High-performance magnesium-based thermoelectric materials:Progress and challenges
Zizhen Zhou,Gung Hn,Xu Lu,Guoyu Wng,Xioyun Zhou,b,d,*
a Center of Quantum Materials and Devices,College of Physics,Chongqing University,Chongqing 401331,PR China
b National Engineering Research Center for Magnesium Alloys,Chongqing University,Chongqing 400044,PR China
c Chongqing Institute of Green and Intelligent Technology,Chinese Academy of Sciences,Chongqing 400714,PR China
d Analytical and Testing Center,Chongqing University,Chongqing 401331,PR China
Abstract Magnesium-based materials have been regarded as promising candidates for large-scale,high-efficiency thermoelectric applications,owing to their excellent dimensionless figure of merit,high abundance,and low cost.In this review,we comprehensively summarize the recent advances of Mg-based thermoelectrics,including Mg2X(X=Si,Ge,Sn),Mg3(Sb,Bi)2,and α-MgAgSb,from both material and device level.Their electrical and thermal transport properties are first elucidated based on the crystallographic characteristics,band structures,and phonon dispersions.We then review the optimization strategies towards higher thermoelectric performance,as well as the device applications of Mg-based thermoelectric materials and the related engineering issues.By highlighting the challenges and possible solutions in the end,this review intends to offer perspectives on the future research work to further enhance the performance of Mg-based materials for practical applications.
Keywords:Thermoelectric;Mg-based materials;Mg2(Si,Ge,Sn);Mg3Sb2;MgAgSb;Thermoelectric device.
1.Introduction
Owing to the global environmental degradation and increased energy consumption,there is a growing demand for clean and sustainable energy conversion technologies.Solidstate thermoelectric(TE)technology,which enables direct and reversible energy conversion between heat and electricity with long life and reliability,provides a fascinating opportunity for the applications in the areas of waste heat recovery and refrigeration[1–3].The crucial challenge of the practical applications for TE conversion technology is to improve the TE performance of materials,which can be gauged by the dimensionless figure of meritzT=S2σT/(κe+κl).HereT,S,σ,κe,andκlare the absolute temperature,Seebeck coefficient,electrical conductivity,electronic thermal conductivity,and lattice thermal conductivity,respectively.It is always challenging to realize high TE performance owing to the strong coupling of the governing electrical and thermal transport parameters.During the past decades,substantial optimization strategies have been proposed to improve thezTvalues of state-of-theart TE materials,such as chemical doping[4–6],electron energy filtering[7,8],quantum confinement effects[9–11],and band/phonon engineering[12–16].Alternatively,a number of novel material systems,including SnSe/SnS[17–20],Cu2Se[21–23],half-Heusler[24–26],skutterudites[27,28],and Zintl compounds[29–31],were explored with promising TE performance.
From the practical application aspects,TE materials should possess high performance and excellent stability at their operational temperature.Current TE materials in commercial practice are mainly Bi2Te3-and PbTe-based compounds[32,33],whereas the scarce or toxic constituent elements pose a potential impediment for their large-scope applications.In comparison,magnesium-based compounds,which commonly consist of Earth-abundant and eco-friendly elements,have attracted intensive interest for potential applications in power generation and refrigeration.In addition,the automotive industry requires TE generators to be as light as possible,which also makes Mg-based compounds with low mass density(e.g.,Mg2Si with a density of~2 g cm-3)to be advantageous over common TE materials[34].The exploration of Mg-based TE materials originated from the discovery of Mg2Si in 1960s[35],which possesses an indirect band gap(Eg)of~0.8 eV and multiple conduction valleys.By alloying with Ge and Sn,peakzTs above 1.0 can be achieved inn-type Mg2Si1-xGexand Mg2Si1-xSnxsolid solutions owing to the band convergence and enhanced phonon scattering by point defects[36–38].Meanwhile,the TE transport properties of another Mgbased compound,Mg3(Sb,Bi)2,were investigated beginning in 2000s[39].Because of the single valence valley with low carrier mobility at Brillouin center,the power factor(S2σ)of hole-doped Mg3Sb2is poor,making it not a goodp-type TE material despite the low lattice thermal conductivity[40–44].In comparison,n-type Mg3Sb2was found to exhibit very promising TE performance due to the larger band degeneracy and much higher carrier mobility[45–47].A peakzTof 1.5 can be achieved inn-type Mg3Sb2-based materials at around 700 K via chemical doping,adding slightly excess Mg,and microstructure engineering[48–52].By alloying with Bi to decrease the band gap,the Mg3Sb2-xBixexhibits excellent near-room-temperature TE performance,making it a promising alternative for solid-state cooling application[53,54].It should be mentioned that Mg3Sb2-xBixcan only make up then-type part of a TE module owing to its poorp-type performance.Fortunately,thep-type component may be replaced by another Mg-based material,α-MgAgSb[55].The TE properties ofp-typeα-MgAgSb were first reported by Kirkham et al.in 2012,which shows the phonon glass electron crystal behavior and exhibits a desirablezTof 0.56 at 500 K[56].Subsequently,a peakzTvalue above 1.0 is achieved through improving the phase purity of hole-doped MgAgSb sample[57–59].Moreover,it possesses an exceptional averagezTover the operation temperature coupled with desirable mechanical properties[60–65],and an excellent TE conversion efficiency of 8.5% is experimentally achieved with a single leg at a temperature difference of 225 K[66].Fig.1 plots the summarizedzTvalues of bothp-andn-type Mg-based materials,demonstrating that the performance of Mg-based thermoelectrics is competitive with that of most typical TE materials.

Fig.1.Dimensionless figures of merit of representative Mg-based TE materials[36,37,48,49,57,66]compared with those of state-of-the-art TE materials[17–19,23–25,32].
Aside from above three types of Mg-based compounds,Mg mainly exists as a doping element in TE materials[67,68].In this review,we summarize the recent progress and perspectives of Mg-based TE materials.We first focus on the structural,electronic,and phonon properties of the three types of Mg-based materials to understand their intrinsic electrical and thermal transport properties.Then,several optimization strategies are introduced to enhance their TE performance,which are considered to be feasible for many other TE materials.Next,we highlight the fabricated TE modules and measured conversion efficiency,which is a vital step towards practical applications.Finally,we shed light on the challenges and perspectives for promoting the development of Mg-based materials for future TE applications.
2.Typical Mg-based thermoelectric materials
2.1.Mg2X(X=Si,Ge,Sn)
Mg2Si crystallizes in an antifluorite structure with the space group ofFm3m(No.225),where four Si atoms occupy the face-centered and vertex sites while eight Mg atoms fill in the center of Si tetrahedrons in a unit cell,as shown in Fig.2a.Mg and Si atoms bond with covalent character,which is implied by the electron localization function isosurfaces[69].Besides,excess Mg can exist at the octahedral interstitial site of Mg2Si[70,71].The Si site can be alloyed by Ge/Sn with varying extent of solubility,forming Mg2(Ge,Sn)xSi1-xsolid solutions,which are the most intensively investigated Mg2Xbased systems owing to their highzTvalues and low cost[72–74].According to the Vegard’s law[75],the lattice parameters(a)of the cubic-structured solid solutions vary based on the fraction of Mg2X(X=Si,Ge,Sn)(refer to Table 1 for theavalues of Mg2X)[76–78].

Table 1The lattice parameters,band gaps,and phononic properties of Mg2X(X=Si,Ge,Sn)[76–78].

Fig.2.(a)Crystal structure model of Mg2X(X=Si,Ge,Sn)[71].(b)Schematic illustration on the band structures of Mg2X in the vicinity ofΓand X points[72].(c)The orbital decomposed band structures of Mg2Si[73].(d)The room-temperature lattice thermal conductivity of Mg2(Si,Ge,Sn)solid solutions.Panel(c)is reproduced with permission from Ref.[73].
