LASS2诱导甲状腺未分化癌8505c细胞自噬、凋亡及相关机制的探讨*
赵青青, 余云艳, 王云, 何维, 杨艳,4, 陈锐, 杨小理,4△, 程晓明△
LASS2诱导甲状腺未分化癌8505c细胞自噬、凋亡及相关机制的探讨*
赵青青1, 余云艳2, 王云1, 何维3, 杨艳3,4, 陈锐1, 杨小理3,4△, 程晓明1△
(1遵义医科大学附属医院普外科,2遵义医科大学免疫学教研室,3遵义医科大学附属医院医学检验科,4遵义医科大学检验医学院,贵州 遵义 563000)
研究LAG1长寿保障同系物2(LASS2)对甲状腺未分化癌细胞株8505c凋亡及自噬的影响及可能的分子机制。用Adv-GFP或Adv-LASS2-GFP重组腺病毒感染8505c细胞,细胞分为阴性对照(NC)组、空载体组(Adv-GFP组)和实验组(Adv-LASS2-GFP组)。感染48 h后提取各组细胞总RNA和蛋白,采用RT-qPCR和Western blot分别检测LASS2 mRNA和蛋白表达水平的变化;CCK-8和集落形成实验评价各组细胞增殖能力的差异;TUNEL染色评估各组细胞凋亡水平;透射电子显微镜观察细胞超微结构及自噬状态;Western blot检测各组细胞凋亡及自噬相关蛋白[细胞色素C(Cyto-C)、Bcl-2、Bax、p62、LC3-I、LC3-II和beclin-1]的表达水平;免疫共沉淀(Co-IP)联合液相色谱-质谱(LC-MS)鉴定与LASS2相互作用的蛋白,结合基因本体论(GO)数据库等分析并经Co-IP进一步验证互作关系。与NC组和Adv-GFP组相比,Adv-LASS2-GFP组LASS2 mRNA和蛋白表达水平均显著上调(<0.01),且随时间延长Adv-LASS2-GFP组细胞增殖能力显著被抑制,集落形成能力降低,细胞凋亡增加(<0.01);透射电镜结果显示,与NC组和Adv-GFP组相比,Adv-LASS2-GFP组细胞可见双层膜结构的自噬小体及大量自噬溶酶体;Western blot结果显示,Adv-LASS2-GFP组Bcl-2和p62蛋白表达水平较NC组和Adv-GFP组均显著下调(<0.01),Bax、Cyto-C和beclin-1表达及LC3-II/LC3-I比值均显著上调(<0.01);Co-IP/LC-MS鉴定及GO等富集分析筛选出参与调控自噬信号通路且与LASS2相互作用的4个蛋白,分别为p62、HSC70、HSP90和CSNK2A1。Co-IP结果显示LASS2与p62不存在直接相互作用。LASS2能促进8505c细胞凋亡并激活自噬,其作用机制可能是通过与p62/HSC70/HSP90/CSNK2A1相互作用而影响自噬和凋亡级联信号通路。
LAG1长寿保障同系物2;甲状腺未分化癌;自噬;细胞凋亡
甲状腺未分化癌(anaplastic thyroid carcinoma, ATC)是甲状腺癌(thyroid carcinoma, TC)中一种罕见的、高侵袭性的恶性肿瘤,尽管其发病率仅占1%~3%,但其中位生存期低于6个月,其死亡病例占所有TC中死亡病例的14%~39%[1]。由于其无放射性碘摄取、促甲状腺激素合成及甲状腺球蛋白合成的生物学功能,目前尚无标准的治疗方案[2],常规治疗收效甚微。因此,关注ATC相关分子生物学如相关癌基因、抑癌基因等分子机制的深入研究有望为ATC的靶向治疗提供潜在治疗靶点。
研究表明,LAG1长寿保障同系物2(LAG1 longevity assurance homolog 2, LASS2)对肝癌[3]、乳腺癌[4]、膀胱癌[5-6]、前列腺癌[7]等恶性肿瘤的浸润和生长具有抑制作用,因此又被称为肿瘤转移抑制基因1(tumor metastasis suppressor gene-1, TMSG-1);它主要调节长酰基链神经酰胺合成,而神经酰胺作为脂质第二信使,参与细胞生长抑制、分化和凋亡等过程。另有研究报道,LASS2能促进神经酰胺合成诱导肺上皮细胞自噬[8]。自噬是溶酶体介导降解细胞内受损细胞器、未折叠蛋白质等细胞代谢产物的生物进程[9],与凋亡之间能被多种应激刺激共同激活、共享多个调节因子,甚至互相协调转换[10],靶向凋亡和/或毒性自噬可促进临床前治疗选择的发展[11]。本课题组前期研究表明,与癌旁正常组织和结节性甲状腺肿组织相比,LASS2在乳头状甲状腺癌(papillary thyroid carcinoma, PTC)中表达降低,并与PTC的临床分期有关,过表达LASS2可抑制人PTC细胞系BCPAP的增殖,促进凋亡并导致G0/G1期阻滞[12]。然而,TC有多种病理分型,LASS2在其它病理分型尤其是ATC中对凋亡和自噬的作用及可能机制尚未见报道。因此,本项工作以人ATC细胞系8505c作为研究对象,体外观察LASS2对8505c细胞增殖、凋亡和自噬的影响,并探讨其相关分子机制,为ATC的分子靶向治疗提供参考资料。
