Multi-solute solid solution behavior and its effect on the properties of magnesium alloys
Jun Wang,Yuan Yuan,Tao Chen,Liang Wu,Xianhuan Chen,Bin Jiang,Jingfeng Wang,Fusheng Pan
National Engineering Research Center for Magnesium Alloys,College of Materials Science and Engineering,Chongqing University,Chongqing 400000,PR China
Abstract The low-density magnesium(Mg)alloys are attractive for the application in aerospace,transportation and other weight-saving-required fields.The mechanical properties and corrosion properties of Mg alloys are the key-property issues for the wide application.It is surprising to find that the solid solution of alloying elements in the α-Mg phase can have multi-effects on the properties of Mg alloys,e.g.,solid solution strengthening,solid solution corrosion-resistance-enhancing,etc.Additionally,the alloy design theory of“solid solution strengthening and ductilizing”proposed by Pan and co-workers has attracted extensive attentions.It is promising that by selected proper multi-alloying-elements(with optimal ratio)solid solutioned in the α-Mg phase,the comprehensive properties of Mg alloys can be synergistically improved.In this work,the solid solution behavior of Mg alloys and the followed solid solution property-enhancing effects were reviewed.The mechanisms proposed recently by researchers for these solid solution property-enhancing behaviors were presented,and the related calculations and predictions were also described.It is shown the demonstrations of the fundamentals for the solid solution property-enhancing of Mg alloys,especially from the atomic inter-reaction aspects,still require elaborated characterization work and calculation work.Additionally,it could be expected that the multi-solute in Mg alloys can bring many possibilities,or,in another saying,“cocktail effects”.With understanding the multi-solute interaction behavior and the corresponded solid solution property-enhancing effects,the good balanced high-performance Mg alloys can be developed.
Keywords:Mg alloys;Solid solution;Strength;Ductility;Corrosion-resistance.
1.Introduction
With the increasing demand of energy saving and environment protection,Mg alloys with the low density,high strength/weight,high stiffness/weight and other unique advantages,have attracted increasing attentions as their promising application in the transportation field and other weight-savingrequired fields[1–13].However,compared with other structural materials e.g.,steels and aluminum alloys,the poor corrosion resistance of Mg alloys[14–17],originating from the low electronic potential of Mg,and the unsatisfactory plasticity originating from the original hexagonal close-packed(hcp)structure of theα-Mg phase,limit the wide and large-scale applications of Mg and Mg alloys[18,19].
With constantly efforts from researchers in recent dozen years,the performance of Mg alloys has been significantly improved[9,20–25]and many Mg alloy series e.g.,AZ,AM,ZK,WE,LA series,have been developed and commercialized.Furthermore,the purity of primary Mg materials has been realized to higher than 99.9%,which makes the corrosion resistance and other properties of Mg alloys been greatly improved[26–30].Moreover,with the rare earth(RE)elements alloying and the controlling of the formation of long-period stacking ordered(LPSO)phase,the ultimate tensile strength(UTS)of Mg-RE-X alloys can be higher than 400 MPa,e,g.,Mg-9.2Gd-3.3Y-1.2Zn-0.9Mn(wt.%)developed by Wang et al.[31]with a UTS around 525 MPa,Mg-8Gd-1Er-0.5Zr(wt.%)developed by Zheng et al.[32]with a UTS around 560 MPa,Mg-8.2Gd-3.8Y-1Zn-0.4Zr(wt.%)developed by Xu et al.[33]with a UTS around 466 MPa.
The common property-enhancing methods for metallic materials mainly include the structure refinement,solid-solution,precipitation,processing,etc.,which were also extensively applied in the properties-tailoring for Mg alloys.Commonly,Mg alloys with fine structure possess high strength and ductility[34,35],and the precipitates formed in Mg alloys can effectively enhance the strength while deteriorate the ductility.In addition,the formation of LPSO phase can make the strength of RE-alloyed Mg alloys much higher than that of Mg-Al-based alloys[31,33,36].Additionally,the special processing mode,for example,the equal channel angular pressing(ECAP),can also make the strength of Mg alloys been improved significantly[37,38].Varied special process and design was found to be able to obviously enhance the strength of the alloys.For example,the dedicate-constructed dual-phase nanostructure of Mg alloys developed by Wu et al.exhibit the high strength up to 3.3 GPa[39].
Naturally,among all the elaborate developed Mg alloys,solid solution always plays a fundamental role in the performance tailoring of Mg alloys[40–42].Through extensive calculations and studies,it was found that the solid solution approach can bring not only the comparable high strength,but also high ductility and corrosion resistance[43–45].In another saying,the comprehensive properties of Mg alloys can be simultaneously improved by the solid solution method to a certain grade.Especially,Mg itself has no allotropy transformation and therefore no martensitic transformation,found in Fe and Ti alloys,was observed in Mg alloys if LPSO transformation is not counted.In this case,the solid solution and precipitation methods are two of the most basic approaches for the properties tailoring of Mg alloys.
In this work,the solid solution-enhancing on the strength,ductility and corrosion-resistance of Mg alloys are reviewed and some prospects for the design of high-comprehensiveperformance Mg alloys by the solid solution method are proposed.(Since this work is mainly completed in the middle of 2021,the literature work after that time may be not exclusively reviewed in this work.)A proposed scheme of the solid-solution property-enhancing of Mg alloys is presented in Fig.1(a).Additionally,except the strength,ductility and corrosion resistance,other properties like damping capacities and electromagnetic shielding effectiveness,etc.of Mg alloys[46–52]can also be tailored by the solid solution effect,as shown in Fig.1(b).While,the effects of solid solution on these properties still require more work to be clarified and are therefore not elaborated in this work.Additionally,the possible effects of the solid solution of different alloying elements and their combinations on the properties of Mg alloys are also summarized in the Fig.1(c).
2.Solid solution behavior of Mg alloys
2.1.Solid solution and thermodynamics
The alloying method is the most commonly applied method to improve the principle properties of alloys.Firstly,the solute atoms in the matrix phase can change the lattice parameters and introduce defects,which further affect series of properties of the alloys.Secondly,the followed precipitation process as the temperature decreasing in the alloy can further dramatically change the microstructure,thereby affecting the final properties of the alloys.
With element alloyed in the materials,there is an equilibrium between solid solution in theα-Mg phase and precipitates formation.Hence,the phase diagrams and thermodynamics,which can provide the information of elemental solution,phases’equilibrium and transformations,are essential information for the materials design.In this section,the solution behavior of alloying elements in Mg alloys was reviewed.
Hume-Rothery et al.[53]have proposed the binary-alloy solid solubility rules based on the experimental solid solubility data of Cu-based and Ag-based alloys,which can be concluded as that the high similarity of the atomic size,electrochemical properties and crystal structures can guarantee a big mutual solubility and otherwise a small solid solubility in each other matrix phases.Those rules are also applicable to Mg alloys[54].
The distribution of the atomic diameters of the main alloying elements,with the atomic diameter of Mg(about 0.32 nm)as the central line and±15% of the diameters’difference as the two parallel lines(about 0.272 nm and 0.368 nm),is shown in Fig.2(a).Thirty elements in total including the Al,Ag,Zn,Li with high solid solubility in theα-Mg phase are found to be consistent with the rule of atomic diameter difference(being in±15%).Furthermore,Darken and Gurry[55]proposed that it is hard to form a high solid solution when the difference of electronegativity between the solute and solvent atoms is more than±0.4.The relationship of electronegativity and atomic number is shown in Fig.2(b).
Recently,our group have further studied the solid solution behavior of binary Mg alloys by the machine learning method and indicated that a total of 11 kinds of features are related to the maximum solid solubility of the alloying element in theα-Mg phase,as shown in Fig.3,and especially the four features electronegativity,formation energy,atomic radius and work function were reported to be the main features for the classification of the solid solution behavior of alloying element in theα-Mg phase[56].
In this work,the tielines for Hcp phase in Mg-X binary systems(X represents the commonly used alloying elements in Mg alloys)were calculated using the available latest commercial Mg-alloy thermodynamic database(TCMg5 in this work at preparation time),performed by the thermodynamic calculation using Thermal-Calc software.As shown in Fig.4,most commonly used alloying elements have limited solid solubility in theα-Mg phase,in which the alloying elements Li,Al,Sc and In show high solid solubilities(>10 at.%).