Because all the Mg2X(X=Si,Ge,Sn)compounds possess identical crystal structure and Brillouin zone,their electronic band structures are similar and the band edges can be described around high-symmetry pointsΓand X.As illustrated in Fig.2b,the valence band maximum(VBM)locates atΓpoint,which consists of two degenerated bands labeled as light-hole(LH)and heavy-hole(HH).Below the VBM,a split-off(SO)band is observed with the energy gap(ESO)ranging from 0.03 to 0.5 eV for the X atoms varying from Si to Sn[72].The conduction band minimum(CBM)locates at X point indicating the indirect band gap.Specifically,the experimentalEgvalues of Mg2Si,Mg2Ge,and Mg2Sn are 0.78,0.60,and 0.33 eV,respectively[79–81].In addition,the CBM at X point is composed of two conduction bands labeled as C1and C2(Fig.2b),respectively.Fig.2c plots the orbital decomposed band structures of Mg2Si,where the band edges at X points are contributed by Mg_sand Si_porbitals.By alloying Sn in Mg2(Si,Ge),the energy gaps between C1and C2can be reduced,and the positions of C1and C2are even inverted in pure Mg2Sn.Combined with the lattice symmetry,a high valley degeneracy of 6 for the conduction band can be achieved in Mg2Si1-xSnx(x≈0.65)and Mg2Ge1-ySny(y≈0.65)solid solutions,leading to exceptionaln-type TE performance[15,82].Despite the larger band degeneracy,the density of state(DOS)effective mass of CBM is still lower than that of VBM owing to presence of the heavy-hole valence bands.
Another important factor in determining TE performance is the phonon-related property,which dominates the lattice thermal conductivity in Mg2Si-based compounds.Low lattice thermal conductivityκlis vital for high TE performance and inherently stems from low phonon group velocity(vp)and large Grüneissen parameter(γ).Pure Mg2X compounds have been found to exhibit relatively high lattice thermal conductivity with a room temperature value of 6–8 W m-1K-1[83,84].As exhibited in Table 1,Mg2X shows higher phonon group velocity and smaller Grüneissen parameter compared with typical TE material Bi2Te3[85],due to the lower mass density and high symmetric lattice structure.To reduce the lattice thermal conductivity in Mg2Si-based compounds,one of the most common strategies is forming solid solutions.Fig.2d plots the room temperature lattice thermal conductivity of Mg2(Si,Ge,Sn)alloys[86].The results indicate that all the solid solutions exhibit much lowerκlthan those of pure systems,which should be attributed to the point defects-induced short-wavelength phonon scattering[36].Besides,first-principles study revealed that the reducedκlin Mg2Si1-xSnxis also related to the softened acoustic phonons[87].Therefore,Mg2Si1-xSnxpossesses the lowest lattice thermal conductivity among these solid solutions,for example,an ultralowκlof 1.4 W m-1K-1has been experimentally obtained in Mg2Si0.375Sn0.625at 300 K[37,88,89].Alternatively,alloying by high content of aliovalent Sb element in Mg2Si also significantly suppressed the lattice thermal conductivity due to the increased Mg vacancies and concomitant strains,which was even lower than that of conventional Mg2Si1-xSnxsolid solutions[90].Detailed microstructure observation revealed the presence of dense dislocations,where the strip-like defects were viewed with isotropic strains thus greatly enhancing the phonon scattering.Other strategies,such as constructing twin boundaries[69],were also theoretically proposed for further reducing the lattice thermal conductivity,which may contribute to the improvement of TE performance in Mg2Si-based materials.

Fig.3.(a)Crystal structure model of Mg3X2(X=Sb,Bi).(b)Band structures and electron Fermi surface of Mg3Sb2[46].(c)The experimental lattice thermal conductivity of both single-crystalline(SC)and polycrystalline(PC)Mg3Sb2 and Mg3Bi2[54,112–114].(d)Phonon dispersions of Mg3Sb2 with the Grüneissen parameter shown through the thickness of the color[115].
2.2.Mg3(Sb,Bi)2
Mg3Sb2and Mg3Bi2Zintl compounds crystallize in a trigonal CaAl2Si2-type structure with the space group ofP3m1 below 1203 K and 976 K,respectively[91].As can be seen from Fig.3a,the tetrahedrally coordinated(Mg2X2)2-(X=Sb,Bi)polyanions form into covalently bonded layers,while the octahedrally coordinated cation Mg2+layers ionically bond with the polyanions framework[92,93].The lattice parameters and the bond lengths between adjacent atoms of Mg3Sb2and Mg3Bi2are exhibited in Table 2[94].In the(Mg2X2)2-network,the vertical Mg-X bonds are usually longer than the three symmetry-equivalent tilted Mg-X bonds.The coexistence of covalent and ionic bonds gives rise to rich electrical and phonon transport properties,which en-ables promising TE performance in Mg3(Sb,Bi)2compounds[95,96].In addition,the two types of Mg atoms occupied in octahedral and tetrahedral sites allow the formation of different solid solutions in a larger compositional range for manipulating the electrical and thermal transport properties.

Table 2The structural characteristics of Mg3X2(X=Sb,Bi)[94],including the lattice parameters(a and c)as well as the lengths of Mg-X ionic bonds(d1),vertical Mg-X covalent bonds(d2),and three symmetry-equivalent tilted covalent Mg-X bonds(d3).
Mg3Sb2has been calculated to be an indirect semiconductor,while Mg3Bi2is predicted to be a semimetal[97–99].Based on different computational methods,the calculated band gap of Mg3Sb2ranges from 0.4 to 0.7 eV[100–103].By fitting the experimental data,the band gap was estimated to be~0.8 eV[104,105].The optical band gaps of Mg3Sb1.5Bi0.5and Mg3SbBi solid solutions were also estimated via the Fourier transform infrared spectroscopy measurement,which yielded a much lower value of 0.28 eV[106,107].More importantly,the angle-resolved photoemission spectroscopy(ARPES)measurements were carried out onn-type Mg3X2single crystals,which directly confirmed the semimetal feature of Mg3Bi2[108]and clearly observed a band gap above 0.6 eV in Mg3Sb2[109].Fig.3b plots the band structures of Mg3Sb2,where the VBM atΓpoint are dominated by thep-orbitals of Mg2+cations and Sb atoms.Unlike the triple degeneratedp-orbitals in cubic system protected by the point symmetry,thepz-orbital is separated frompx-topy-orbitals in Mg3X2caused by the crystal field effect,leading to a single valence valley atΓpoint.First-principles predictions revealed that this crystal filed splitting energy can be decreased by alloying Ca,Sr,Ba,or Eu at octahedral Mg site to increase the valence band convergence and improve the TE performance ofp-type Mg3Sb2[99,110].The CBM of Mg3Sb2locates away from the high-symmetry point,giving rise to a high band degeneracy of 6 for electron pockets.The larger band degeneracy of CBM also reflects the better TE performance forn-type Mg3Sb2.In addition,alloying Bi in Sb site was found to generate more dispersive CBM owing to the weakened Mg-Mg ionic bonds.Theoretical simulations based on first-principles calculations illustrated that the DOS effective mass of CBM decreased from 1.52me(Mg3Sb2)to 1.23me(Mg3SbBi)and 0.87me(Mg3Bi2)by increasing Bi content in Mg3X2[111].Combined with the reduced band gap,alloying Bi in Mg3Sb2will enhance the carrier mobility but suppress the Seebeck coefficient,suggesting that a balance Bi content is crucial for high TE performance.