材料和方法
1 细胞
8505c细胞购自湖南丰晖生物科技有限公司,采用含10%胎牛血清(fetal calfserum, FBS)的DMEM培养液,置于37 ℃、5% CO2孵箱中培养,液氮保存。
2 主要试剂与仪器
Adv-GFP和Adv-LASS2-GFP重组腺病毒购自北京百奥川生物科技有限责任公司;DMEM培养液和FBS购自Gibco;Trizol和RT-qPCR试剂购自TaKaRa;鼠抗β-actin单克隆抗体、兔抗Bcl-2单克隆抗体、兔抗Bax单克隆抗体、兔抗细胞色素C(cytochrome-C,Cyto-C)单克隆抗体和兔抗p62单克隆抗体均购自杭州华安生物技术有限公司;兔抗beclin-1单克隆抗体和兔抗LC3(LC3-I和LC3-II)单克隆抗体均购自CST;鼠抗LASS2单克隆抗体购自Santa Cruz;全蛋白提取试剂盒购自江苏凯基生物技术有限公司;CCK-8试剂购自北京索莱宝科技有限公司;BCA蛋白定量试剂盒和免洗考马斯亮蓝染液购自上海雅酶生物科技公司;IP裂解/洗涤缓冲液购自Thermo Fisher;GFP纳米抗体偶联琼脂糖珠购自成都阿帕克生物科技有限公司;TUNEL细胞凋亡染色试剂盒购自上海碧云天生物有限公司。HITACHI 7800透射电子显微镜购自HITACHI;CFX96 Real-Time PCR仪和ChemiDoc MP凝胶成像系统均购自Bio-Rad。
3 主要方法
3.1细胞感染及实验分组细胞传至第4代且处于对数生长期时,用Adv-GFP和Adv-LASS2-GFP分别感染8505c细胞,分成阴性对照(negative control, NC)组、空载体组(Adv-GFP组)和实验组(Adv-LASS2-GFP组),感染48 h后按各实验目的进行收样。所有实验重复3次。
3.2RT-qPCR检测LASS2 mRNA表达情况感染48 h后,提取各组细胞总RNA,测定RNA浓度与纯度,并逆转录合成cDNA,储存于-80 ℃ 冰箱中备用。以β-actin作为内参照,RT-qPCR检测分析LASS2 mRNA相对表达水平,扩增体系25 μL,反应程序为:95 ℃ 30 s;95 ℃ 5 s,60 ℃ 34 s,共40个循环;熔解曲线:95 ℃ 15 s,60 ℃ 60 s,95 ℃ 15 s。以2-ΔΔCt值表示基因相对表达量。β-actin的上游引物序列为5'-TCCTGTGGCATCCACGAAACT-3',下游引物序列为5'-GAAGCATTTGCGGTGGACGAT-3';LASS2的上游引物序列为5'-ATCGTCTTC GCCATTGTT-3',下游引物序列为5'-CGGTCACTGCGTTCATCT-3'。
3.3CCK-8法检测细胞增殖能力收集各组8505c细胞,调整浓度至2.5×107/L,以每孔100 μL的量接种至96孔板中,37 ℃、5% CO2培养过夜,次日感染Adv-GFP或Adv-LASS2-GFP,继续培养24、48和72 h,各时间点弃旧培养液,加入100 μL DMEM及10 μL CCK-8试剂,37 ℃孵育2 h后,在酶标仪450 nm处测定各组值并制作生长曲线[13]。
3.4TUNEL染色检测细胞凋亡严格按照TUNEL凋亡检测试剂盒说明书进行操作。收集细胞,PBS洗1次,4%多聚甲醛固定细胞30 min后,制备细胞涂片。0.3% PBS稀释的Triton X-100室温孵育样品5 min透化,PBS洗3次,每次5 min。在样品上分别加入适当浓度的TUNEL检测液37 ℃避光孵育60 min,PBS洗3次,每次5 min,封片,荧光显微镜下观察8505c细胞形态变化和TUNEL阳性颗粒[14]。采用ImageJ软件计数阳性细胞数并统计分析。