Fig.1.(a)and(b)Schematic illustration of the solid-solution property-enhancing of Mg alloys and(c)summary for the possible effects of the solid solution of different alloying elements and their combinations on the properties of Mg alloys.

Fig.1.Continued

Fig.2.The atomic characteristics of elements:(a)the atomic diameter of the element and the alloying element whose size is favorable in high solid solution of the α-Mg phase and(b)the relationship between element electronegativity and the atomic number.
According to the available literature information,only the element Cd can form an infinite solid solution with Mg at high temperature range(>253 °C)[27].There are some alloying elements have not been included in TCMg5 and are therefore not considered in this work.It is supposed all the included Mg-based binary systems in TCMg5 have been fully thermodynamic assessment and some differences between the calculated values and the reported experimental values from literatures may exist.More comprehensive information can be found in our recently work[56],where all available values of the solid solubility of different alloying elements in theα-Mg phase from literatures and calculation results have been collected and analyzed.
2.2.Multi-solute Mg alloy
The solid solution of alloying elements has been reported to effectively improve the strength,ductility and/or corrosion resistance of Mg alloys,and significant increasing studies about the solid solution effect have been conducted to develop Mg alloys with high performance and/or comprehensive properties[57–66].However,these researches mainly focus on the effect of solid solution of a single element on properties.And notably,it was reported that the solid solution of a single element is not conducive to the improvement of corrosion resistance[67,68],while the simultaneous solid solution of two elements can exhibit better corrosion resistance than the solid solution of a single element[62,63,69].Therefore,it is suggested that the tailoring of performances of Mg alloys can be greatly expanded with multi-alloying-element addition[45,70–73].However,since there are enormous possible combinations of elements according to the Periodical Table,and the interaction effects between the alloying elements on the solid solubility and further the combined properties are not clear,few related studies have been reported[74,75].

Table 1Variation of the strengthening parameter kY in Mg alloys in presence of various solute elements[129].

Fig.2.Continued

Fig.3.The features related to the maximum solid solubility of alloying element in the α-Mg phase.
The recent work in our group have found that some combinations of alloying elements can increase the total solid solubility of alloying elements in theα-Mg phase based on the thermodynamic calculation results[45].For example,the solid solubility of RE elements in theα-Mg phase was predicted to be possibly increased by the addition of the third alloying element Li,as shown in Fig.5.From this aspect,properties of Mg based alloys can be further tailored with the adjustment of the combination of alloying elements.It also should be noted that the calculation results require more experimental verification due to the possible inaccuracy ternary thermodynamic database in which most are just simply extrapolated from binary systems.The related experiments are in progress in our group.Furthermore,considering the lack of multi-component solid solution theory for Mg alloys and the huge workload of system-by-system research,the firstprinciples calculations combined with machine learning methods are also conducting to study the solid solution behavior of ternary Mg alloys in our group.

Fig.4.The tielines for Hcp phase in Mg-X binary systems calculated by the thermodynamic database TCMg5.
3.Solid solution strengthening Mg alloys
3.1.Solid solution strengthening mechanism
Solute atom in a crystalline lattice can reside in either interstitial or substitutional sites,which depends on their sizes relative to the solvent atoms.The most relevant mechanism for a random distribution of the solute atoms in these theories is supposed to be the elastic interaction due to the size misfit,and/or,the modulus misfit.Since the solute atoms have different shear modulus and sizes from the solvent atoms,additional strain fields on the lattice of the surrounding matrix can be imposed and restrict the dislocation motion in the local lattice,resulting the solid solution strengthening[76].The electronic contribution to the solid solution strengthening was also presented,which could be attributed to the difference in valence electrons between the solvent and solute atoms[77–79].Furthermore,the electronic work function,which is related to the effect of different solute atoms on the mechanical properties of materials,has also been applied to describe the solid solution behavior of Mg systems[80].
Some models have been proposed to quantitively or semiquantitively describe the solid solution strengthening effects.Fleischer[81]and Labusch[82]have proposed empirical theories for solid solution strengthening.Based on previous models,Wesemann et al.[83]recently proposed a general equation for the critical resolved shear stress(CRSS)increase,

whereAis a constant,Gis the shear modulus,εis a mismatch parameter,xis the solute content in mole fraction,and exponentspandqare decided by the theory considered.
The combined mismatch parameter,εL,suggested by Fleischer and exponentsp=4/3 andq=2/3 taken from Labusch have been applied in some studies[83–85].Varvenne et al.[86]have modified the Labusch model and applied in the light alloy systems.

Fig.5.The solid solubility of X in α-Mg phase with and without Li addition calculated using PanMg2013 database.
And the effect of solid solution strengthening of a solutejon the yield stressΔδSSHjhas been suggested to be described as[87],


whereA′is a constant different fromA,xjis the mole fraction of solutej,and especially,the combined mismatch parameterεLjis expressed as,whereεGj′is the shear modulus,εbjis the lattice constant,andαis a parameter decided by the dislocation type(screw dislocations:3<α<16 and edge dislocations:α>16).
Based on the Eq.(1),Gypen and Deruyttere proposed a general multi-solid-solution-strengthening model[88],which is commonly applied in dilute solid solutions,and they considered the contribution of each solute atomxjto the total solid solution strengthening effect,as,

3.2.Alloying element effects
Commonly alloying elements,e.g.Al,Zn,Ca and RE elements,have been widely added to Mg alloys to obtain the high strength and hardness[42,73,89,90].Three strengthening methods,solid solution strengthening,precipitation strengthening and refinement strengthening,are commonly combined to take the effects.However,it is hard to separate these strengthening effects quantitively and,needless to say,the low-temperature-precipitation-strengthening is also usually applied based on the theory of the change of alloying element solubility with temperature.Some empirical formula with relation with the content of the added alloying elements have been proposed in the literature.Hence,in this work,their strengthening effects are just reviewed as addition of alloying elements without the distinguish of the absolute solid solution strengthening effects.
In earlier years,the strengthening behaviors of single crystal Mg solid solution alloys with dilute concentration studied by Akhtar and Teghtsoonian[91,92]and Vanderplanken and Deruyttere[93]showed the CRSS of basal slip of Mg at 298 K or room temperature can be enhanced with the solid solution of Al,Zn and Sn.Specifically,the increment is proportional to(c-c0)n,in whichcis the solute content,c0is the minimum content required for solid solution strengthening andn=1/2 or 2/3.And in recent several years,it is further proposed that the strengthening effect of Al and Zn solutes in Mg alloys at 373 K can be described using the Labusch model[59,82,86],in which the increment of CRSS of basal slip is proportional toc2/3.

wherecis the content of the solute element(in at.%)andKis a constant that depends on the alloying element and temperature[59,86].Furthermore,it was suggested by Wang et al.[94]that the random solid solution effects that control the strengthening dilute Mg-Al solid solution alloys could be extrapolated to the supersaturated regime,while it’s not the same case for alloying element Zn.The strong strengthening effect of Zn at high solute concentrations was suggested coming from the tendency of solute clustering[95]and short range ordering[96]of Zn atoms inα-Mg phase.And in this work,we have summarized the relationship between the CRSS of basal slip of Mg alloys and different alloying elements with different concentrations ascn(n=1/2 or 2/3),as shown in Fig.6(a)and(b),for direct comparison.