The intrinsically low lattice thermal conductivity of Mg3(Sb,Bi)2is equally important for its promising TE performance.Fig.3c depicts the experimental lattice thermal conductivity of both single-crystalline and polycrystalline Mg3X2,revealing that the room-temperature values are smaller than 2 W m-1K-1[54,112–114].Despite the lower density and relatively simple crystal structure,such lowκlis even comparable to that of state-of-the-art TE material Bi2Te3,which has its origin of the softened acoustic branches and strong vibrational anharmonicity.Fig.3d shows the phonon dispersion relations of Mg3Sb2,where the thickness of the color lines indicates the degree of anharmonicity[115].Clearly,the low-frequency acoustic phonons exhibit strong anharmonicity.Based on the experimentalκland considering the three-phonon scattering process,the Grüneissen parameter of Mg3Sb2is estimated to be 1.83,which is larger than those of good TE materials with lowκl(e.g.,PbTe[116]),thus reflecting the strong anharmonicity.Another reason for the low lattice thermal conductivity of Mg3Sb2is the small average phonon group velocity of 2587 m s-1[115],and the values of longitudinal modes are almost identical along intralayer and interlayer directions[45].This observation is consistent with the nearly isotropic thermal conductivity despite the layered structure[117],which is further supported by the chemical bonding analysis[49].Nevertheless,Li et al.measured the lattice thermal conductivity ofp-type Mg3Sb2single crystal and observed slight anisotropy,which was attributed to the different averaged group velocities along intralayer and interlayer directions[118].More interestingly,it was found that replacing octahedral site Mg with heavier Ca/Yb led to a striking abnormal increase in the lattice thermal conductivity by a factor of 2–3[119]contrary to common mass-trend expectations.Combining extensive phonon measurements with calculations,Ding et al.uncovered that compared with the ternary analogs,the large phonon flattening and softening of the low-frequency transverse acoustic phonons in Mg3X2rooted from the unique Mg-X bonds,which markedly suppresses the group velocity,enlarges the scattering phase space,and in turn enables the lowκl[120].This anomalous phonon transport behavior also provides exotic insights for lowering the lattice thermal conductivity that is expected to obey the common reliance on heavy elements.
2.3.MgAgSb
As first reported in 2012[56],MgAgSb was found to exist in three different crystal structures:a room-temperature(300–560 K)αphase with space groupI4c2,a mediumtemperature(560–630 K)Cu2Sb-relatedβphase with space groupP4/nmm,and a half-Heuslerγphase with space groupF43mat high temperature(630–700 K).As shown in Fig.4a,all the three crystal structures exhibit the Mg-Sb pseudocubes filled with Ag atoms[56,63].γ-MgAgSb tends to possess a highly disordered structure with the mixed occupation of Mg,Ag,and vacancies,which will continuously decompose with the elevated temperature[121].The actual composition ofβ-MgAgSb usually largely deviates from the nominal stoichiometric ratio due to the high concentration of Ag and Sb vacancies,leading to a metallic transport behavior.α-MgAgSb possesses a complex lattice structure consisting of a distorted Mg-Sb rocksalt lattice rotated by 45° along thecaxis and Ag atoms inside the polyhedrons.The doubled Mg-Sb rocksalt lattice accommodates a mixed ordering of filled Ag atoms,forming one-dimensional chains in three different directions[122].Owing to the promising TE performance,most reports focused on the electrical and thermal transport properties ofα-MgAgSb as well as the physical origins.
α-MgAgSb is experimentally demonstrated to be a semiconductor with an estimated band gap of 0.16 eV[57].Based on the first-principles calculations,the band structures ofα-MgAgSb exhibit an indirect band gap of 0.1 eV by using the local density approximation exchange-correlation function[58,123].For the standard Perdew-Burke-Ernzerhof(PBE)functional in generalized gradient approximation,α-MgAgSb is predicted to be a semimetal with a band overlap between VBM along Z-A line and CBM atΓpoint[59,62,124,125].It is well known that the standard density functional theory(DFT)calculations usually underestimate the band gap seriously due to the presence of the derivative energy concerning the number of electrons[126].Indeed,by considering more accurate methods such as modified Becke-Johnson(MBJ)[127]and Heyd-Scuseria-Ernzerhof[128]functional,the calculated results show a band gap of~0.3 eV inα-MgAgSb[129–131].Fig.4b compares the PBE and MBJ computed band structures[131],where the MBJ method does not change the positions of band edges.The CBM locates atΓpoint,implying a single conduction valley in the whole Brillouin zone.In comparison,the VBM is found to locate along the Z-A high symmetry line pointing out a high band degeneracy of 8,which indicates the better TE performance ofp-typeα-MgAgSb.

Fig.4.(a)The crystal structure models of α-,β-,and γ-MgAgSb[56].(b)The electronic band structures[131],(c)phonon dispersions[136],and(d)Grüneisen parameter(γ)of α-MgAgSb along high symmetry lines[136].Panel(a)is reproduced with permission from Ref.[56].Panel(c)and(d)are reproduced with permission from Ref.[136].
Benefiting from the complex crystal structure and unique lattice dynamics properties,α-MgAgSb exhibits glass-like phonon transport behavior and low lattice thermal conductivity[132].The room temperatureκlofα-MgAgSb is in the range of 0.51~0.76 W m-1K-1,which has been well verified both theoretically and experimentally[62,133–138].With the elevated temperature,theκlcan be decreased to below 0.3 W m-1K-1.The ultralow lattice thermal conductivity has its physical origins from three aspects.First,α-MgAgSb possesses a complex crystal structure with 24 atoms in a primitive cell,indicating that there are 3 acoustic phonon branches and 69 optical branches in the phonon dispersions.Note that the optical phonons have negligible contributions to the lattice thermal transport in the bulk materials owing to the low group velocity.Such a fact implies that the heat energy transport inα-MgAgSb is greatly limited due to the low fraction of acoustic modes,thus leading to the low lattice thermal conductivity.Second,the deep non-interactive 4delectrons and the abnormal occupied sites of Ag atoms give rise to the weak Ag-Sb bonds[56,60,130],as demonstrated by the low elastic module of~50 GPa inα-MgAgSb[61,130].These bonding characteristics are closely related to the low phonon group velocity and strong lattice vibrational anharmonicity.Fig.4c,d plot the phonon dispersion relations and corresponding Grüneisen parameter along high symmetry lines,respectively[136].The cutoff frequency of acoustic phonons responsible for heat transport merely reaches~30 cm-1,determining an ultralow mean phonon velocity of 1276 m s-1and playing a pronounced role in the low lattice thermal conductivity.Besides,Fig.4d reveals highγof the longitudinal acoustic(LA)and optical(LO)modes,and the averaged values is as high as 2.0,suggesting the strong anharmonicity and thus high phonon scattering.Third,both the DFT calculations on formation energy and the Rietveld refinement in high-resolution synchrotron radiation power X-ray diffraction imply that the intrinsic Ag vacancies exist as the dominant point defects inα-MgAgSb owing to the weak Ag-Sb bonds[121,131,139].These point defects largely suppress the phonon mean free path(MFP)via effectively scattering the short-wavelength phonons,which therefore significantly decreases the lattice thermal conductivity as also observed in various materials[140–144].
3.Strategies for improving the performance of Mg-based thermoelectrics
3.1.Carrier concentration optimization
The coupling between the electrical and thermal transport properties makes it difficult to improve the TE performance,which are all directly affected by the carrier concentration.Moreover,the concentration of majority carriers plays essential roles in the electrical transport properties at high temperature at which bipolar effect may take effect.It is thus of vital importance to optimize the carrier concentration for maximal TE performance through doping matrix with acceptor or donor element.It should be noted that dopants with tiny contents usually have negligible effect on the intrinsic band structures.