3.5Western blot法检测各组细胞蛋白的相对表达水平感染48 h后,按照全蛋白提取试剂盒说明书提取各组细胞总蛋白,BCA法定量蛋白浓度,经蛋白变性、分装并暂存于-80 ℃。每个泳道上样量为20 µg,10%或12.5% SDS-PAGE分离后转移至PVDF膜,4 ℃湿转、封闭、洗膜、Ⅰ抗(鼠抗LASS2单克隆抗体稀释倍数1∶500,鼠抗β-actin单克隆抗体稀释倍数1∶5 000,兔抗Bcl-2、Bax、p62、beclin-1、LC3和GFP单克隆抗体稀释倍数1∶1 000),4 ℃孵育过夜、洗膜、Ⅱ抗(羊抗鼠、羊抗兔Ⅱ抗稀释倍数均为1∶5 000)室温孵育2 h,洗膜、成像,Gel-Pro软件测量条带灰度值并统计数据,计算LASS2、Bcl-2、Bax、p62、beclin-1、LC3和Cyto-C蛋白相对表达量。
3.6透射电镜观察细胞超微结构变化感染48 h后,去除培养液、用灭菌PBS轻柔漂洗细胞2次,以500×离心3 min收集各组细胞,分别加入2.5%戊二醛4 ℃固定、丙酮逐级脱水、包埋、切片及枸橼酸铅染色后,透射电镜下观察细胞超微结构变化并拍片[15]。
3.7Co-IP/LC-MS鉴定与LASS2互作的蛋白及互作网络的建立与验证将各组8505c细胞用预冷的IP裂解/洗涤缓冲液裂解5 min、离心、取上清(总蛋白)并采用BCA法测定蛋白浓度。将25 μL已平衡的GFP纳米抗体偶联琼脂糖珠加入1 mg总蛋白中,4 ℃旋转孵育60 min、4 ℃离心弃上清、预冷的IP裂解/洗涤缓冲液重悬、离心弃上清、加入2×SDS-PAGE蛋白上样缓冲液重悬、95 ℃煮沸10 min,再次离心、沉淀GFP纳米抗体偶联琼脂糖珠并吸取上清(即为与LASS2互作的免疫复合物)。制胶、上样、用SDS聚丙烯酰胺凝胶电泳分离复合物,清洗去除杂质、加入免洗脱考马斯亮蓝染液染色并清洗、观察并拍照;将上述制备好的胶条经蛋白酶解、高效液相色谱-串联质谱(LC-MS)检测并鉴定与LASS2互作的免疫复合物,并行GO分析、直系同源蛋白数据库(cluster of orthologous group, COG)、真核生物同源蛋白簇(eukaryotic orthologous groups, KOG)功能注释、信号通路(pathway)功能注释及STRING筛选、建立与LASS2互作自噬相关的关键蛋白网络,采用Co-IP首先验证该网络中p62与LASS2互作关系。
4 统计学处理
采用SPSS 22.0软件进行实验数据统计分析, Graph Pad Prism 8.0作图。结果以均数±标准差(mean±SD)表示。组间差异比较采用单因素方差分析。以<0.05为差异有统计学意义。
结果
1 LASS2在8505c细胞中过表达
Adv-LASS2-GFP组LASS2 mRNA和蛋白相对表达水平分别较NC组和Adv-GFP组均显著上调(<0.01),NC组与Adv-GFP组未见显著差异(0.05),见图1。

Figure 1. Validation of LASS2 overexpression in 8505c cells. A: overexpression of LASS2 showed green fluorescence (scale bar=200 μm); B: relative mRNA expression of LASS2 in 8505c cells assessed by RT-qPCR; C: the protein expression of LASS2 in 8505c cells assessed by Western blot. Mean±SD. n=3. **P<0.01 vs Adv-GFP group.
2 过表达LASS2对8505c细胞增殖及集落形成能力的影响
CCK-8结果显示,与NC组和Adv-GFP组相比,Adv-LASS2-GFP组在转染48和72 h后,过表达的LASS2显著抑制8505c细胞增殖(<0.01),而NC组与Adv-GFP组之间无显著差异(>0.05),见图2A。集落形成实验结果表明,Adv-LASS2-GFP组集落形成率较NC组和Adv-GFP组显著降低(<0.01),见图2B。

Figure 2. Effect of LASS2 overexpression on the proliferation and colony formation of 8505c cells. A: the viability of 8505c cells detected by CCK-8 assay; B: the colony formation assay. Mean±SD. n=3. **P<0.01 vs Adv-GFP group.