Fig.6.The effect of different alloying elements with different concentrations on the CRSS of basal slip(a)n=1/2 and(b)n=2/3 of Mg-X alloys[41,91-94];the yield strength(c)n=1/2 and(d)n=2/3 of Mg-X alloys[41,42,57,96,97].
Generally,the strengthening effect of alloying elements below their solubility limits on Mg alloys include solid solution strengthening as well as grain refinement strengthening,and therefore the effect of grain refinement strengthening should be extracted to properly assess the solid solution strengthening effect of alloying elements on Mg alloys.According to the Hall-Petch formula,the effect of grain refinement strengthening can be obtained.The solid solution of alloying elements such as Al[41],Zn[96],Sn[97],Gd[42]and Y[57]in Mg with polycrystalline structure were also reported to enhance the yield strength of Mg alloys linearly withcn,as shown in Fig.6(c)and(d),wherecis the solute atom concentration andn=1/2 or 2/3,after the correction of grain refinement strengthening effect.
It can be concluded from Fig.6 that both the CRSS and yield strength of Mg-X binary alloys have a strong linear relationship withcn(n=1/2 or 2/3).Especially,it shows that the solid solution strengthening effect of different alloying elements can be sequenced as Gd≈Y>Zn>Sn>Al.Additionally,the direct relationships between alloying element contents and the properties of Mg-X alloys(CRSS,yield strength and Vickers-hardness)are also given in Fig.7 for a better understanding of the strengthening effects of different alloying elements.As shown in Fig.7,the CRSS,yield strength and hardness all also show obvious linear relationships with the alloying element contents,and the solid solution strengthening effect on the yield strength is Y>Gd>Zn>Sn>Al and the hardness is Gd≈Y>Zn>Sn>Al.
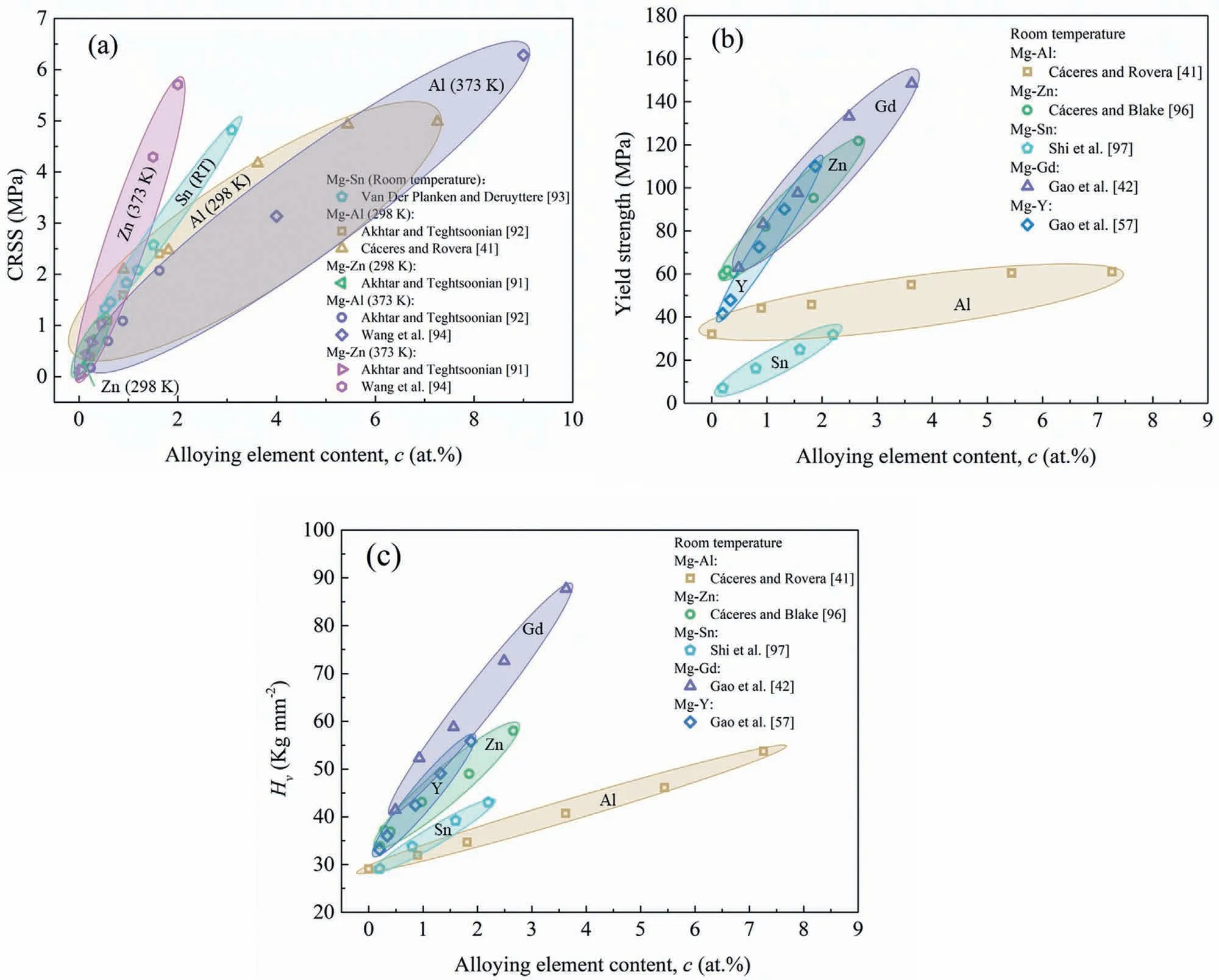
Fig.7.The alloying element contents on the properties of Mg-X alloys:(a)CRSS[41,91–94],(b)yield strength and(c)hardness[41,42,57,96,97].
Mg-Al-X system:The alloying element Al has been the most common alloying element in Mg alloys for its great effect on the enhancement of mechanical properties and corrosion resistance,etc.And the high solubility of Al in theα-Mg phase also make Al be the excellent alloying element for the solution-property-enhancing in Mg alloys.Wang et al.[94,98]presented that the Al addition in Mg alloys can increase the CRSS of both basal slip and pyramidal slip through micropillar compression tests and thus simultaneously enhancing their strength and reducing the plastic anisotropy.Some empirical formula for the strengthening effects of Al in Mg alloys have also been proposed in the literature(not defined to the solid solution range).It was reported that the hardness of Mg-Al alloys can be increased with function asHv10(kg mm-2)=29+3Al(wt.%)and the yield strength was also enhanced to~70 MPa from~30 MPa when the Al content was up to 8 wt.%[41].Cáceres and Rovera[41]further suggested that the yield strength increases linearly withcn,after correcting for grain strengthening effects.
The combined effects of Al and X in the Mg alloys have also been extensively studied and the solid solution strengthening effects have been noted.The tensile yield strength of Mg-1Mn(wt.%)alloy can be improved steadily from 27.4 to 122.9 MPa with the increasing Al content from 0 to 9 wt.%,mostly due to the solid solution strengthening behaviors[35].And Wan et al.[48]confirmed the tensile strength of Mg97Zn1Y2(wt.%)can be enhanced with Al addition and especially the hardness increased by 40% by addition of 3 wt.% Al.Rahulan et al.[99]reported that the as-extruded Mg-5Li-4Al alloy possessed an enhanced UTS~250 MPa.
Mg-Zn-X system:Zn,which has the same crystal structure as Hcp-Mg(hexagonal close-packed structure)and a low cost,also becomes an important alloying element on the tailoring properties of Mg alloys[90,96,100–102].The solid solution strengthening mechanism of Zn addition in the single crystal of Mg were studied by Akhtar and Teghtsoonian[91]and two strengthening stages have been reported,where both linear functions ofc1/2(the square root of the solute concentration)with different slopes have been presented and the transition point was 0.025 at.% Zn.It was proposed that the strengthening of stageⅠwas attributed to the increase of the forest dislocation density which depend on the solute concentration,and that of stageⅡwas due to the change in the thermally activated mechanism occurred from intersection to single solute atom dislocation pinning mechanism.
The hardness of Mg-Zn alloys was reported to increase linearly with the alloying element concentration asHv10(kg mm-2)≈33+9Zn(at.%)and the yield strength also increases linearly withcn,after correcting for grain strengthening effects[96].It is reported that,compared with pure Mg,the hardness and ultimate strength in yield,tension and compression of as-cast Mg-Zn alloys increase with the increasing Zn content up to 5 wt.%(around 6 wt.% solubility in Hcp-Mg at 300°C)and the 1 wt.% Zn addition make the yield and tensile strength almost be doubled[102].With more elaborated studies,Stanford and Barnett reported that the solute strengthening of the basal slip system increases with the increasing Zn content and no change is observed in stress to activate the twin system[103].The further strengthening effects of addition of Zn in Mg-X systems are also reported,e.g.Mg-Ca-Zn system[104],Mg-Li-Zn system[105],and etc.It was reported that the as-extruded Mg-3.5Li-2Zn(wt.%)alloy have a UTS~250 MPa and the elongation>20%[106].
The comparison of strengthening effects coming from Al and Zn were also been studied.Meng et al.[107]have reported the as-cast Mg-8Li-1Zn alloy with tensile strength 81.04 MPa and elongation 33%,and the as-cast Mg-8Li-1Al alloy with tensile strength 87.48 MPa and elongation 40%.Xu et al.[108]have reported the as-cast Mg-7Li-3Al(wt.%)alloy with tensile strength 178.37 MPa and elongation 14%,and the as-cast Mg-7Li-3Zn(wt.%)alloy with tensile strength 125.69 MPa and elongation 8%.If the solution unit of atompercent is to be used,the strengthen effects of Al and Zn with the similar atom-percent additions are to be similar[107].
Additionally,it was reported that there is a solutedislocation interaction,which is also an effective strengthening mechanism in the deformed Mg alloys,presented in Al and Zn solid solutioned Mg alloys.Xu et al.[109]have reported that in a AZ31 alloy,both the twin boundary dislocation interaction and twin boundary solute-dislocation interaction(Al and Zn solute atoms)can significantly enhance the yield stress of recompression along ND of pre-strained sample and also decrease the hardening peaks during a detwinning predominant deformation.Additionally,Cui et al.[110]indicated that the secondary twins formed remarkably in the interior of the primary twins in Mg alloys with a high concentration of solute atoms.
Mg-Mn-X system:Mn,though with limited solubility in theα-Mg phase,is also a common alloying element to improve the strength of Mg alloys.For example,Gu et al.[111]reported that the yield strength of as-cast Mg can be improved from~20.7 to~28.9 MPa with 1 wt.% Mn solid solutioned in theα-Mg phase.
Mg-Zr-X system:Similar to the effect of Mn,Zr also shows certain solid solution strengthening effect though with limited solubility.Gu et al.[111]have indicated that the yield strength and UTS of as-cast Mg can be improved from~20.7 and~87.2 MPa to~67.5 and~171.9 MPa,respectively,with the solution of 1 wt.% Zr.
Mg-Sn-X system:Alloying element Sn,as alternative replacement of RE elements,has attracted many attentions because of its high-temperature stable Mg2Sn compound[112–115].On the other hand,the addition of Sn in Mg alloys also shows significant solid-solution-strengthening effect.It was reported that with a content 1 wt.% Sn solid solutioned in theα-Mg phase,the yield strength and UTS can be improved around twice as much that of pure Mg in as-cast state[111].The UTS of solution-treated Mg-1Sn(at.%)solid solution alloy as 130 MPa was also reported to be more than twice that of pure Mg[49].Shi et al.[97]proposed that the hardness of Mg-Sn binary alloys increases with the Sn concentration with a function asHv0.5(kg mm-2)=28.34+6.88c(at.%),and the yield strength of Mg-Sn binary alloys follows the relationship withcnat room temperature,where the strengthening of the basal planes take the effect[97].
Mg-Ag-X system:Ag,with the maximum solid solubility 4.355 at.% shown in Fig.4(b),can also effectively strengthen the alloy.The 1 wt.% Ag solid solutioned in as-cast Mg can effectively enhance the yield strength and UTS to~24 and~116 MPa,respectively[111].
In addition to the commonly used alloying element,some other alloying elements were also be studied in literature.For example,the yield strength and UTS of as-cast pure Mg can be improved to~36 and~147 MPa,respectively,with the solid solution of 1 wt.% In[111];the UTS and YTS of Mg-1Ga(at.%)binary solid solution alloys were more than twice than that of pure Mg[49].The 0.5 wt.% Ti addition to AZ91 alloy can improve its UTS and UYS from 144 to~170 MPa and from 95 to~140 MPa,respectively,in which the increased solid solution of Al in theα-Mg phase with the addition of Ti as well as the solid solution of Ti has a contribution[116].
Mg-RE-X system:RE elements,with big atomic sizes,surely have strong strengthening effects for the solution in Mg phase[42,117–119].As shown in Fig.2,the atomic size of most RE elements is close to that of Mg and thus generally,RE elements show relatively high solid solubility in theα-Mg phase while the solid solubility drops significantly with the decreasing temperature.As the commonly used alloying elements in Mg alloys,RE elements can improve the binding energy of Mg atoms and decrease the diffusion velocity of atoms[120].The experimental studies show the hardness of Mg-Gd alloys increases linearly with the increasing Gd content asHv0.5(kg mm-2)≈37+14c(at.%)and the yield strength increase linearly withcn[42,121].The same models can also applied to Mg-Y system only with different coefficients[57].Particularly,Stanford et al.[89]have reported that the addition of Y in Mg can strengthen all three deformation modes,including basal slip,pyramidal slip and twinning modes.It should be noted that the strengthening effect of Gd and Y is significantly higher than that of Al and Zn[42,57,122],where in addition to the classical size and/or modulus misfits model,the valence effect may account for the enhanced strengthening effect of Gd and Y in Mg.
With continuous pursuing the higher strength,multialloying-element systems,particularly,the systems with both RE elements and non-RE elements have been extensively studied.Generally,with multi-solutes in theα-Mg phase,the dislocations-slip in the system becomes much more complicate and correspondingly,the strength is further enhanced.Cai et al.[122]indicated that only small addition of Gd(0.6 wt.%)in AZ91 alloy can effectively improve its tensile strength.And Yao et al.[23]have successfully prepared a high strength as-extruded(ED)Mg-1.4Gd-1.2Y-0.4Zn(at.%)alloy sheet(yield strength 303MPa,UTS 373MPa and elongation to failure 11.0%),where it is suggested that the solid solution strengthening effect of RE solute atoms(Gd,Y)accounts for the largest part of the high strength.Mo et al.[60]indicated that for Mg-0.5Gd-1.2Ca alloy,the solid solution strengthening can be thermally stabled.The volume fraction of theα-Mg phase can be increased with the addition of Y and/or Ce,and the UTS of the LA81 alloys can be enhanced with the addition of Y and/or Ce and especially the as-rolled LA81-0.6Ce-0.6Y alloy possessed a high UTS value as 278.7 MPa[73].The comprehensive properties of Mg-Li alloys can also be significantly improved by the combined alloying elements Al,Zn and RE elements[123–127].Wu et al.[126]reported the tensile strength and elongation of as-cast LAZ532 alloys can be enhanced with Ce addition and reach the maximum value 218.54 MPa and 15.36%,respectively,with 1 wt.% Ce addition.They also pointed out through solution treatment at 370°C for 12 h for the solution strengthening effect,the tensile strength and elongation of LAZ532 alloys can be further enhanced and reach 233.05 MPa and 18%,respectively,with 0.5 wt.% Ce addition[126].Recently,Król et al.[120]reported the enhanced hardness of Mg-Li-Al alloys with the addition of RE elements(Ce+La+Nd)for the added solid solution of the intermediatesized RE atoms.It should be noted that here the focused point is the solution-strengthening effects,hence some very high-strength Mg alloys(400~600 MPa)were not included here,where the precipitate-strengthening and some special structure-strengthening may take the main effects.
3.3.Calculations and predictions
Some models have also proposed to select the appropriate alloying elements for the design of Mg alloys.Yasi et al.[128]constructed a predictive mesoscale model for solute strengthening of Mg alloys by quantum-mechanical firstprinciples calculations,which was validated with available experimental data for several solutes such as Al and Zn.It is indicated that maximizing strength requires optimization of the tradeoff between high strengthening capacity(i.e.large solute misfits)and high solubility(low misfit magnitudes).A“design map”proposed by Yasi et al.[128]for the strengthening potencies of 29 different solutes combined the firstprinciples data for size-and chemical-misfits(i.e.,the change of stacking fault energy(SFE))with the simple solid-solution strengthening model as follows,is shown in Fig.8.
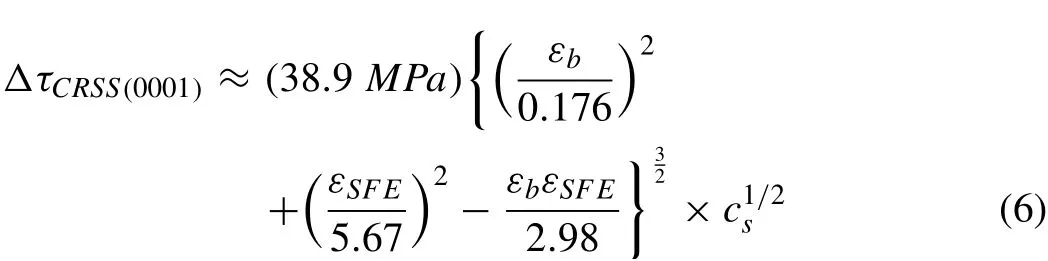
whereΔτCRSS(0001)is the change of the CRSS of the basal slip in the solid solution alloy in comparison with pure Mg in MPa,εbandεSFEare the size misfit and chemical misfitresulted by the solute atoms,respectively,andcsis solute concentration in at.%.
Toda-Caraballo et al.[129]proposed a general model that can be applied to estimate the strengthening effect of each solute on Mg,shown as:

whereσYis the yield strength,σCRSSis related to the CRSS of the predominant slip system,σpis a residual stress related to thermomechanical processing,Biis a constant for elementi,Xiis the atomic fraction of solutei,kY,Mg=0.21(MPa m0.5)is the strengthening coefficient of pure Mg,ΔkY,iis the variation onkYlisted in Table 1 andDgbis the final grain size.The predicted results using the model showed good agreement with the experimental results,and they suggested the atomic size and the elastic misfit combined controlling the solid solution hardening and the strengthening coefficient of the Hall-Petch relationship and especially,the atomic size misfit can take the larger effect on solid solution strengthening.
Besides,there are various type of first-principles calculation of the strengthening effects of defined elements in Mg alloys.The strengthening effect of Al have been explained by the formation of strong covalent bonds between Al and Mg atoms[130].With the ideal shear resistance and generalized SFE calculations,Garg et al.[131]reported that Al can increase the critical ideal shear resistance of all slip systems except the prismatic system and the energy barrier changes in opposite way,indicating the enhanced prismatic slip system.Though the ideal shear resistance other non-basal slip systems may be reduced with the solutes’addition,the basal slip system is still the easiest one to be activated and hence the solutestrengthening effects mainly attribute to the strengthening of the basal slip[58,132].Additionally,Liu et al.[133]have established the supercell models of Mg and Mg35Al to perform the first-principles pseudopotential plane wave calculations and reported the increase of the bulk modulus,Young’s modulus and shear modulus of the Mg-Al solid solution alloy.
As for Zn,based on the polycrystal plasticity model,Raeisinia and Agnew[134]have reported that the CRSS of basal slip increased with the increasing Zn concentration.According to the simulation results of the strain-stress curve of crystal cell along[0001]for Mg-1.85 at.%Zn alloy,Luo et al.[135]indicated the minimum CRSS of Mg,Mg-1.85 at.% Zn for pyramidal plane slip systems is 2.24,2.72 GPa,respectively.And the calculation for solute elements Sn and Zn in Mg were also performed by Garg et al.[58,132].