Numerous investigations have reported the elemental dopants forp-andn-type TE performance in Mg2Si-based materials in terms of both theoretical prediction and experimental achievement.DFT calculations revealed that the optimal hole and electron concentration for maximumzTin Mg2Si are 5.3×1019cm-3and 3.7×1020cm-3,respectively,which are much higher than those of undoped samples[145].Experimentally,the most commonn-type dopants for Mg2Si-based compounds are Bi and Sb occupied in Si site[36,146–150],which contribute one external electron per substitution.Gao et al.prepared Sb-doped Mg2Si0.7Sn0.3materials,where the carrier concentration dramatically increased with the enhanced Sb content,resulting in improved power factors andzTvalues[151].In the following year,Liu et al.significantly enhanced the carrier concentration of undoped Mg2Si1-xSnxsolid solutions by two orders of magnitude through Bi doping[152].Bi doping was found to increase the carrier concentration,which also slightly suppressed the lattice thermal conductivity,thereby giving rise to a maximumzTof 0.87 at 630 K[152].Othern-type dopants such as La was also reported in Mg2Si-based compounds[153],whereas the doping efficiency was lower than those of Sb and Bi.For thep-type doped Mg2Si-based compounds,it has been argued that the rigid band picture is not suitable for all dopants.For example,Ag doping on Sn site is likely to form a resonant state in the band structure of Mg2Si1-xSnxaccording to the DFT calculations[154,155],whereas most of the experimental results showed no indicator for resonant state behavior,suggesting that Ag should occupy the Mg site[156–160].Using synchrotron and neutron diffraction,Prytuliak at al.studied the dopants substitution of different lattice sites of Mg2Si and surprisingly found that Ag occupies the Si site[161].This contradiction implies that the synthesis conditions(for example,the ratio of Mg and Si used for preparation)have deep effect on the preferential substitution site of Ag doping.By doping 5% Ag in melt-spun Mg2Si0.4Sn0.6,a peakzTof 0.45 can be obtained at 690 K with a carrier concentration of 6×1019cm-3[162].Li is another effectivep-type dopant for Mg2Si-based materials[163],which preferentially occupies Mg site according to the DFT calculations and experimental measurements[147,148,164].Zhang et al.achieved a high power factor of 15 μW cm-1K-2and an improvedzTof 0.5 at 750 K via Li doping inptype Mg2Si0.3Sn0.7[165].Most recently,Huang et al.prepared Na-doped(Mg2Sn0.9Si0.1)0.93(Na2S)0.07sample and realized a maximumzTof 0.52 at 673 K,representing one of the highestzTvalues forp-type Mg2(Si,Sn)[166].Otherp-type dopants such as K,Ga,and In can also tune the carrier concentration,while the obtained TE performance is poor owing to the relatively lower carrier concentration[167–169].To the best of our knowledge,Table 3 summarizes the reportedp-andn-type dopants utilized for the TE performance enhancement of Mg2Si-based materials.
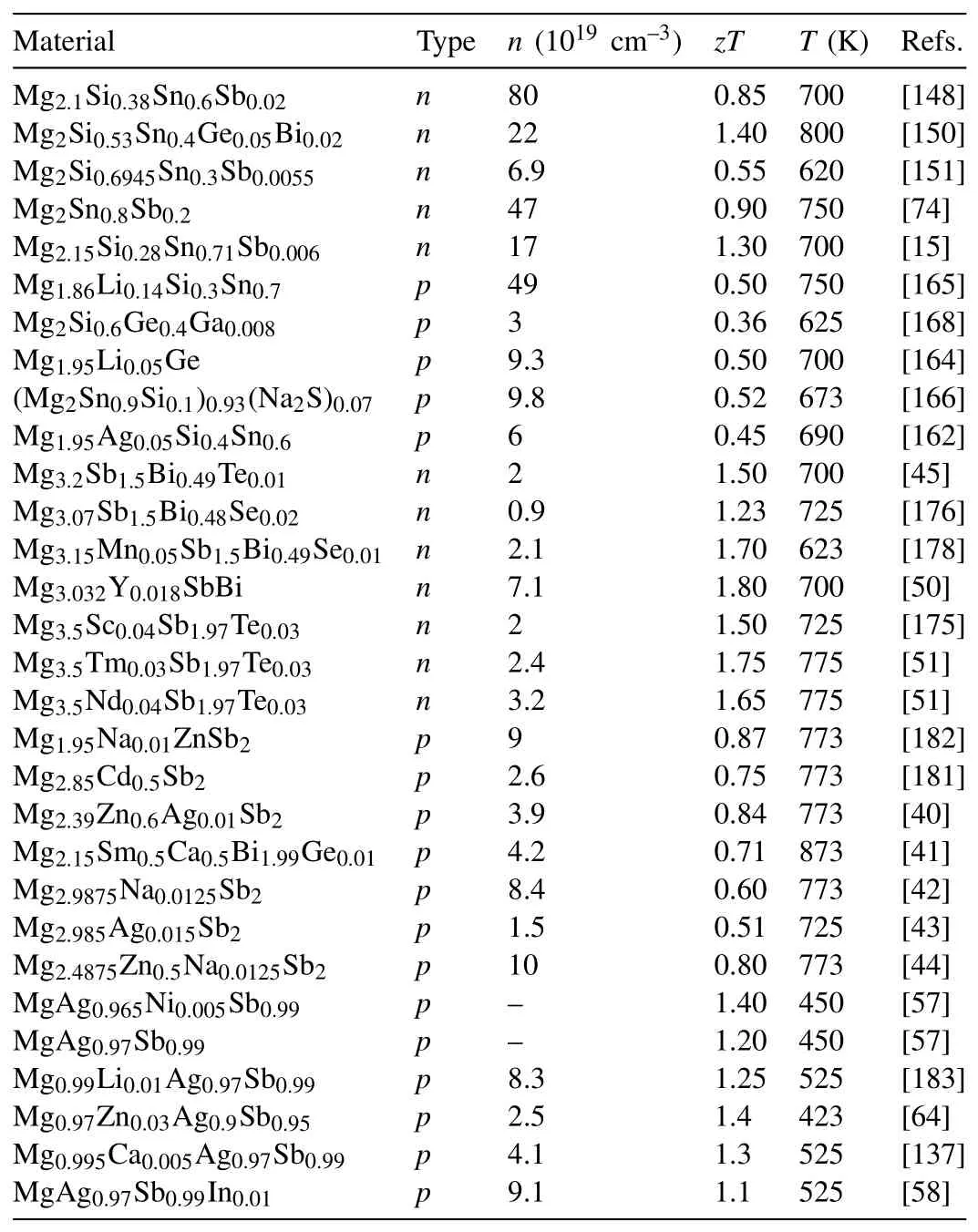
Table 3A summary on the representative dopants for Mg-based TE materials and corresponding zT values.
Similarly,the carrier concentration and doping efficiency of Mg3(Sb,Bi)2are vitally affected by the native defects determined by the chemical environment of synthesis[45,109].Although the intrinsic defects provide some carriers,the carrier concentrations are usually either much lower or higher than the optimal values,suggesting the necessity of adding external dopants.Mg3Sb2single crystal synthesized by the vertical Bridgeman method exhibited the transport behavior of ap-type semiconductor[170].Initially,Te element replacing the Sb site was first chosen as then-type dopant for Mg3Sb2[46,48],which needed the chemical environment of Mg excess according to the comparison on the calculated formation energy of various defects[115].Indeed,it was found that Tedoped Mg3Sb2single crystal grown from the Sb-flux approach still showedp-type transport behavior,which changed tontype through a post-annealing process under Mg vapor[114].Note that much Mg excess may lead to the formation of Mgrich phase[171],which could increase the thermal conductivity and affect the stability of Mg3Sb2samples[172].Hence,controlling the content of Mg is rather important for achieving high TE performance inn-type Mg3Sb2.Both the DFT calculations and single crystal studies revealed that only slightly excess Mg(less than 0.1%)is needed to guarantee efficientntype doping[109,173,174].In contrast,n-type polycrystalline samples were obtained with the excess Mg in the range from 0 to as high as 16% in the initial synthesis due to the possible loss of Mg during the complicated synthesis conditions.By doping with 3% Te,Zhang et al.achieved a highzTof~1.5 at 773 K inn-type Mg3Sb2-based materials[175].Compared with Te,its isoelectronic chalcogen dopants(i.e.,Se,S)were found to be less efficient because of the reduced carrier concentrations and mobility[176–178].The group IIIB elements,including Sc and Y,are among the most effective dopants enabling the realization of very high carrier concentrations,which is attributed to their similar ionic radius to that of Sb[179].Benefiting from the similar electronegativity,these dopants have quite weak influence on the carrier transport,thereby effectively contributing to the enhanced TE performance.Attempts on tuning the hole concentration of Mg3Sb2include doping Zn,Ag,and Na on Mg site[43,44,169,180].The TE performance forp-type Mg3Sb2-based materials is always lower than that ofn-type ones.Xiao et al.optimized the TE performance ofp-type Mg3Sb2by codoping of Cd and Ag,which obtained an enhanced peakzTof~0.75 at 773 K[181].Moreover,Hu et al.enhanced thezTofp-type Mg3Sb2to 0.87 through Na doping and Zn alloying[182].The potential dopants for achievingp-orn-type behavior are summarized in Table 3.