3 过表达LASS2对8505c细胞凋亡能力的影响
使用TUNEL染色标记凋亡细胞,TUNEL标记中红色荧光为凋亡阳性细胞。结果显示,与NC组和Adv-GFP组相比,Adv-LASS2-GFP组细胞红色荧光显著增多(<0.01),见图3。
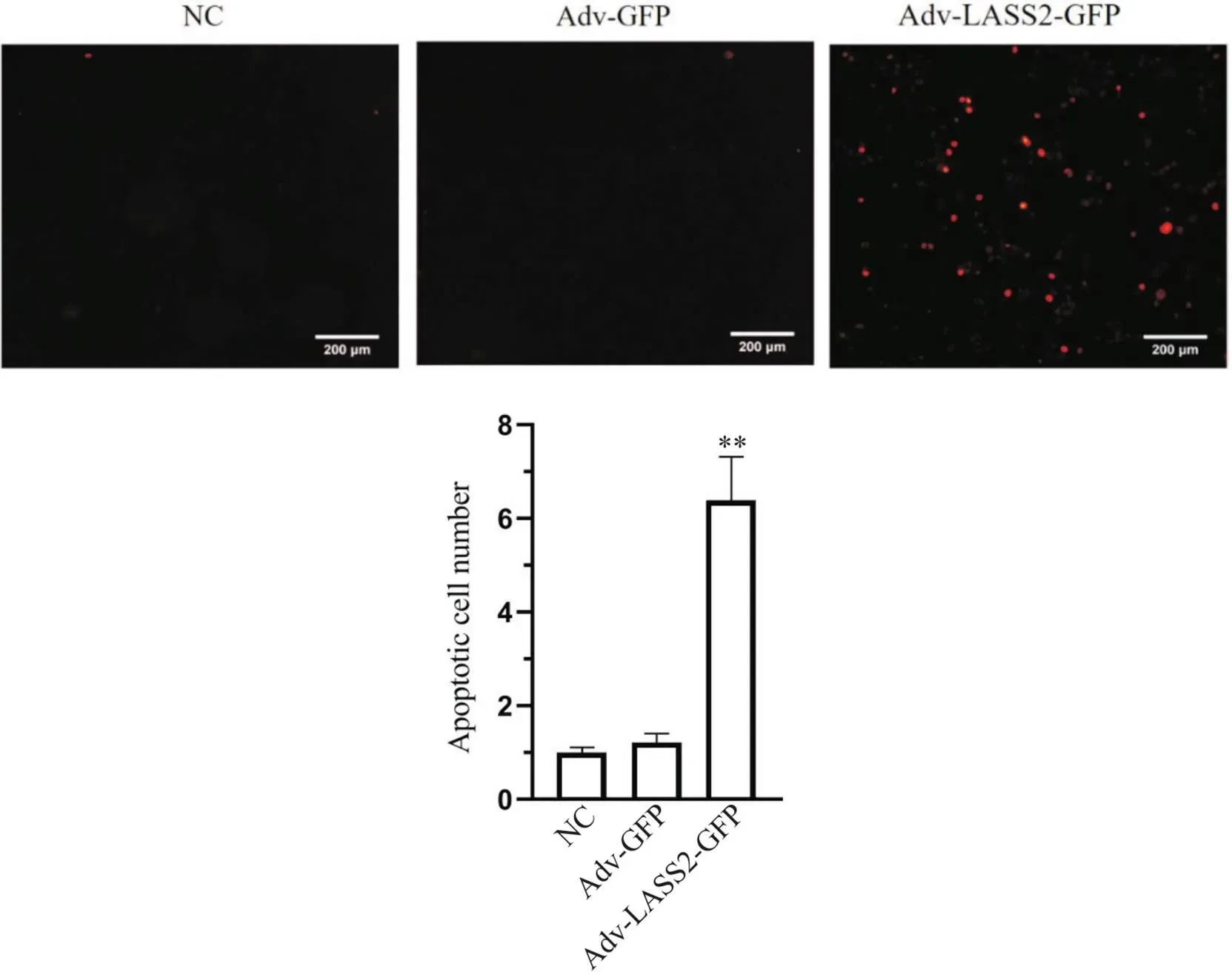
Figure 3. Effect of LASS2 overexpression on the apoptosis of 8505c cells detected by TUNEL staining (scale bar=200 μm). Mean±SD. n=3. **P<0.01 vs Adv-GFP group.
4 过表达LASS2对8505c细胞中凋亡相关蛋白表达的影响
Western blot结果显示,与NC组和Adv-GFP组相比,Adv-LASS2-GFP组Bcl-2蛋白表达显著下调,Bax和Cyto-C蛋白表达显著增加(<0.01),而NC组与Adv-GFP组之间无显著差异(>0.05),见图4。

Figure 4. Effect of LASS2 overexpression on the expression of apoptosis-related proteins Cyto-C,Bax and Bcl-2 in 8505c cells. Mean±SD. n=3. **P<0.01 vs Adv-GFPgroup.