Fig.8.Solid-solution strengthening potency(contours)vs.their size(εb)and chemical(εSFE)misfits for 29 different solutes in Mg,copied from Ref.[128].
Due to the relatively high solid solubility of some RE elements such as Gd and Y in Mg,the solid solution strengthening effect of RE elements on Mg alloys can be strong.Using the viscoplastic self-consistent model,Agnew et al.[136]reported that Mg-Y binary solid solution alloys show higher strength in comparison with pure Mg.Recently,Luo et al.[135]have reported that the minimum CRSS of Mg-1.85 at.%Y for pyramidal plane slip systems is 2.96 GPa,much higher than that of Mg 2.24 GPa and Mg-1.85 at.% Zn 2.72 GPa,indicating a strong strengthening effect of Y in Mg alloys.Liu et al.[133]also reported that the bulk modulus,Young’s modulus and shear modulus of Mg increase with Er addition and the solid solution strengthening effect of Er is much greater than that of Al.
Here,to better differentiate the strengthening effects of different alloying elements in Mg alloys,the information of commonly used Mg-X binary alloys[56,137–147](including the solid solubility,lattice parameters,volume difference and bulk modulus,etc.)is given in the Table S1.

Table 2CRSS of the possible deformation modes in Mg under compression along the[0110],[0116]and[0001]orientations[98].
3.4.Section summary
Though after extensive studies on the solid solution strengthening Mg alloys,the clear strengthening mechanism is still hard to be concluded.One reason is the mixed effects coming from the solute,grains,and precipitations,which are hard to be experimentally quantified.Secondly,the calculations are mainly on the CRSS,which have no direct quantitative relation with the strength.Needleless to say,no calculation has been performed on varied amount solute addition and mixed solutes addition,the details of the solute-substitutions in lattice and then the effects on the strengthening of slip system.Additionally,it should be noted that the SFE is a fundamental parameter in metal and alloys,which is usually employed to understand the effect of different solutes on the activation of basal and non-basal slip systems in alloys and further the ductility of alloys.That also means,the increase of the SFE of the slip system in Mg alloys resulted by specific solid solution atoms can make the slip system more difficult to activate,thereby effectively strengthening the alloy system.Therefore,the SFE could be also an effective indirect parameter to measure the strengthening behavior of Mg alloys.
Generally,the stronger strengthening effects coming from RE elements have been observed compared with that coming from other common transition elements and main group metallic elements in the first five rows,where the big size of the atoms and the strong valance bonds may take the main effects.
4.Solid solution ductilizing Mg alloys
4.1.Solid solution ductilizing mechanism
As the lightest structural materials at present,Mg alloys have been widely applied in the areas of aerospace,transportation,3C production,biology and energy sectors.However,the deformation ability of Mg alloys especially at room temperature is severely limited due to their limited number of activatable slip systems at room temperature,which extremely hinders the large-scale production of Mg alloy products[2,4,43,45,58].The correlated studies on Mg single crystals showed that the basal slip is the easiest slip system to be activated with commonly accepted only about 0.5 MPa of stress to yield,while other possible deformation modes are significantly difficult to be activated due to the much higher CRSS values,as shown in Table 2[148–153].Therefore,the plastic deformation of Mg alloys at room temperature and low temperature range is dominated by basal slip.
At room temperature,the only two independent slip systems(less than five)in base〈a〉slip cannot satisfy the Von-Mises criterion,which is a major reason for the poor plastic deformation ability of Mg alloys at room temperature and low temperature range[154].In addition,the two independent slip systems are in the same slip plane and cannot coordinate the deformation along thec-axis,and the CRSS of the basal slip is much lower than that of the non-basal slip,which makes it easy to produce basal texture during the subsequent deformation processing of Mg alloys,leading to poor uniform forming ability and serious deterioration in performance uniformity.The CRSS ratio of non-basal to basal slip systems was reported to be 40–100 for single crystals and 4–6 for polycrystals of pure Mg[103,155].Therefore,reducing the CRSS difference between the basal and the non-basal slip systems to promote the activation of the non-basal slip systems to satisfy the Von-Mises criterion is an essential method to improve the uniform plastic deformation ability and finally the ductility of Mg alloys[44,94,122].
Since 2002,our group has performed significant research on the effects of alloying elements on the ductility of Mg alloys.It is found that some specific elements can reduce the ratio of the slip resistance between basal and non-basal slip systems and be helpful to the activation of non-basal slip and thus improving the ductility of Mg alloys[44,156].An alloy design theory of“solid solution strengthening and ductilizing”for Mg alloys was proposed,and more detailed results can been seen in the reference[44].
It can be seen from Fig.9 that the slip resistance of the basal or non-basal plane can be increased or decreased when the alloying elements are solid solutioned in theα-Mg phase.And the difference of the slip resistance between the basal and the non-basal plane of solid solutions((Δτ′)),between the basal and the non-basal plane of Mg(Δτ)can be concluded as the three cases:Δτ′≈Δτ,Δτ′<ΔτandΔτ′>Δτ,when the slip resistance of the basal or non-basal plane is increased or decreased after the solid solution of alloying elements.
CaseⅠ:Δτ′≈Δτ.As shown in Fig.9(a)and(d),the solid solution of alloying elements has little effect on the difference of slip resistance between the basal and the non-basal plane,and therefore cannot promote the activation of non-basal plane and improve the ductility of Mg alloys.