Unlike Mg2Si and Mg3Sb2with better TE performance for theirn-type materials,previous studies mainly focused on thep-type doping onα-MgAgSb determined by its highly degenerated valence bands near Fermi level.Because of the large hole DOS effective mass,a relatively high carrier concentration of~9×1019cm-3is required to realize the optimal TE performance inα-MgAgSb according to the theoretical estimation based on the single parabolic band(SPB)model and deformation potential theory[63].Various dopants have been adopted to increase the pristine carrier concentration(~2.7×1019cm-3at 300 K),including Li,Na,Ca,Yb doping on Mg site and As,Bi,Sn,Pb doping on Sb site.In addition,the carrier concentration can also be tuned via controlling the Sb content[134–137].Among the investigated dopants,the most effective one to provide holes toα-MgAgSb is Li,which gives rise to a maximum carrier concentration of 1.2×1020cm-3,more than five times larger than that of undoped compound[183].This can be attributed to the close ionic radius between Mg(66 pm)and Li(60 pm).With the chemical doping on Mg site,the electrical transport mechanism is dominated by the acoustic phonon scattering at low temperature,as the electrical conductivity decreases with the temperature with the dependence ofT2/3before intrinsic excitation.Additionally,both the phase stability and the strong bipolar effect lead to suppressed TE performance at high temperature.In the whole temperature range from 300 to 548 K,a high power factor of~25 μW cm-1K-2was realized through Li doping[134].It has been found that the power factors obtained after doping Li at Mg site are basically in accordance with the theoretically evaluated values based on a rigid band picture,which however is not the case for doping on the Sb site.This critical difference in the electrical transport behavior is attributed to the perturbation on the valence band edges when doping is realized on the Sb site,which is similar to the Anderson localization[184].Such a fact is in agreement with the DFT calculations that the valence band edges are predominantly decided by thep-orbital of Sb atoms[58].Despite the effect on the band structures,doping on the Sb site with In element achieved an optimal carrier concentration and resulted in a peakzTof~1.1 at 525 K[134].In addition,decreasing the content of Sb can also optimize the carrier concentration;combined with Ni or Cu doping at Ag site,an exceptionalzTof~1.4 can be achieved[57].The related dopants and corresponding carrier concentrations are all exhibited in Table 3.More importantly,the presence of complicated phase transitions in MgAgSb makes it hard to obtain pureα-MgAgSb,which is harmful to TE performance.For example,Kirkham et al.showed that theα-MgAgSb with high content of second phase of Ag3Sb possessed a low peakzTof only 0.5 at 425 K[56].Through a two-week annealing treatment at 553 K,a pureα-MgAgSb phase was obtained resulting in higherzTof 0.8[58].It is thus of vital importance to select appropriate synthesis conditions forα-MgAgSb TE materials[185].
3.2.Band engineering
Band structure engineering has been widely utilized to improve the power factors of TE materials,which can be achieved by forming solid solutions,chemical doping,and even introducing point defects[37,38,186].Among various modifications of band structures,band convergence is considered to be most effective in enhancing the TE performance.When two bands are converged with the energy difference lower than 2kBT,the band degeneracyNvis increased,which can enhance the DOS effective massm*DOSwithout suppressing the carrier mobilityμ[13].The Seebeck coefficient can therefore be improved at a certain carrier concentration with the electrical conductivity unaffected since[13,187,188]
HerekBis the Boltzmann constant,ћis the reduced Planck constant,eis the electron charge,andris the scattering parameter.To directly elucidate the mechanism of the improved TE performance via band convergence,the TE quality factorB,which is positively related to the maximumzT,is introduced and expressed as[33]

wheremI,Cl,andEare the inertial effective mass,average longitudinal elastic moduli,and deformation potential coefficient,respectively.Clearly,the increase inNvcaused by band convergence could lead to higherB,thereby benefiting the TE performance.
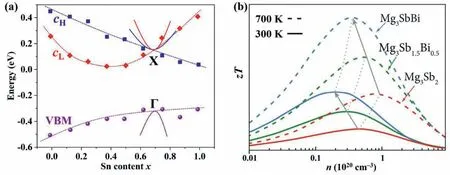
Fig.5.(a)The relative positions of light(CL)and heavy(CH)conduction bands as well as the VBM as a function of the Sn content in Mg2Si1-xSnx solid solutions[15].(b)Predicted zT as a function of carrier concentration for Mg3Sb2-xBix based on the SPB model[96].
Mg2Si(Mg2Ge)and Mg2Sn possess two low-lying conduction bands(light band and heavy band)with their respective band edges reversed in the two systems,which allows the possibility of band convergence.Many studies focused on the band structures and electrical transport properties of Mg2Si1-xSnx,where complete band convergence are reported to realize at the compositions ofx=0.6–0.7,as can be seen from Fig.5a[36–38,189].The converged conduction bands lead to the increase ofNvand thus improved power factor,which are originated from the hybridization of Sn_4dand Mg_3sstates as well as the crystal lattice strain.Liu and Tan et al.comprehensively investigated the band structures and the TE transport properties of a series of Mg2Si1-xSnxsolid solutions doped with Sb[38].Through transport property measurements,they revealed progressively enhanced Seebeck coefficients with the peak atx=0.7 and an exceptionalzTvalue of 1.3 at 700 K.DFT calculations confirmed that the two low-lying conduction bands coincide in energy at the Sn content ofx=0.7,which nicely agrees with the experimental results.In addition,alloying Ge in Mg2Si1-xSnxsolid solutions also achieved band convergence and improved the TE performance[190].By doping Sb in Mg2(Sn,Ge)solid solutions,the TE performance of Mg2Sn0.73Ge0.25Sb0.02was much higher than that of Mg2Sn because of the enhanced band degeneracy[38].In addition,Ge alloying was found to increase the weighted mobility and widen the band gap,which was highly desirable for achieving largezT[191].
As Mg3Sb2and Mg3Bi2are semiconductor and semimetal,respectively,the band structures of Mg3Sb2-xBixcan be effectively modified whenxchanges from 0 to 2.This offers a practical opportunity to optimize the TE performance via modulating the band structures of Mg3Sb2-xBixsolid solutions.Note that the CBM and VBM of Mg3Sb2are largely contributed by Mg_3sstate and Sb_5pzstate,respectively,where the band gap is originated from their energy difference.Through alloying Bi in Mg3Sb2,the band gap is decreased due to the smaller energy difference between Mg_3sand Bi_6pzorbitals.In addition,both the CBM and VBM become more dispersive,leading to smaller band effective mass.As mentioned above,n-type Mg3(Sb,Bi)2compounds exhibit promising TE performance owing to the high conduction band degeneracy.Using the experimentally measuredn-type Seebeck coefficient and Hall carrier concentration,the DOS effective mass of Mg3Sb2-xBixcalculated based on the SPB model indeed decreased with the increase of Bi content[96].First-principles calculations on the evolutions of the band structures for Mg3Sb2-xBixcarried out by Han et al.showed the same trend[192].The lowered DOS effective mass caused by Bi alloying will benefit the weighted carrier mobility according to the experimental measurements[96].It should be mentioned that the alloying scattering in solid solutions is usually harmful to the carrier mobility.Fortunately,the alloying scattering introduced by Bi alloying in Mg3Sb2is too weak to affect the electron carrier mobility.This can be attributed to two aspects:(i)the conduction band edges are mainly contributed by Mg_3sstates,which means that substituting Bi in Sb site has minor effect on the electron transport;(ii)the small radius difference in the host and alloying atoms due to lanthanide contraction gives rise to a weak alloy scattering potential as also observed in half-Heusler solid solutions[193].Fig.5b plots the schematic carrier concentration dependence ofzTfor Mg3Sb2-xBixfitted by experimental results and the SPB model[96],demonstrating that the TE performance is sharply enhanced with the increase of Bi content fromx=0 tox=1.Besides,the optimal carrier concentrations are reduced by alloying with Bi,which is easier to be achieved in experiment.To improve the hole-doped TE performance of Mg3Sb2,the basic idea of the orbital engineering is to minimize the splitting energy betweenpz-orbital andpx,y-orbitals atΓpoint caused by crystal field effect and spin orbital coupling.Tan et al.proposed an effective and straight strategy to weakening the crystal field splitting,that is,substituting a more metallic elements on octahedral Mg2+site[99].The band structure calculations of MMg2Sb2(M=Mg,Ca,Sr,Ba)suggested that thepz-orbital gradually shifted downward,which was even lower thanpx,y-orbitals in BaMg2Sb2.As a result of band convergence,the peak power factor ofptype Mg3Sb2alloying with 1/8 Ba was almost twice as high as that of pristine system.Similar finding can also be confirmed in Mg3Bi2,where the orbital splitting can be reduced by alloying with Ca or Eu,thereby leading to larger valence band degeneracy and higher TE performance for itsp-type materials[110].