5 透射电镜观察过表达LASS2对8505c细胞自噬相关结构的影响
透射电镜结果显示,与NC组和Adv-GFP组比较,Adv-LASS2-GFP组可见具有双层膜结构的自噬小体及自噬溶酶体结构,见图5。

Figure 5. Effect of LASS2 overexpression on the autophagy of 8505c cells was observed under transmission electron microscope. Scale bar=1 μm.
6 过表达LASS2对8505c细胞自噬相关蛋白beclin-1、LC3和p62表达的影响
Western blot结果显示,Adv-LASS2-GFP组p62蛋白表达水平显著低于NC和Adv-GFP组(<0.01),而beclin-1蛋白表达水平和LC3-II/LC3-I比值相对NC和Adv-GFP组显著上调(<0.01),见图6。
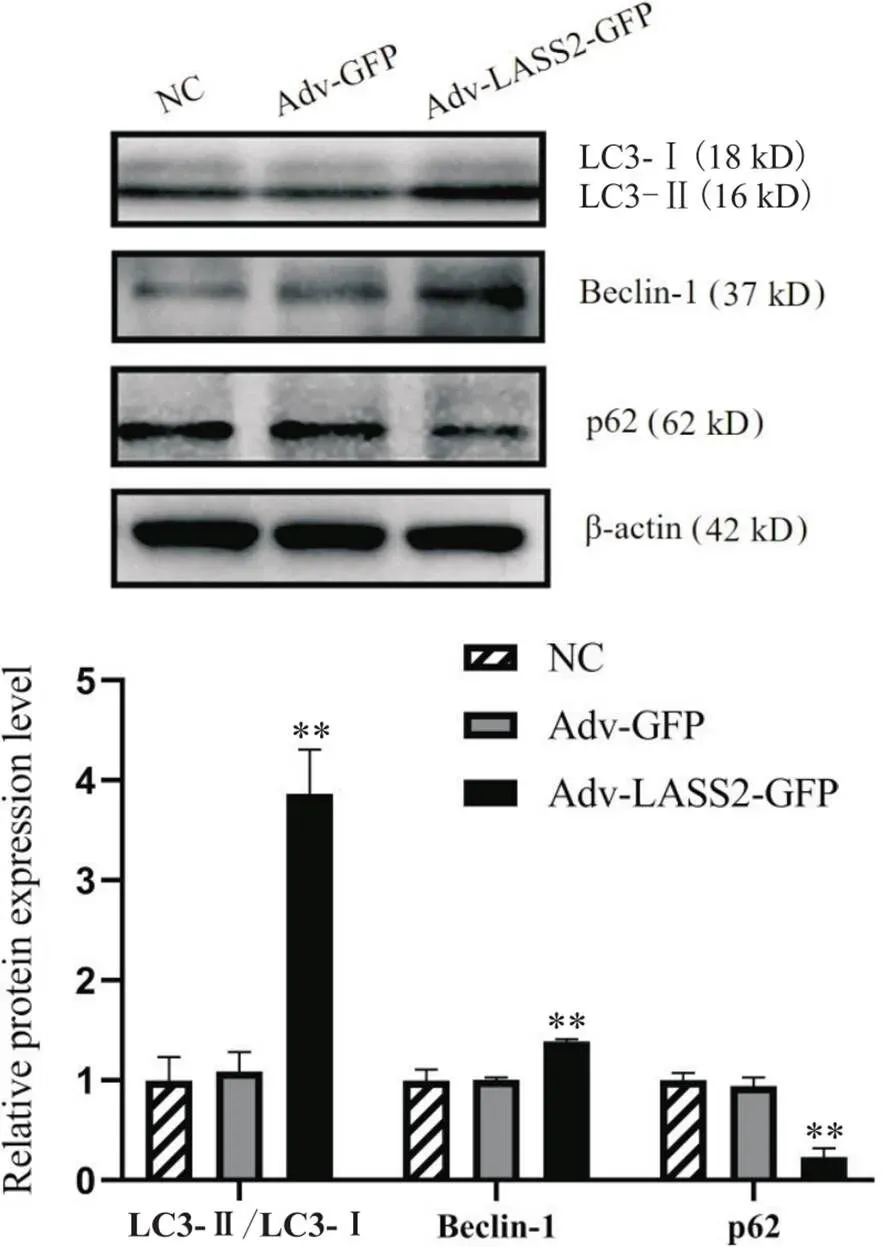
Figure 6. Effect of LASS2 overexpression on the protein expression of LC3, beclin-1 and p62 in 8505c cells. Mean±SD. n=3. **P<0.01 vs Adv-GFP group.
