scenario,multidrug co-assembled CFNsviasupramolecular interactionsmediated nucleation are robust to perform multifunctional integration and preserve drug bioactivity.Among a variety of therapeutic agents,ICG is a water-soluble PTT/PDT agent approved by FDA due to the excellent absorption in the near-infrared region and the deep tissue penetrationin vivo[70].Beneficial from the sufficientπmoieties and hydrophilic groups,ICG exhibits strongπ-πstacking and electrostatic interaction to promote nucleation in multidrug systems.The co-assembly of ICG,UA and PTX by nanoprecipitation produced stable ICG@UA/PTX nanodrugs[Fig.6(A)][60].In contrast,UA and PTX cannot form stable nanodrugs in the absence of ICG,indicating that the supramolecular interactions between ICG and UA/PTX molecules promote the nucleation both kinetically and thermodynamically.ICG/PTX nanodrugs were further prepared without UA,confirming the key role of complementary supramolecular interactions by selecting correct co-assembled agents[64].Through a similar strategy,pyropheophorbide-a,a photosensitizer agent,was combined with the prodrugs of single thioether-or selenoether-linked CTX and oleic acid by nanoprecipitation to achieve synergistic chemo-photodynamic therapy[71].
Ce6,another conventional PTT/PDT agent,was co-assembled with GA and FA to form multifunctional GA-Ce6-FA CFNs through the synergism ofπ-πstacking,hydrophobic interaction and electrostatic interaction[61].The preparation process of GA-Ce6-FA CFNs should be mentioned to highlight the role of complementary supramolecular interactions.Experimentally,GA methanol solution was dropped into Ce6 aqueous solution.Despite the preexistence of Ce6 molecules in system,can nucleation and subsequent nanodrug emergence be observed only after the addition of GA[61].This phenomenon firmly supports that in comparison to multidrug systems,the interactions between single-drug molecules are not enough for generating stable nuclei according to the discussion in Section 3.1.GA molecules can offset the originalπ-πstacking and electrostatic interaction between Ce6 molecules by participating the assembly of Ce6,thus facilitating the formation of stable nuclei.
4.1.5 Enrichment of CFN Types As mentioned above,single chemotherapeutic drugs usually lead to drug resistance and subsequent tumor metastasis and recurrence.Combination therapy using multiple drugs is efficient to solve this problem,but still suffers from the differential distribution and metabolism of different drugsin vivo.The co-assembly of drugs to produce CFNs can not only avoid these shortcomings,but also integrate the drugs lacking of nucleation ability into CFNs,thus greatly enriching the types of drug candidates.As an example,ICG has been employed as a template to construct co-delivery multidrug CFNs due to the strongπ-πstacking of ICG molecules[Fig.6(B)][62].A variety of drugs with immunotherapeutic and chemotherapeutic efficacy can be co-assembled with ICG,such as PTX.The dominant interaction between ICG and PTX was verified as hydrophobic interaction rather thanπ-πstacking,which permitted the formationof stable PTX-ICG nuclei through enhanced intermolecular interaction[Fig.6(C)][62].Nevertheless,the strongπ-πstacking of ICG molecules constructs the framework of CFNs during nucleation.Owing to the liposolubility of most drugs,this strategy can be expanded to all of the ICG-templated nanodrugs.Another model is demonstrated by simulating the formation of CFNs from BBR and CA for multidrug-resistantStaphylococcus aureustreatment[Fig.6(D)][72].To be specific,the primary formation of CA-BBR tiny nuclei was driven by hydrogen bonding,while the subsequent growth of CFNs was mediated by the inter-CA and inter-BBRπ-πstacking[Fig.6(E)and(F)].In all,taking the advantages ofπ-πstacking-driven nanodrug nucleation,the low-cost and highly efficient preparation of diversified CFNs are achieved.

Fig.6 Representative π-system multidrug co-assembled CFNs characterized with function integration and type enrichment
4.2 Metal Ions-involved Coordinated Drugs
In comparison to the nucleation process ofπ-system drugs,which involves complicated supramolecular interactions and incompletely understood mechanism,the nucleation of metal ions-involved coordinated nanodrugs is easy to elucidate.In this method,drug molecules possess carboxyl,hydroxyl and/or amino groups,which can potentially coordinate with a variety of metal ions,such as Fe3+,Cu2+,Mn2+,Zn2+and Ca2+.As immersing drug organic solution in metal ions-contained aqueous solution,the coordination of drugs with metal ions will promote the nucleation and the formation of CFNs.The bonding energy of coordination bonds is 50—200 kJ/mol,which is high enough to decreaseΔHsiand subsequentΔGcrit-new[45].Besides quasi-spherical particles,the orientation of drug-metal coordination can also manipulate the nucleation of CFNs with rod-like and onion-like morphologies[73].In addition,metal ions intrinsically possess biological functions,such as Fenton and Fenton-like reaction activity,magnetic resonance and photoacoustic imaging,and chemotherapeutic efficacy.These advantages of metal ions-involved coordinated drugs make them as competitive candidates forthe applications in combination therapy and cascade therapy.