Forα-MgAgSb,DFT calculations have revealed that the valence bands show two different valleys at X and M highsymmetry points with close energy with that of VBM located along Z-A line[59,125].This suggests that the TE performance of hole-dopedα-MgAgSb can be further improved via band engineering.Based on the orbital decomposed band structures analysis,Tan et al.suggested two strategies to realize band convergence by tuning the Mg_sor Ag_dorbital[62].They pointed out that substituting Zn on Mg site could increase the effectives-pinteraction,which would lower the VBM and thus enhance the band degeneracy.Meanwhile,alloying Pd or Rh for Ag would shift the valence valleys at X and M high-symmetry points,thereby leading to the band convergence as well.Owing to increased band degeneracy,the peakzTnear 2.0 can be potentially achieved at 575 K when Zn or Pd is alloyed inα-MgAgSb,which exceeds the experimental results and implies the room to further enhance the TE performance.Similarly,Feng et al.reported that the formation of Ag-Mg antisite defects could increase the band degeneracy near the Fermi level,which would significantly improve the TE performance ofα-MgAgSb[131].Note that the prediction of band convergence inα-MgAgSb is all based on first-principles calculations,which are waiting for further experimental verification.
3.3.Defect and nanostructure engineering
Low thermal conductivity is necessary to achieve highzTvalues in materials.The lattice thermal conductivity is weakly coupled with other TE transport properties and usually dominates the heat transport in TE material.Therefore,reducing the lattice thermal conductivity without largely affecting the Seebeck coefficient and electrical conductivity is a general principle for promoting TE performance,which can be realized by forming crystal defects such as point defects,dislocations,interfaces,etc.[194–196].For instance,point defects can dramatically scatter short-wavelength phonons,thus leading to additional thermal resistance[197].The mesoscale grains are more effective for scattering longwavelength phonons[198].As first reported in 1950s,the synthesis of nanostructured TE materials through mechanical alloying or high-energy ball milling can largely reduce the lattice thermal conductivity,owing to the reduced grain size of powder particles.Combined with the slightly affected carrier mobility,an overall enhancement inzTvalues can be achieved in nanostructured materials[199].
Mg2Si,Mg2Sn,and Mg2Ge are known to have high lattice thermal conductivity,which can be significantly reduced via alloying with each other as discussed above.The reduction of the lattice thermal conductivity in these solid solutions is mainly originated from the introduced mass difference scattering.Owing to the larger mass difference between Si and Sn,Mg2Si1-xSnxsolid solution has been regarded to be the most promising candidate for TE application in Mg2Si-based compounds.Beyond that,alloying with small amount of Ge in Mg2Si1-xSnxsolid solution was found to have a notable impact on heat transport,as additional Ge could lead to the formation of Ge-containing secondary phases.By controlling the synthesis and processing techniques,the morphology and size of these secondary phases were tuned to induce nanoprecipitates at the grain boundaries and distortions in the lattice,which greatly enhanced the phonon scattering and thus lowered the lattice thermal conductivity[200].Further study by Zhang et al.demonstrated that the thermal conductivity was reduced from 2.2 to 1.3 W m-1K-1via Sb doping in MgSi0.4Sn0.6,contributing to a peakzTof 1.1 at 773 K[88].Zhu et al.also observed the decreased lattice thermal conductivity caused by Sb dopant and microstructure,which gave rise to a maximumzTof 1.1 at 770 K[201].Liu et al.reported an exceptionalzTas high as 1.3 at 740 K inn-type Mg2.16(Si0.4Sn0.6)1-ySbydue to the optimized carrier concentration and lowered lattice thermal conductivity[152].They found that Sn-rich precipitates in the range of tens of nanometers were dispersed in the matrix and served as phonon scattering centers,which resulted in low lattice thermal conductivity in the temperature range of 300–700 K.Using spark plasma sintering,n-type Sb-doped Mg2Si0.5Sn0.5was fabricated and exhibited a 37% porosity,which led to an ultralow lattice thermal conductivity of 0.486 W m-1K-1and a promisingzTas high as 1.63 at 615 K[202].Apart from alloying and doping,the lattice thermal conductivity can be further reduced via structural modification at the nanoscale level known as nanostructuring,including reduction of grain size and introduction of nanoprecipitates/nanoinclusions.The intentionally generated features,having similar dimensions with the wavelength of phonons,effectively scatter the phonons.DFT calculations have revealed that the lattice thermal conductivity of Mg2Si can be reduced by 40% with the phonon MFP minimized to 20 nm,implying that nanostructuring is a potential pathway towards improving TE performance[203].In addition,the existence of nanoprecipitates can be generated viain situformation or by introducing nanoparticles during the synthesis process[204,205].Farahi et al.reported the introduction of SiC nanoparticles inn-type Bidoped Mg2Si1-xGex.Despite the high thermal conductivity of SiC,these nanoparticles significantly suppressed the heat transport and lowered the room temperature lattice thermal conductivity from 3.1 to 1.8 W m-1K-1,which consequently led to increasedzTvalues[206].
As discussed above,Mg3Sb2and Mg3Bi2possess intrinsically low lattice thermal conductivity.Similar to Mg2Sibased compounds,Mg3Sb2and Mg3Bi2can form solid solutions in a full composition range,and the experimental lattice thermal conductivity of Mg3Sb2-xBixsystem also exhibits a“U-shape”curve.Theoretical investigation verified that the enhanced phonon transport resistance stems from the mass fluctuation-induced point defect scattering[207].Based on the point defect strategy,the lattice thermal conductivity of Mg3Sb2was decreased to~0.4 W m-1K-1via alloying with Mg3Bi2,which even approached the amorphous limit of Cahill model[208].Besides,alloying Bi in Mg3Sb2has been considered to soften the lattice,where the sound velocity will be decreased gradually from 2587 to 2055 m s-1.Utilizing a melt centrifugation technique,the formation of dislocations and increasing porosity was achieved inn-type Te-doped Mg3Sb2-xBixsolid solution,which resulted in a significant reduction of 50% in the thermal conductivity and yielded a peakzTof 1.63 at 723 K[52].Furthermore,additional phonon scattering from grain boundaries can be achieved by the nanostructuring effect,leading to the fact that the lattice thermal conductivity of nanostructured Mg3Sb1.8Bi0.2is lower than that of the pristine sample[209,210].Apart from the solid solutions,Zhang et al.discovered that Tm and Te codoping simultaneously improved the power factor and reduced the lattice thermal conductivity,giving rise to a highzTof 1.75 inn-type Mg3.5Tm0.03Sb1.97Te0.03[51].Kanno et al.found high-density Frenkel defect with ionic charge neutrality in Mg3Sb2[211].DFT calculations and X-ray scattering revealed that strain field-induced disorder and anharmonicity are responsible for the low relaxation time and restrained phonon transport.