7 LASS2通过与p62/HSC70/HSP90/CSNK2A1互作而激活自噬
为了进一步探讨过表达的LASS2对8505c细胞自噬和凋亡作用的相关机制,我们采用Co-IP/LC-MS鉴定与LASS2互作的蛋白并通过GO富集分析、STRING等,筛选与LASS2互作的凋亡和自噬相关关键蛋白并建立互作网络图。结果提示,LASS2与p62/HSC70 (heat shock cognate protein 70)/HSP90 (heat shock protein 90)/CSNK2A1 (casein kinase 2 alpha 1)存在直接或间接互作关系。Co-IP进一步验证LASS2与p62是否存在互作关系,实验结果表明LASS2与p62之间不存在直接互作关系。见图7。

Figure 7. Isolation of LASS2 protein complex and validation of interaction between LASS2 and p62. A: LASS2 protein complex was isolated by Co-IP SDS-PAGE and stained with Coomassie bright blue; B: the interaction network of LASS2 was constructed by STRING software based on the identification results of Co-IP/LC-MS (SQSTM1: p62; HSPA8: HSC70; HSP90AA1: HSP90); C: the interaction between LASS2 and p62 was verified by Co-IP.
讨论
治疗或干预后肿瘤细胞存活或死亡的分子机制是复杂的,细胞死亡过程大致分为细胞凋亡、自噬性细胞死亡和坏死,其中细胞凋亡可能是最广泛的特征形式程序性或调节性细胞死亡[16],诱导肿瘤细胞凋亡是肿瘤防治的手段之一。细胞凋亡是多基因精确调控、由经典的内源性和外源性凋亡途径执行,其中内源性途径是通过激活有毒的BH3结构域蛋白如Bax和(或)Bak来启动的,这些蛋白在线粒体外膜中形成孔从而导致线粒体功能障碍,伴随Cyto-C释放到胞质中[16]。Huang等[6]报道LASS2诱导膀胱癌细胞(J82、BIU87)线粒体融合,抑制线粒体分裂,降低线粒体膜电位。前期研究显示,过表达的LASS2降低HepG2细胞线粒体膜电位并引起胞内Ca2+超载,提示其可能通过调控线粒体功能抑制肝癌的发展[3]。因此,在本实验中检测了线粒体途径相关的内源性凋亡,结果显示LASS2过表达抑制8505c细胞活力和集落形成能力,细胞凋亡率及细胞凋亡标志蛋白Bax和Cyto-C表达水平升高,抗凋亡蛋白Bcl-2表达水平降低,这与本课题在PTC体外前期研究[12]的结果一致,提示LASS2诱导细胞凋亡是其抑制ATC的重要机制之一。
自噬是维持细胞稳态、进化上保守的重要分子途径,在肿瘤细胞中自噬发挥双重作用,取决于不同肿瘤的类型、阶段或遗传背景。一方面,作为细胞质量控制机制,防止肿瘤发生,尤其是在肿瘤发生的早期阶段,即自噬可作为一种肿瘤抑制因子。另一方面,肿瘤进展到晚期,自噬可作为肿瘤细胞存活的主要因素促进肿瘤的转移或作为一种细胞防御机制可能降低化疗药物的治疗效果。本研究结果显示,LASS2过表达促进8505c细胞自噬小体和大量自噬溶酶体形成,beclin-1表达水平和LC3-II/LC3-I比值升高,自噬底物蛋白p62表达水平降低,说明胞内自噬激活。
自噬作为一种程序性细胞死亡,与凋亡密切相关,参与肿瘤发生过程中的多个信号通路并与凋亡存在复杂的串扰。已有研究表明,beclin-1与Bcl-2蛋白相互作用可增强或破坏自噬和凋亡之间的串扰[16]。或自噬溶酶体释放的组织蛋白酶可以切割有毒的BH3结构域蛋白BID,从而将Bax和Bak从保护性BH3结构域蛋白如Bcl-2中置换出来[10]。因此,自噬和凋亡两者之间存在合作、促进和对抗交互作用关系。本实验结果显示,过表达LASS2促进8505c细胞凋亡及激活自噬,提示两者可能互为促进或合作关系、促进细胞程序性死亡。为进一步明确LASS2调控自噬、凋亡的分子机制,本研究通过Co-IP/LC-MS鉴定与LASS2的互作蛋白,率先揭示LASS2与p62/HSC70/HSP90/CSNK2A1存在直接或间接互作关系。鉴于p62在细胞凋亡、自噬和肿瘤发生等信号转导过程中发挥重要作用[17],本实验采用Co-IP首先验证LASS2与p62之间的互作关系,结果显示LASS2与p62不存在直接互作,可能存在中间蛋白介导两者互作。Matsumoto等[18]观察到CSNK2能直接磷酸化p62的Ser403,并促进蛋白泛素化的选择性自噬降解。Miyata等[19]表明,HSP90可诱导CSNK2聚集物解离,形成可溶的HSP90-CSNK2复合物,并显著增强CSNK2激酶活性。本实验仅检测了LASS2与p62的互作形式,有待更多、深入的研究去证明其中的互作关系。
综上所述,LASS2可显著诱导人ATC细胞系8505c凋亡和自噬,其作用机制可能是通过与p62/HSC70/HSP90/CSNK2A1互作而影响自噬和凋亡级联信号通路。
[1] Saini S, Tulla K, Maker AV, et al. Therapeutic advances in anaplastic thyroid cancer: a current perspective[J]. Mol Cancer, 2018, 17:154.