4.2.1 Metal-polyphenol Coordinated CFNs Owing to the unique electronic structures of phenol,the coordination interaction between phenolic hydroxyls and metal ions is rather strong.It has been widely reported that as mixing hydrophilic polyphenols and multivalent metal ions in aqueous media,a metal-phenolic networks(MPNs)would be formed through metal-polyphenol coordination[74,75].With respect to lipophilic polyphenols,moreover,the multivalent metal ions in aqueous media are capable to promote the nucleation of polyphenols,exhibiting the process like nanoprecipitation.Shikonin,also known as“Zicao”in traditional Chinese medicine,is a lipophilic natural polyphenol,which presents good antitumor bioactivity.However,the poor watersolubility greatly limits the practical applications of shikonin.We found that the injection of shikonin ethanol solution into FeCl3aqueous solution produces Fe(Ⅲ)-shikonin CFNs,wherein the metal-polyphenol-coordinated interaction is the driving force[76].Thermodynamically,the Fe(Ⅲ)-shikonin coordination decreases the ΔHsiand subsequentΔGcrit-new,leading to stable nucleation[Fig.7(A)].Kinetically,the Fe(Ⅲ)in aqueous solution acts as“seed”,which facilitates the heterogeneous nucleation.In the experiments,with the increase of Fe(Ⅲ)feeding amount,the size of as-prepared CFNs became smaller,obeying the general principle of nucleation mechanism of nanoprecipitation[Fig.7(B)].Thanks to the coordination interaction,the CFNs from lipophilic quercetin and Fe(Ⅱ)were also prepared through coordination-mediated nucleation[77].Note that although both of the driving force is coordination interaction,the process of coordination-mediated nucleation of nanoprecipitation is distinct from the formation of classical MPNs.The former undergoes an interdiffusion between lipophilic polyphenols from organic solvent and metal ions from water,while the latter only involves the coordination between hydrophilic polyphenols and metal ions in water.Furthermore,the composed metal ions of CFNs bring additional therapeutic and imaging functionalities,such as the ferroptosis inducibility and T1-weighted magnetic resonance imaging from the Fe(Ⅲ)of Fe(Ⅲ)-shikonin CFNs[76].Very recently,nanoprecipitation method was reported to prepare Fe(Ⅲ)-tannic acid and Fe(Ⅲ)-luteolin@poly(vinylpyrrolidone)CFNs for against oxidative stress and photothermal/immune therapy,respectively,validating its flexibility forconstructing metal ion-lipophilic polyphenols[78,79].