Owing to the disordered rocksalt sublattice and natural point defects,α-MgAgSb has intrinsically low lattice thermal conductivity with the room temperature value in the range of 0.51–0.76 W m-1K-1.Native Ag vacancies are the main intrinsic point defects inα-MgAgSb,which can cause large strain fluctuation and mass difference in lattice and in turn enhance the phonon scattering.Generally,the Ag vacancy concentration can be manipulated by controlling the hot-press temperature because of the recovery effect,which is commonly regarded as the thermal relaxation process in semiconductors for countering native defects[123].Higher hot-press temperature means stronger recovery effect and thus decreases the concentration of Ag vacancies,as evidenced by the reduction of hole concentrations[123].By decreasing the hot-press temperature,Liu et al.found that the room-temperature lattice thermal conductivity ofα-MgAgSb decreased from 0.74 to 0.54 W m-1K-1,which was attributed to the elevated Ag vacancy concentration[123].As a result,they achieved a high peakzTof 1.3 and an averagedzTof 1.1 in the temperature range of 300–500 K.Besides,chemical doping-induced point defects inα-MgAgSb could also generate additional phonon scattering and suppress the lattice thermal conductivity.It was discovered that several dopants could lower the lattice thermal conductivity,where Yb was the most effective one[136,137].Hence,the averagezTwas profoundly improved to as high as 1.2 from 300 to 548 K.Lei et al.fabricated a series of carbon nanotubes(CNTs)compositedα-MgAgSb hybrid materials,which introduce additional interfaces between CNTs and the matrix and in turn increased phonon scattering[138].Compared with those of pristine sample,the lattice thermal conductivity of MgAg0.97Sb0.99/0.1 wt.% CNTs was reduced by 15%,leading to a 50% improvement in room-temperaturezTvalue.As a widely applicable approach,multiscale nanostructuring is also effective forα-MgAgSb.The lattice thermal conductivity of nanostructuredα-MgAgSb was 20% lower than that of the sample with coarse grains,which was ascribed to the stronger phonon scattering originated from high-density dislocations,stacking faults,nanoscale precipitates,and microscale grain boundaries[183].All the microstructures inα-MgAgSb were directly observed via transmission electron microscopy images.As these crystal defects and precipitates could effectively scatter phonons in all frequency range but have negligible influence on carrier mobility,nanostructuredα-MgAgSb exhibits superior TE performance than that with coarse grains[58,137].
4.Mg-based thermoelectric modules
The significant advances achieved in improving the TE performance of materials stimulate practical device applications,including cooling/heating and power generation.A TE couple consists of bothp-andn-type materials,which are connected electrically in series via metallic contacts and thermally in parallel utilizing thermally conductive but electrically insulating ceramic plates.A temperature differential is thus allowed through the length of the couple,and a TE device can be constructed by combining several or more couples.For the lowgrade heat that is uneconomic to utilize through traditional Ranking cycle,thermoelectric generator(TEG)is believed to be the most promising pathway for waste heat recovery.On the milliwatt scale,TEGs can be utilized to provide electricity for low-power applications such as mobile electronic devices and wireless sensors.Using body heat,Seiko incorporates thin bulk TE devices into wrist watches,which reduces the energy consumption by~1 μW.In domestic situations,TEGs have also been used for waste heat recovery in diesel power plants and woodstoves.In addition,benefiting from the precise control of temperature and noise-free operation process,TE cooling devices have also been broadly utilized in electronic equipment,such as infrared detectors,car seats,diode lasers,and refrigerators.
In terms of practical applications,TE materials in devices suffer from very large mechanical stress rooted from the high temperature gradient and thermal cycling.Additionally,the effects of high temperature on the electrical and thermal transport properties are also of great concern.Due to the diffusion of elements as well as chemical reactions at interfaces,it is necessary to utilize an intermediate layer as diffusion barriers.The electrical contact of TE legs using a simple solution,which requires direct bonding between TE materials and electrodes,are not feasible at high temperature[212].As a result,excellent stability and good thermal and electrical contacts are necessary for a TE material.Owing to strict requirements in addition to highzT,Bi2Te3-based materials still dominate the market for TE applications used in low temperature range.
To reduce the thermal expansion coefficient discrepancies between two legs and decrease the complexity of used electrode materials,it is preferred to choose the same TE compound for bothp-andn-type legs.Unfortunately,most compounds that include Mg-based materials do not possess highzTvalues for theirp-andn-type materials simultaneously.For instance,n-type Mg2Si-and Mg3Sb2-based compounds can realize a highzTnear 1.5,whereas theirp-type counterparts can hardly reach azTof 0.8 at the same temperature.In comparison,p-typeα-MgAgSb exhibits superior TE performance.The mismatched performance is a serious obstacle for the construction of TE devices,and most of the reported modules used Mg-based TE materials on a single leg.Furthermore,there are several important engineering challenges for practical device application,including interface optimization,electrode fabrication,and protective coating[213],which requires further solutions relying on the basic physical and chemical properties of Mg-based TE materials.
The mechanical properties of Mg-based thermoelectric materials are also important for device applications.For Mg2Sibased solid solutions,the fabrication of thermoelectric devices is challenging owing to their poor mechanical properties,which can be improved by introducing SiC additives to strengthen the matrix.Schmidt et al.incorporated~2 vol.%SiC nanoparticles into Mg2Si,and found that the fracture toughness was improved by~33%[214].Besides,Yin et al.added SiC nanowires and nanoparticles into Mg2Si0.3Sn0.7matrix.Owing to the excellent mechanical properties of SiC,the flexural strength,compression strength,Vickers hardness,and fracture toughness of SiC-added samples were all significantly improved,leading to desirable mechanical properties,while the thermoelectric performance was hardly influenced[215].Mg3(Sb,Bi)2alloys exhibit intrinsic good mechanical properties rooted from the robust bonding strength between cations and polyanions[92].The mechanical properties ofα-MgAgSb are largely related to its microstructures such as grain boundaries and vacancies.Using uniaxial compressive loading,nano-indentation,and Vickers indentation-crack measurements,Liu et al.demonstrated the excellent mechanical properties of nanostructuredα-MgAgSb with high density of grain boundaries[216].Since relatively less research focused on improving mechanical properties of Mg-based compounds,there is still a great demand for exploring suitable additives and better synthetic methods,which may improve their mechanical and thermoelectric properties simultaneously.
With the great advances made in Mg-based TE devices,the conversion efficiency has been significantly improved,which is comparable with that of commercial Bi2Te3devices as summarized in Fig.6a.For the development of Mg2Sibased TE device,efforts have mainly been focused on the optimization of bonding technique and materials.Kim et al.used Bi-doped Mg2Si as the hot-siden-type material in a segmented leg,in which Bi2Te3was bonded to Mg2Si via evaporating 1 nm Ag and 50 nm Ti[217].Here Ag acts as“glue”and Ti enhances the adhesion between the semiconductors.Skomedal et al.reported the bonding of Mo electrode on the hot side of high-performance Mg2(Si0.4Sn0.6)0.99Sb0.01and Mg2Si0.53Sn0.4Ge0.05Bi0.01,and deposition of thin layers of Pb,Ni,and Cr were adopted to improve the adhesion and contact[218].Combined with ap-type material MnSi1.75Ge0.01,a maximum power output of 3.24 W was achieved at 1008 K with an estimated efficiency as high as 5.3%.Withn-type Mg2Si0.4Sn0.6andp-type MnSi1.73both doped with Sb,the constructed TE module exhibited a high efficiency of more than 6.5% under a temperature difference of 520 K.