[2] Jiao C, Li L, Zhang P, et al. REGγ ablation impedes dedifferentiation of anaplastic thyroid carcinoma and accentuates radio-therapeutic response by regulating the Smad7-TGF-β pathway[J]. Cell Death Differ, 2020, 27(2):497-508.
[3] Yang Y, Yang X, Li L, et al. LASS2 inhibits proliferation and induces apoptosis in HepG2 cells by affecting mitochondrial dynamics, the cell cycle and the nuclear factor‑κB pathways[J]. Oncol Rep, 2019, 41(5):3005-3014.
[4] Fan SH, Wang YY, Wu ZY, et al. AGPAT9 suppresses cell growth, invasion and metastasis by counteracting acidic tumor microenvironment through KLF4/LASS2/V-ATPase signaling pathway in breast cancer[J]. Oncotarget, 2015, 6(21):18406-18417.
[5] Chen Y, Wang H, Xiong T, et al. The role of LASS2 in regulating bladder cancer cell tumorigenicity in a nude mouse model[J]. Oncol Lett, 2017, 14(5):5149-5156.
[6] Huang L, Luan T, Chen Y, et al. LASS2 regulates invasion and chemoresistance via ERK/Drp1 modulated mitochondrial dynamics in bladder cancer cells[J]. J Cancer, 2018, 9(6):1017-1024.
[7] Xu X, Liu B, Zou P, et al. Silencing of LASS2/TMSG1 enhances invasion and metastasis capacity of prostate cancer cell[J]. J Cell Biochem, 2014, 115(4):731-743.
[8] Mizumura K, Justice MJ, Schweitzer KS, et al. Sphingolipid regulation of lung epithelial cell mitophagy and necroptosis during cigarette smoke exposure[J]. FASEB J, 2018, 32:1880-1890.
[9] Wang Y, Xiong H, Liu D, et al. Autophagy inhibition specifically promotes epithelial-mesenchymal transition and invasion in RAS-mutated cancer cells[J]. Autophagy, 2019, 15(5):886-899.
[10] Booth LA, Roberts JL, Dent P. The role of cell signaling in the crosstalk between autophagy and apoptosis in the regulation of tumor cell survival in response to sorafenib and neratinib[J]. Semin Cancer Biol, 2020, 66:129-139.
[11] Emdad L, Bhoopathi P, Talukdar S, et al. Recent insights into apoptosis and toxic autophagy: the roles of MDA-7/IL-24, a multidimensional anti-cancer therapeutic[J]. Semin Cancer Biol, 2020, 66:140-154.
[12] Zeng F, Huang L, Cheng X, et al. Overexpression of LASS2 inhibits proliferation and causes G0/G1cell cycle arrest in papillary thyroid cancer[J]. Cancer Cell Int, 2018, 18:151.
[13] 张暑军, 李青青, 乔春林, 等. M1型巨噬细胞对小鼠膀胱癌MB49细胞活力、迁移、侵袭及凋亡的影响[J]. 中国病理生理杂志, 2022, 38(1):1-10.
Zhang SJ, Li QQ, Qiao CL, et al. Effects of M1 macrophages on the viability, migration, invasion and apoptosis of mouse bladder cancer MB49 cells[J]. Chin J Pathophysiol, 2022, 38(1):1-10.
[14] Zhang W, Xiong H, Pang J, et al. Nrf2 activation protects auditory hair cells from cisplatin-induced ototoxicity independent on mitochondrial ROS production[J]. Toxicol Lett, 2020, 331:1-10.