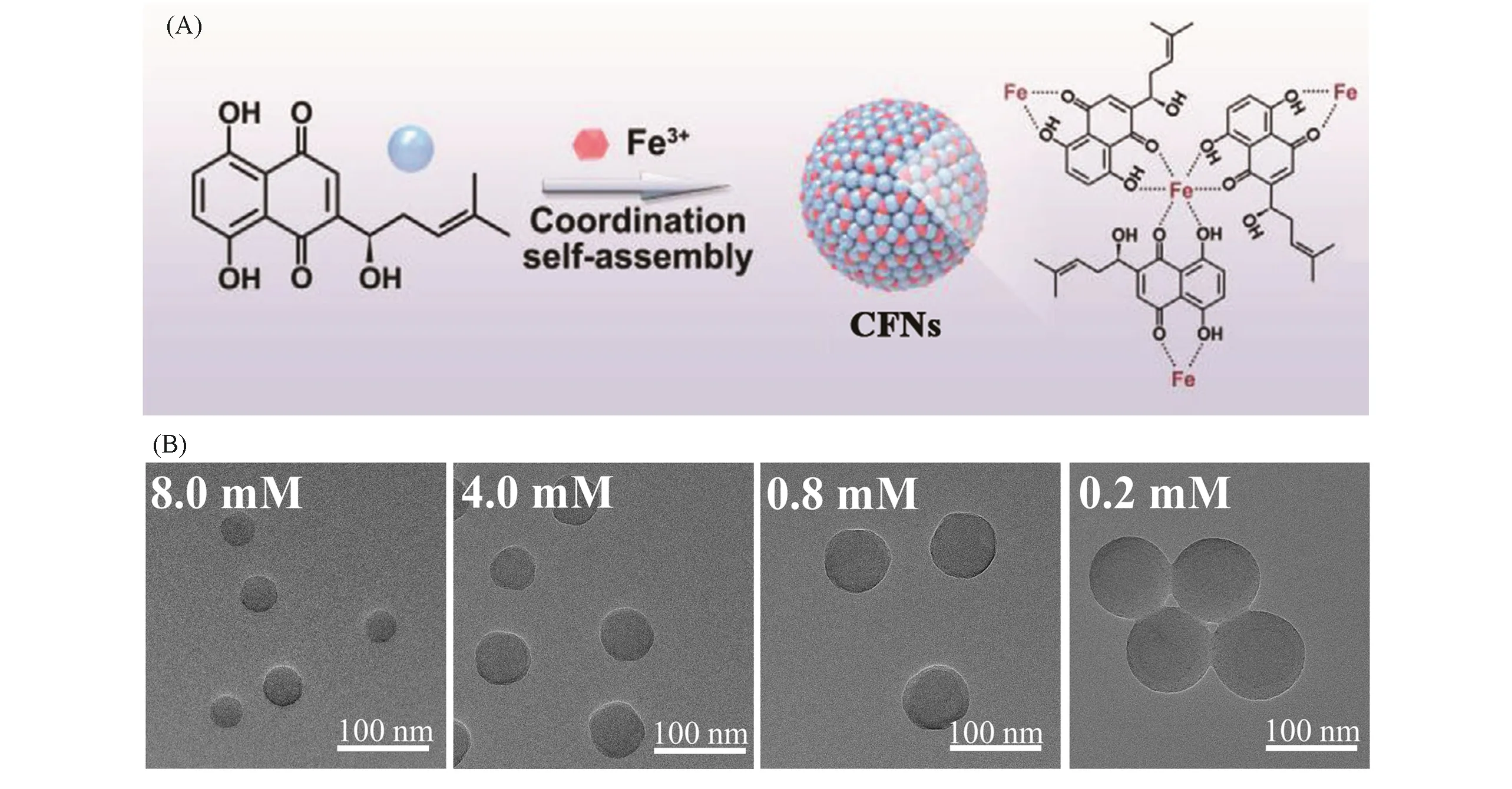
Fig.7 Schematic illustration of the formation of Fe(Ⅲ)-shikonin CFNs driven by coordination interaction(A)and TEM images of Fe(Ⅲ)-shikonin CFNs prepared with different Fe(Ⅲ)concentrations(B)[76]
4.2.2 Other Metal Coordination CFNs Besides metal-polyphenol coordination,the nucleation of CFNs can be promoted by other metal-drug coordination,thus greatly broadening the territory of nanoprecipitation.Most natural drugs and in particular modified drugs possess coordination groups with metal ions,which is convenient for performing coordination-mediated nucleation and further employed as the bridges for combining other supramolecular interactions.Taking Fe(Ⅲ)-MTX-ICG CFNs as the example,MTX provides the carboxyl groups for coordinating with Fe(Ⅲ)and the amino groups for the electrostatic interaction-driven assembly with ICG[80].After the formation of Fe(Ⅲ)-MTX complexes through coordination interaction,the strong electrostatic attraction between positively charged amino groups of MTX and the negatively charged sulfonate groups of ICG promotes the final nucleation.In this process,theπ-πstacking and hydrophobic interaction between MTX and ICG also contribute to nucleation.The synergism of metal coordination,electrostatic interaction,π-πstacking and hydrophobic interaction reduce theΔGcrti-newtogether.Similar to Fe(Ⅱ)and Fe(Ⅲ),Cu(Ⅱ)and Mn(Ⅱ)with good coordination ability and specific biological functionalities have also been employed for coordination-driven nucleation.For instance,assisted byπ-πstacking and hydrophobic interaction,coordination-driven nucleation and assembly of Cu(Ⅱ)-DOX and Mn(Ⅱ)-ICG-Ce6 CFNs are achieved[81,82].