Fig.9.Alloy design theory of“solid solution strengthening and ductilizing”:(a)and(d)Δτ′≈Δτ;(b),(e)and(h)Δτ′<Δτ;(c),(f)and(g)Δτ′>Δτ,redraw from[44].
CaseⅡ:Δτ′<Δτ.As shown in Fig.9(b),(e)and(h),the solid solution of alloying elements can reduce the difference of slip resistance between the basal and the non-basal plane,which is beneficial to the activation of the non-basal slip,and therefore improving the uniform plastic deformation ability of Mg alloys.
CaseⅢ:Δτ′>Δτ.As shown in Fig.9(c),(f)and(g),the solid solution of alloying elements can increase the difference of slip resistance between the basal and the non-basal plane,making the activation of the non-basal slip more difficult,which is not conducive to the enhancement of the ductility of Mg alloys.
However,it is worth noting that the decrease in the slip resistance of basal slip decreases the strength-enhancing effects of Mg alloys.As shown in Fig.9(e),the ductility of the alloys can be improved while the strength can be deteriorated to some extent.Nevertheless,the case shown in Fig.9(b)and(h)should be the development direction,and especially the case shown in Fig.9(h)is the most conducive circumstance to simultaneously improving the strength and ductility of Mg alloys.Therefore,alloying elements that can not only increase the slip resistance of basal plane,but lower the difference of slip resistance between the basal and non-basal plane should be selected for the alloys design,which can have a strengthening effect to enhance the strength and promote the activation of non-basal plane to improve the ductility,thus realizing the aim of alloys design“solid solution strengthening and ductilizing”.
Recently,it is reported the poor ductility of Mg alloys has been further attributed to the pyramidal
Notably,the effect of solutes on the ease of cross slip of a dislocation was believed to be the reason for the solute softening effect[103].Yasi et al.[156]also confirmed it using an atomic scale modeling and the solute effect on the improved ability of dislocations to form a jog-pair was suggested to be the reason for the observed solute softening in Mg single crystals[103,157].Furthermore,Wu et al.[158]recently verified the solute-enhanced cross-slip mechanism by in-situ observations of deformation of Mg-Y alloy,and presented the competing pyramidal-to-basal transition and pyramidal II-I crossslip processes during the expansion of an L×L
Most recently,they have also proposed an theoretical mechanism for the ductility enhancement of solid-solution Mg alloys that solute can accelerate the cross-slip of pyramidal
Additionally,the solute-enhanced
4.2.Alloying element effects
Three important microstructure factors,including grain size,texture and slip activity,have been concluded to take effects on the ductility of Mg alloys[160].As far back to 1962,Chapman and Wilson[161]have reported that the ductility enhancement can be realized by the reduction of grain size and the promising effect of small grain sizes on the ductility of Mg alloys was also confirmed by the experimental results[162].The enhancement of ductility of fine-grained Mg alloys could be ascribed to the decreased prevalence of twinning in refined structures[163,164].
In addition to the grain size,the texture also shows potential effect on the ductility of Mg alloys[165–169].It was reported that the ductility of Mg alloys with weakened texture can be improved when the grain size was normalized[166]while the specific reason for this effect is unclear now[160].Perez-Prado et al.[160]have suggested the most possible reason for the improvement of ductility of Mg alloys with weaker texture is the reduction in the activation of twinning.Besides of the above two points,the activity of slip system is considered as a fundamental factor on the ductility of Mg alloys[128,147,158,170].Solutes in theα-Mg phase can effectively increase the activity of the non-basal slip systems,and especially the
Mg-Al-X system:As for alloying element Al,Akhtar and Teghtsoonian[157]have early reported at low temperatures,the CRSS for{1010}<1120>prismatic slip decreased continuously with the addition of Al while at higher temperatures,the CRSS increased at low Al solute concentrations and then decreased at the higher one.The results can be explained in terms of an increase of a decrease of the thermally activated component of the flow stress with the addition of Al.Furthermore,the drop in Peierls’stress with the addition of Al was suggested to be a general solution effect.However,the decrease in the height of Peierls’barrier,which was shown to be the rate controlling step in prismatic slip of Mg and its alloys,is not necessarily related to the decrease of axial ratio(c/a,the decreased c/a ratiowas reported to promote the activation of the pyramidal
Mg-Zn-X system:Same as alloying element Al,the CRSS for{1010}<1120>prismatic slip can be decreased continuously with the addition of Zn at low temperatures while at higher temperatures,the CRSS increased at low Zn solute concentrations and then decreased at the higher one[157].It is indicated a small amount addition of Zn(<0.5 at.%)can simultaneously strengthen and soften the Mg-Zn alloys because of the strengthening of the basal planes and the activation of prismatic and possibly pyramidal planes[172].
As mentioned above,Al cannot significantly improve the ductility of Mg alloys.However,the combined addition of Al and Zn was suggested to reduce the slip anisotropy by selectively strengthening basal slip[173,174]and thus making the prismatic and/or pyramidal slip systems easier to be activated in comparison with the basal slip,finally improving the ductility of Mg alloys effectively.Chino et al.[175]reported the room temperature stretch formability of as-rolled Mg-xZn-0.2Ce(wt.%)alloys can be improved with Zn addition up to 1.5 wt.% for the improved texture and softening effect of Zn solutes.Furthermore,the combined strengthening and softening effect with small amount addition of Zn was confirmed in Mg-Zn-Ca-Ce alloys[104],which can be attributed to more non-basal slip systems being easier to be activated,and especially the prismatic slips with the increasing Zn addition up to 1.5 wt.%.The 1.5 wt.% Zn addition can improve both the strength and ductility of Mg-Zn-Ca-Ce alloys with tension yield strength(TYS)and tension fracture elongation(TFE)about 131.0 MPa and 42.4%,respectively,in extrusion direction(ED).Similarly,the ductility could be deteriorated with further Zn addition for the dominant solid solution hardening[104].
Mg-Ca-X system:Ca also plays an important role on the ductility enhancement of Mg alloys.The Ca addition in Mg can promote the activation of non-basalslip as a result of the reduction of energy barrier fordislocations to cross slip to non-basal planes(Ca can reduce the unstable SFE for all slip modes in Mg),thus improving the ductility[64,66].In their study[64],although the basal slip{0001}<1120>is still the major deformation mode,the fraction of nonbasal slip systems({1100}prismatic planes and{1101}pyramidalⅠplanes)increases with the increase of strain,which also plays an essential role in the deformation of Mg-0.47Ca(wt.%)alloy,and the pyramidal I slip lines were associated withinstead of
Since Zn and Ca can both increase the ductility of Mg alloys,the effects of combined Zn and Ca on the ductility of Mg alloys have attracted much attention.Chino et al.[177]have reported that the elongation to failure of Mg-Zn alloys increased with Ca addition and especially,the incremental amount at 90°(the angle between the tensile direction and the RD)was larger than those at 0 and 45° corresponded to titling of the basal planes to the transverse direction(TD).Also,the stretch formability of Mg-Zn alloys can also be significantly enhanced with Ca addition due to the promotion of the prismaticand
Chai et al.[182]have also reported that the ductility of Mg-1Sn-0.7Ca alloy in tension along the ED as 17.3% can be increased to 30.5% after the addition of 0.5 Zn wt.%,attributing to the increased activation of prismatic slip and some other factors like enhanced grain boundary cohesion and improved intergranular strain propagation capacity.Additionally,Cheng et al.[183]further indicated that the alloying element Zn can reduce the unstable SFE of prismatic and pyramidal slip systems in Mg-Sn alloys,thus possibly activating the prismatic and pyramidal slip systems and finally effectively improving the ductility of Mg-Sn alloys.The significantly improved ductility of Mg-1Gd(wt.%)alloy with the synergistic alloying of Ca and Zn as 28.5% in ED was also reported by Zhao et al.[65].Furthermore,one high strength(YS=165 MPa in TD)and high ductility(EL=18% in TD)as-rolled alloy Mg-2Al-1Zn-0.1Ca(wt.%)was proposed by Nandy et al.[66],and the high ductility is mainly due to the decreased CRSS ratio between the basal slip and non-basal slip after Ca addition.
Other TM-contained system:The ductility of Mg-3Ni(wt.%)alloy can be improved with Mn addition up to 1 wt.%,where,besides the refinement effect ofα-Mg dendrites,the solid solution ductilizing with Mn addition takes the main part[184].Also,the Sn addition can also improve the ductility of Mg alloys.Gu et al.[111]have reported that 1 wt.% Sn solid solutioned in theα-Mg phase can improve the ductility from~13%to~20%in as-cast alloy while deteriorate the ductility in as-rolled alloy.The solid solution of Sc,though exhibiting limited solid solution strengthening effect on Mg alloys for the small atomic size,weak solute-dislocation and solute-twin boundary interactions,can effectively improve their roomtemperature ductility due to the decreased SFE after Sc addition[185].Silva et al.indicated the maximum strain increases from 15% for pure Mg to 30% for Mg-0.4Sc(at.%)alloy at 298 K[185].One high strength-ductility XAZM-300(Mg-1.0Ca-1.0Al-0.2Zn-0.1Mn(wt.%))Mg alloy was developed by Pan et al.[186]in which the TYS can be up to~425 MPa and the elongation is reasonably high as~11%.The high density of activated stable pyramidal dislocations,