[15] 刘欢, 梁丽英, 刘显, 等. 白花丹醌对人结肠腺癌Caco-2细胞凋亡、自噬及PI3K/Akt/mTOR信号通路的影响[J]. 中国病理生理杂志, 2021, 37(2):255-262.
Liu H, Liang LY, Liu X,et al. Effects of plumbagin on apoptosis, autophagy and PI3K/Akt/mTOR signaling pathway in human colon adenocarcinoma Caco-2 cells[J].Chin J Pathophysiol, 2021, 37(2):255-262.
[16] Fairlie WD, Tran S, Lee EF. Crosstalk between apoptosis and autophagy signaling pathways[J]. Int Rev Cell Mol Biol, 2020, 352:115-158.
[17] Islam MA, Sooro MA, Zhang P. Autophagic regulation of p62 is critical for cancer therapy[J]. Int J Mol Sci, 2018, 19(5):1405.
[18] Matsumoto G, Wada K, Okuno M, et al. Serine 403 phosphorylation of p62/SQSTM1 regulates selective autophagic clearance of ubiquitinated proteins[J]. Mol Cell, 2011, 44:279-289.
[19] Miyata Y, Yahara I. The 90-kDa heat shock protein, HSP90, binds and protects casein kinase II from self-aggregation and enhances its kinase activity[J]. J Biol Chem, 1992, 267:7042-7047.
LASS2 mediates autophagy and apoptosis of anaplastic thyroid carcinoma 8505c cells and its related mechanisms
ZHAO Qing-qing1, YU Yun-yan2, WANG Yun1, HE Wei3, YANG Yan3,4, CHEN Rui1, YANG Xiao-li3,4△, CHENG Xiao-ming1△
(1,,2,,3,,4,,563000,)
To investigate the effects of LAG1 longevity assurance homolog 2 (LASS2) on the apoptosis and autophagy of anaplastic thyroid carcinoma 8505c cells and its possible mechanisms.The 8505c cells were divided into negative control (NC) group, Adv-GFP group and Adv-LASS2-GFP group, which were not infected or infected with recombinant adenoviruses Adv-GFP or Adv-LASS2-GFP. Total RNA and protein of each group were extracted 48 h after infection. The relative LASS2 mRNA and protein expression levels were determined by RT-qPCR and Western blot, the proliferation of the cells was examined by CCK-8 and colony formation assays, and the apoptosis of the cells was examined by TUNEL staining. Cell ultrastructure and autophagy state were observed by transmission electron microscopy. The expression of apoptosis- and autophagy-related proteins, cytochrome-C (Cyto-C), Bcl-2, Bax, p62, LC3-I, LC3-II and beclin-1, was determined by Western blot. Co-immunoprecipitation (Co-IP) combined with liquid chromatography-mass spectrometry (LC-MS) was used to identify the proteins interacting with LASS2. The proteins were analyzed by Gene Ontology (GO) database and further verified by Co-IP.Compared with NC and Adv-GFP groups, the relative mRNA and protein expression levels of LASS2 in Adv-LASS2-GFP group were significantly up-regulated (<0.01), the cell apoptosis was significantly promoted (<0.01), and the proliferation and colony formation abilities were significantly decreased (<0.01). Transmission electron microscope results showed that double-membrane autophagosomes and a large number of autolysosomes were found in Adv-LASS2-GFP group compared with NC and Adv-GFP groups. The results of Western blot showed that, compared with NC and Adv-GFP groups, the expression level of Bcl-2 and p62 was significantly down-regulated, while the expression of Bax, Cyto-C and beclin-1, and the ratio of LC3-II/LC3-I were up-regulated in Adv-LASS2-GFP group (<0.01). Co-IP/LC-MS identification and GO enrichment analysis screened 4 proteins involved in regulating autophagy signaling pathway and interacting with LASS2, including p62, HSC70, HSP90 and CSNK2A1. Co-IP showed that LASS2 did not directly interact with p62.LASS2 promotes apoptosis and autophagy of 8505c cells possibly through p62/HSC70/HSP90/CSNK2A1 signaling pathway.
LAG1 longevity assurance homolog 2; Anaplastic thyroid carcinoma; Autophagy; Apoptosis
1000-4718(2022)09-1577-08
2022-04-13 [
2022-08-01
杨小理 Tel: 0851-28608154; E-mail: xiaoliyang1216zy@163.com; 程晓明 Tel: 0851-28608980; E-mail: cxm1688@ sina.com
R736.1; R363.2+1
A
10.3969/j.issn.1000-4718.2022.09.006
[基金项目]国家自然科学基金资助项目(No. 81960494);贵州省卫健委科技项目(No. gzwjkj2019-1-192);遵义市科技计划课题项目[遵市科合HZ字(2019)68号]
(责任编辑:余小慧,李淑媛)