5 Summary and Outlook
In this overview,the contribution of supramolecular interactions to CFN nucleation is emphasized on the basis of the CNT,which determines the nucleation process,physicochemical properties and biological functionalities of the final products.In this context,the elaborated discussions of single-drug self-assembled CFNs including natural drugs and modified drugs reveal the dominant role of the synergism of various supramolecular interactions,which eventually reduces the critical free energy of nucleation and therewith promotes the formation of CFNs.With respect to multidrug co-assembled CFNs composed ofπ-system drugs or metal ion-coordinated drugs,abundant supramolecular interactions endow them with novel functionalities and synergistic bioactivity,thus opening a new door to construct next-generation CFNs.Despite these significant advances,the inherent imperfections of CFNs still limit the practical applications comparable to NDDSs represented by the lipid NPs used for mRNA COVID-19 vaccine[83].
Due to the carrier-and surfactant-free features of CFNs,they exhibit the behaviors of nonspecific cellular uptake and nonresponsive disassembly.Although supramolecular interactions partially enhance the physiological stability of CFNs,the premature leakage of drugs and unpredictable side effects still exist.These drawbacks are considered to be solved mainly based on two alternative strategies.Namely,encapsulation of as-prepared CFNs with cytomembranes owning the capability of conjugating with the specific receptors on pathological cells,and/or integration of targeting drugs or nano-building blocks into CFNs.The former is conductive to avoid immunogenicity and enhance biocompatibility,but in the face of complex preparation,time and economic costs,and low encapsulation efficiency.While the latter is more practicable by supramolecular interactions-mediated homogeneous or heterogeneous nucleation,allowing for designing CFNs with dual-or even multi-responsiveness as well as precise drug delivery and release.With the aim of biosafety and bioregulation,the nano-building blocks from nontoxic and bio-endogenic molecules with specific receptors are most satisfactory for the assistant preparation of CFNs.In this scenario,AAs and PAAs are perfect hosts not only for preparing a rich variety of CFNs with controllable size,morphology,components and responsiveness,but for further conjugating with the upregulated AA receptors on tumor cells,such asL-type AA transporter family[84].Taking the advantages of safe delivery and controlled release,the methodology for the preparation ofAA-and PAA-involved CFNs should be systematically built in the future investigations.
Owing to the different reactivity of multidrugs in supramolecular interactions-mediated CFN nucleation,the feeding ratio and actual ratio of multidrugs in the as-prepared CFNs are usually inconsistent,which greatly impacts the therapeutic efficacy and fails in individual therapy.Theoretical simulations prior to complicated experiments are welcome to predict the mode and strength of supramolecular interactions during nucleation,thus screening the parameters and optimizing the conditions for CFN preparation with accurate components.Unfortunately,the theoretical prediction is suitable only for simple CFN systems,while it is poorly operable for complex multidrug co-assembled CFNs because of the diversified structures and synergetic supramolecular interactions.Very recently,polymeric prodrugs of DOX,CPT and PTX have been designed with the same frameworks and co-assembled into CFNs with controlled feeding ratios,while the therapeutic efficacy of the co-assembled CFNs with ratiometric drug loading is enhanced in comparison with the combination therapy of either molecular drugs or self-assembled CFNs[85].However,this strategy is too sophisticated to be expanded to other multidrug systems.Given the quantifiable coordination centers and drugs,the CFN nucleation driven by metal coordination exhibits the potential for stoichiometric loading of drugs and metal ions.The library of CFNs characterized with controllable components and individual therapy is expected to be greatly broadened by associating multidrugs with specific coordination functionalities and various metal ions.
Similar to NDDSs,the development of CFNs also faces the bottleneck of uncertain pharmacokinetics,resulting from the gap between material sciences and clinical needs.It should be noted that more attentions should be paid for exploring the fate of CFNin vivoby establishing new methods,for instance,the noninvasive monitoring of real-time drug distribution,release and activation by functional molecular imaging technique.Besides complementary supramolecular interactions,multimodal imaging functionalities from biosafe molecules and nano-building blocks should be further introduced in the CFNs to perform imaging-guided therapy,permitting precise and individual theranostics with optimized prescription.
On the whole,an in-depth study of supramolecular interactions-mediated CFN nucleation links basic design and clinical demands of next-generation nanodrugs,which will lead us to the ultimate goal of nanomedicine featured with high biosafety,multiple functionalities,maximal efficacy,and minimal side effects.It is believed that the aforementioned overview will attract more attentions to conventional nanoprecipitation and arouse the flourishing field of CFNs both for material explorations and biomedical applications.