Fig.10.Identification of the dislocations associated with pyramidal I slip lines in a specimen of Mg-0.47Ca(wt.%)alloy after 4% strain,(a)Pyramidal I slip lines are identified in Grain A whose orientation(in Euler angles)is shown.A TEM thin foil across these slip lines was extracted by FIB.(b)The location of the thin foil to be lifted out.(c)After FIB cutting of a large trench,the foil emerged and was extracted using a microprobe.(d,e)Two-beam dark-field images taken along the[1010]zone axis under g=and g=(0002),respectively.Copied from[64].

Fig.11.Wavy slip lines observed during the in-situ test for the specimen of Mg-0.47Ca(wt.%)alloy at 8% strain:(a)cross-slip ofdislocations between basal and prismatic planes,(b)cross-slip ofdislocations between basal and pyramidal I planes,copied from[64].

Fig.12.Secondary electron SEM micrographs and corresponding EBSD maps showing non-basal slip traces in grains with(a,b)high and(c,d)low Schmid factors for basal slip in Mg-0.3Zn-0.1Ca alloy sheet subjected to(a,b)0.06 and(c,d)0.12 plastic strain.Schematic diagrams showing(e)pyramidal slip and(f)basal slip in grain A,and(g)pyramidal slip in grain B,copied from[178].
which are less possible to transform into the immobile one for the low moving resistance in XAZM-300 Mg alloy,provided additional deformation mechanisms along thedirection,and thus is believed to contribute to the improved ductility.
Mg-Li-X system:Mg-Li alloys,as the lightest structure materials in the present,have attracted much attention[187–190],where the alloy formability at room temperature have been significantly improved with Li addition[191].The maximum solid solubility of Li in theα-Mg phase is 6.25 wt.%at 236°C and the structure starts to change from hexagonal close-packed(HCP)to body center cubic(BCC)when the content of Li is more than 5.7 wt.%[189],and the structure of Mg-Li alloys transformed completely into the body center cubic(BCC)when the content of Li is more than~10.3 wt.%or the Mg/Li ratio by mass is less than 8.7[192].The existence ofβphase and the decrease of the axis ratio(c/a)of the lattice originated from the addition of Li into Mg enabling prismatic slips to be activated easier can significantly enhance the deformation ability of Mg-Li alloys[136,191].The reasons for the improvement of ductility of Mg-Li alloys have been studied extensively[136,171,193–197].Agnew et al.[136]have reported that the addition of Li in Mg alloys has a positive effect on the randomization of the texture and can improve the ductility at room temperature due to the enhanced non-basal slip activity,prismaticslip especially.Furthermore,Al-Samman[195]found that 4 wt.%Li addition can effectively enhance the activity of non-basal slips leading to the development of a weakened texture,which is beneficial to improve the ductility and reduce the plastic anisotropy of Mg-4Li sheet.Haferkamp et al.[194]indicated that the addition of Li in Mg alloys can reduce thec/aratio of Mg alloys,which has an effective influence on the reduction of the CRSSs,and therefore enhancing the activity of nonbasal slips.In addition,Agnew et al.[136,171]also reported that the addition of Li in Mg alloys can reduce the CRSSs of non-basal slips and enhance the
Mg-RE-X system:RE elements have attracted much attention on the improvement of ductility of Mg alloys as main effects on weakened texture and activation of non-basal slips[43,198–201].Shi et al.[174]examined the rolling textures of six Mg alloys with different content of Zn and RE elements(Ce,La,Nd,and Y)and the results showed that the overall texture strength and the basal pole intensity of REcontaining Mg alloys aligned with the sheet normal direction is lower than those Mg alloys without RE addition.In addition,the planar anisotropy of RE-containing Mg alloys was reduced(r~1),in which the lowerr-values mean a larger volume fraction of grains that are favorable to accommodate in-plane tensile deformation by basal slip and twinning,and therefore the formability of Mg sheets with lowerr-values could be improved under straining conditions which call for thinning of the sheet[174].Hantzsche et al.[175]reported that Mg alloy sheets with weak textures can be obtained by the solid solution of Nd,Ce and Y in theα-Mg phase and thus the sheet formability can be improved.
Ce is considered as one effective alloying element to improve the ductility of Mg alloys.Chino et al.[202]have already reported that 0.2 wt.% Ce addition can improve the room temperature ductility of Mg-0.2Ce(wt.%)alloy for the homogeneous deformation due to the promotion of non-basal slips.And the Mg-0.2Ce(wt.%)alloy shows the samec/aratio 1.6244 with that of pure Mg,therefore the change of atomic binding states resulted from the addition of Ce was suggested to be the most important factor in the enhancement of non-basal slips[202].However,the ductility was deteriorated at 573 K due to the suppression of dynamic recrystallization and the low diffusion with 0.2 wt.% Ce addition.Recently,Sabat et al.[200]indicated that small addition of Ce(0.2 wt.%)in Mg can reduce the relative CRSS values of prismatic,pyramidal
Gd and Y are the typical alloying elements that can not only enhance the strength,but improve the ductility of Mg alloys,and the improved ductility of Mg-Gd and Mg-Y alloys during deformation was suggested to be related to the enhanced activity of non-basal slip systems and in particular the
Wu et al.[209]has reported one Mg-(2 and 3)Gd-1Zn(wt.%)alloy with excellent room temperature ductility and formability,the ultimate elongation~50% and a uniform elongation greater than 30% at room temperature as a result of the non-basal texture and low texture intensity that can lead to a high strain hardening exponent,large uniform elongation,etc.Additionally,Nagarajan et al.[210]indicated that the notional friction stress,δ0,was unaffected by the Gd content up to 0.4 at.% Gd compared with the pure Mg,but increased rapidly afterwards.The two-stage behavior was consistent with the solid solution softening of the prismatic planes at the lower concentrations and strong solution hardening by short-range order,on both basal and prismatic slip at the higher ones[210].
Based on the results from electron backscatter diffraction and transmission electron microscopy analysis,Sandlöbes et al.[205]attributed the improved ductility of Mg-Y alloys to the significantly large amount of slip with
Notably,Gd and Y show the high potential to improve the strength and ductility of Mg alloys simultaneously.Kula et al.[198]have reported that the Gd and Y solutes can improve both the strength and ductility of Mg-Gd and Mg-Y alloys,respectively.It is worth noting that compared to Mg-Y alloys,Mg-Gd alloys showed higher strength and ductility under tension and compression due to the more effective solid solution strengthening and grain-boundary strengthening effects of Gd solutes[198].
Though three factors grain size,texture and slip activity are mainly considered in this work for their relationships with the ductility of Mg alloys,it should be noted that many other interrelated factors like work hardening rate and strain rate sensitivity also have significant effects on the ductility and formability of Mg alloys[160].And both work hardening rate and strain rate sensitivity provide a material with macroscopic resistance to necking[160].It is reported by Xie et al.[154]that the macroscopic ductility is commonly determined by the onset of necking which is very sensitive to the work hardening rate.Additionally,the ductility limit in superplasticity can be commonly explained by the necking instability[215,216],which is determined by the Hart condition as,