For the application of Mg3(Sb,Bi)2TE materials,the most important issues are related to their weak stability,including Mg oxidization and loss,the performance deterioration and decomposition at high temperature,as well as the possible deliquescence in humid environment.It has been concluded that the small ionic radius of Mg2+(octahedral site)should be largely responsible for the relatively unstable lattice.Increasing the Bi content in Mg3Sb2–xBixwould weaken the interlayer bonding and lead to worse thermal stability[45].Besides,the precipitation of Sb/Bi phase and Mg loss can be observed at elevated temperature owing to the high vapor pressure of Mg.Fortunately,suitable doping on octahedral Mg2+site were found to improve the thermal stability of Mg3Sb2–xBix.The effective dopants include Ag forp-type Mg3Sb2and La/Y/Sc forn-type Mg3Sb2–xBix,which might be attributed to the larger ionic size of these elements compared with that of Mg[50].In addition,the thermal stability could also be meliorated by increasing the nominal content of Mg.The excess Mg is expected to suppress the formation of Mg vacancies thus enabling better thermal stability.As demonstrated by thermal cycling tests,the sample with more Mg excess exhibited much more stable Seebeck coefficient and electrical conductivity than the one with less Mg excess[94].However,too much Mg-excess will lead to the oxidization and thus increased thermal conductivity,which means that a balance content of Mg is of vital importance.As shown in Fig.6b(blue line),Mao et al.reported a TE module consisting ofn-type Mg3.2Bi1.498Sb0.5Te0.002andp-type Bi0.5Sb1.5Te3[54].A maximum temperature difference as high as 91 K was achieved by the Peltier effect with the hot side maintained at 350 K and an input electrical current of 9 A.The efficiency of this module is even superior to the commercial Bi2Te3-based devices.Yang et al.fabricated a full-scale cooling device usingn-type Mg3.2Bi1.4975Sb0.5Te0.0025andptype(Sb0.75Bi0.25)2(Te0.97Se0.03)3,which achieved a maximum temperature difference of 76 K with the hot side at 350 K(green line in Fig.6b)[219].In addition,Liu et al.designed a wearable TE generator constructed byp-type Bi0.4Sb1.6Te3andn-type Mg3.2Bi1.498Sb0.5Te0.002legs[220].When placed on a human arm with the ambient temperature of 289 K,the proposed device provided a peak power density of 20.6 μW cm–2.Despite the high efficiency and low-cost raw elements,the process cost of Mg3(Sb,Bi)2-based materials are still high due to the strict synthesis conditions and weak stability,which need to be comprehensively considered before practical application.

Fig.6.(a)Reported conversion efficiency of Mg-based thermoelectric modules(comprising p/n materials)compared with that of SnSe module and commercial Bi2Te3 module[6,55,66,217,218,221].The hot-side temperatures are labelled at the top of each column,while the cold-side temperature is room temperature.(b)The cooling performance of Mg-based thermoelectric devices as a function of current[54,219,222].
Similar to Mg2Si,previous efforts were mainly focused on the optimization of electrical and thermal contacts to realize the expected efficiency ofα-MgAgSb-related TE devices.Typical methods for the fabrication of contact layers such as sputtering is not appropriate for the fabrication of the contact layers forα-MgAgSb,especially when the operating temperature is high and a thick contact layer is required.Using a one-step hot-press technique,Kraemer et al.constructed a singleα-MgAgSb leg with an Ag pad on both the bottom and top surfaces,leading to high mechanical strength and low resistance[66].The resistance of the Ag contact pad was below 10 μΩcm2measured via a home-made scanning voltage probe under a current of 0.1 A.The advantages of Ag contact include three aspects:i)Ag possesses high thermal and electrical conductivity and is easy to solder due to its softness;ii)Ag andα-MgAgSb have almost identical thermal expansion coefficient,which can thus reduce the interface stress significantly;iii)Ag could decrease the elemental diffusion because of a small concentration gradient.As a result,the fabricatedα-MgAgSb single leg exhibited a TE conversion efficiency as high as 8.5% operated between 293 and 518 K,which was even higher than that of a reference Bi2Te3-based module with an imposed temperature difference of 225 K.At a current of 1.48 A,the power output reached 46.2 mW.Note that all the above discussed TE modules only use Mg-based materials on a single leg.Recently,Liu et al.fabricated a TE module with Cu-doped Mg3Sb1.5Bi0.5andα-MgAgSb as then-andp-type legs,respectively[55].This Mg-based TE module enabled a high conversion efficiency of~7.3% with the hot-side temperature of 593 K in comparison to other advanced TE modules.Using a scalable processing routine,Ying et al.reported a TE module consisting ofp-typeα-MgAgSb andn-type Mg3(Sb,Bi)2,which displayed a high conversion efficiency of~7% under a temperature difference of 250 K[221].The high efficiency highlights the promising prospects of low-cost Mg-based TE devices for large-scale applications in low temperature region[222],which was significantly benefited from the advanced optimization strategies in both material and device levels as summarized in Fig.7.
5.Summary and outlook
To summarize,Mg-based thermoelectric materials have attracted intense interests in recent years due to highzTvalues at low to medium temperature range,together with the abundant,low-cost,low-density and environmentally friendly nature of the constituted elements.In this timely review,we comprehensively summarize the most important research advances of this group of TE materials,including their crystal structures,electronic band structures,thermal and electrical transport properties,as well as effective strategies to improve theirzTvalues and the conversion efficiency of designed devices.The prominent points can be briefly summarized as follows:
(1)Both Mg2Si-and Mg3Sb2-based compounds show highly degenerated conduction bands and can be alloyed and/or doped to form a wide range of compositions,providing many effective strategies for enhancing their thermoelectric performance,such as band engineering and defect/nanostructure induced phonon scattering.HighzTvalues of near or above 1.5 have been realized in theirn-type materials at the temperature of 700–800 K.
(2)Mg3Sb2andα-MgAgSb exhibit phonon glass electron crystal behavior,originating from their unique crystal structures.The intrinsically low lattice thermal conductivity is caused by the low group velocity and strong vibrational anharmonicity,which are related to the weak interlayer bonding and structural distortions for Mg3Sb2andα-MgAgSb,respectively.Further chemical doping inα-MgAgSb can optimize the carrier concentration and introduce additional point defects,making the peakzTas high as 1.4 near 550 K forp-typeα-MgAgSbbased materials.
(3)Thermoelectric devices,applied for both power generation and cooling,have been successfully fabricated using Mg-based materials,and the obtained conversion efficiency can be comparable with that of Bi2Te3-assembled modules.
Despite the rapid progress in thermoelectric performance enhancement,the practical applications of Mg-based compounds are still at an early stage,which therefore requires more efforts concentrating on this area.Some current challenges and future directions are summarized into the following two aspects.
(1)From materials level,the mismatchedzTvalues forpandn-type materials of the same Mg-based compounds largely limit their applications,which require the breakthrough to effectively control the Fermi level and engineer the band and phonon structures.Especially,zTvalues forp-type Mg3Sb2-based materials should be urgently improved.This may be achieved by isoelectronic substitution to realize band convergence.Besides,operating the valence band anisotropy may also improve thep-type thermoelectric performance.Considering the narrow temperature range in whichα-MgAgSb exists,more attention should be paid to tuning the phase transition temperature,which could be realized by controlling the preparation condition and/or appropriate alloying.
(2)The long-term stability of Mg-based thermoelectric devices,which involves stable thermal,electrical and mechanical properties at high temperature,is essential to ensure the future applicability.This implies that further investigation on Mg-based thermoelectric devices should focus on joint connection,interface material deposition,and thermal/mechanical stability.
Owing to the promising advantages especially from the commercial point of view,these challenging tasks for developing Mg-based thermoelectric devices are well worthy of systematic investigation,which should speed up their practical applications for cooling and energy harvesting.
Conflic of interest
There are no conflicts to declare.
Acknowledgments
We are grateful for financial support from the National Natural Science Foundation of China(Grant Nos.52125103,52071041,12104071,11874356,U21A2054).
 Journal of Magnesium and Alloys2022年7期
Journal of Magnesium and Alloys2022年7期
- Journal of Magnesium and Alloys的其它文章
- Effects of heat treatment on the corrosion behavior and mechanical properties of biodegradable Mg alloys
- Multi-solute solid solution behavior and its effect on the properties of magnesium alloys
- Calcium phosphate conversion technique:A versatile route to develop corrosion resistant hydroxyapatite coating over Mg/Mg alloys based implants
- The role of solutes in grain refinement of hypoeutectic magnesium and aluminum alloys
- Characterization on the formation of porosity and tensile properties prediction in die casting Mg alloys
- Biomass-derived carbon doping to enhance the current carrying capacity and flux pinning of an isotopic Mg11B2 superconductor