As discussed in the work of Pérez-Prado et al.[160],the true meaning of work hardening rate and strain rate in the microstructure of Mg alloys is not as clear as in that of Al alloys though they are relatively easy to measure.For Mg alloys,the activation of twinning can increase the work hardening rate,while it is not entirely clear whether this has the same effect on ductility as an alloy that deforms completely by slip.The microstructural factors resulting the hardening effect in these two cases are significantly different,since Mg alloys do not always fail due to the ductile mode of plastic instability.Conversely,Mg alloys can transition into a more brittle fracture mode when the flow stress becomes very high.Notably,the addition of solutes could also have an effect on the work hardening rate and strain rate sensitivity in consistent with the effect on the texture and relative slip activity,and these effects cannot easily be decoupled.
4.3.Calculations and predications
It is worth noting that the key to activating the non-basal slip systems is to reduce the difference of CRSS between the basal and non-basal slip systems.The SFE,as the energy corresponding to a stacking fault formed by a specific relative sliding displacement,is directly related to the CRSS of each slip system and has been widely studied in recent years.Numerous studies have indicated that the change of SFE is related to the activation of the basal and non-basal slip systems and the ability of the plastic deformation.The effects of different solute atoms on the SFE of Mg alloys have been extensively studied by our group,in which(i)the alloying elements like Al,Bi,Ca,Dy,Er,Ga,Gd,Ho,In,Lu,Nd,Pb,Sm,Sn,Y and Yb are reported to significantly reduce the SFE of I1,(ii)the alloying elements like Ca,Dy,Er,Gd,Ho,Lu,Nd,Sm,Y and Yb are reported to greatly reduce the unstable SFE of the prismatic slip system and are beneficial to the reduction of the CRSS of the prismatic dislocation and(iii)the alloying elements like Ag,Al,Ca,Dy,Er,Ga,Gd,Ho,Li,Lu,Nd,Sm,Y,Yb and Zn are reported to be beneficial to promote the activation of the pyramidal slip system and can improve the intrinsic ductility of Mg alloys[44].Han et al.[217],based on the first-principles study,also reported that the Al addition can improve the ductility of Mg-Al alloys attributed to the decrease of the SFE on the basal plane.Furthermore,thegeneralized SFEs and(0001)surface energies of Mg alloyed with nonmetallic elements including H,C,N,O and Si were computed by Zhang et al.[218]through first-principles calculations and it was predicted that Si,O and N exhibit potential in modifying the ductility of Mg alloys by considering the competition between pyramidal dislocation emission and basal crack propagation.
Additionally,Sandlobes et al.[170]reported that the ductility of Mg increase with the addition of Y due to the high activity of pyramidal
To better explain the relationship between SFE and the activation of the basal and non-basal slip system and the deformation ability of Mg alloys,our group has further studied the effects of different solute atoms(Al,Zn and Y with a content of 0–3 at.%)on the SFE of Mg at 0–500 K by the molecular dynamics simulation,in which the generalized SFE of basalslip systems,prismaticslip system and pyramidalslip system were studied[44].The modified plastic forming parametersχ1andχ2shown in Eqs.(9)and 10)were employed to explain the relationship between the SFE and deformation ability of Mg alloys at the same temperature:the parameterχ1is related to the activation of basal and prismatic slip systems andχ2is related the activation of basal and pyramidal slip systems.

whereγisBis the stable SFE of the basalslip system,γusBis the unstable SFE of the basalslip system,γisMis the substitution value of the stable SFE and unstable SFE of the prismatic slip system,γisPyrandγusPyrare the stable SFEγisand unstable SFEγusfon the second-order pyramidal plane,respectively,and the subscript Mg indicates pure Mg and X indicates Mg-X alloy.For Eqs.(9)and(10),once the values ofχ1andχ2of the alloy are greater than 1,the ductility of the alloy is better.The results show that the values ofχ1andχ2of the Mg-Al alloy at various compositions and temperatures are mostly less than 1,and both decrease slightly with the increase of Al content.Nevertheless,the values ofχ1andχ2of the Mg-Zn and Mg-Y alloys are mostly greater than 1,and especially the values ofχ1andχ2increase with the increasing Zn/Y content indicating that the tendency of prismatic slip increases.Notably,the values ofχ1andχ2increase more rapidly with the increasing Y content than that with the increasing Zn content.It can be concluded that the increase of Al content cannot effectively promote the activation of non-basal slip systems while the increase of Zn content can be beneficial to the activation of non-basal slip systems,and particularly the increase of Y content can obviously increase the possibility of the activation of the non-basal slip systems in Mg alloys at the same temperature.The simulation results further confirm that the solid solution strengthening and ductilizing of Mg alloys can be realized by the solid solution of Zn and Y.
Besides,Zeng et al.[21]calculated the theoretical critical shear strength(τmax,reflecting the value of CRSS)of pure Mg and Mg-Sn models for basal and pyramidal slip systems by first-principles.The calculated results showed that after doping Sn atoms to pure Mg,theτmaxin basal slip increased from 114 to 149 MPa while that in pyramidal slip decreased from 1571 to 446 MPa.Hence,the decreasedτmaxratio of the pyramidal slip to the basal slip can make the pyramidal slip systems more likely to be activated and therefore leading to an improvement of the formability of Mg-Sn alloys.Moreover,Buey et al.[20]studied the core structure and solute strengthening of second-order pyramidal
In addition to the SFE,some other factors and models have also been studied to investigate the effect of different solute atoms on the ductility of Mg alloys.Yasi et al.[156]developed a geometry-based model from first-principles data for the interaction of solutes with a prismatic screw dislocation core,and predicted the possibility of thermally activated cross-slip stress above room temperature in Mg alloys.From the interaction data,they predicted six binary Mg alloys(Mg-Ca,K,Na,Sc,Y and Zr)with softening prismatic slip and suggested that Mg-Li alloys could also have lower crossslip stress above room temperature.The softening of thermal cross-slip was primarily assigned to a reduction of the easy prismatic SFE.
Later,Tsuru and Chrzan[220]computed the changes in electronic structure associated with the motion of the dislocation over its Peierls barrier and reported that these changes occurred primarily in p-states right below the Fermi level for thescrew dislocation in Mg.Hence,alloying additions with electronic state in this energy range were likely to interact strongly with the dislocation core and thereby influencing its motion.These additions,in turn,can be identified through simple computation of their electronic structure when embedded within an otherwise perfect bulk.Therefore,They[220]suggested that the substitutional Y,Ca,Ti and Zr were expected to interact strongly with the screw dislocation cores in Mg while Zn and Al with little interaction.Furthermore,they also indicated that Y can stabilize the screwdislocation compact core in Mg and thus enabling cross-slip ofdislocations from the basal plane to non-basal planes.
In the works of Garg et al.[58,131,132],alloying elements Ce,Y and Zr were reported to decrease the ideal shear resistance along different slip systems of Mg alloys,and lower the dislocation motion energetics on all the slip systems thus enhancing the dislocation motion.Besides,the fraction of loading orientations that exhibited a preference for non-basal slip systems increased with the addition of Ce,Y and Zr solute atoms indicating a decrease in the plastic anisotropy(i.e.the activation of non-basal slip systems)of Mg alloys,and the dominant slip system in Mg was also changed from the basal partialto prismaticwith the addition of Ce,Y or Zr solute atoms.Furthermore,the electronic density of states and valence charge transfer were suggested to provide a quantum insight into the underlying factors influencing the observed softening/strengthening behavior[58,132]and for example,the electronic density of states calculation showed that the contribution fromdstates of Ce,Y and Zr solute atoms decreases the electronic structure stability of their respective solid solution and thus enhancing the slip activity.
The viscoplastic self-consistent model was applied by Agnew et al.[136]to interpret the differences in the mechanical behavior of hexagonal close packed Mg alloys,they found the compressive ductility of all tested alloys(3,5 Li,and 1,3 Y wt.%)are improved significantly in comparison with pure Mg due to the increased
Kim et al.[173]have investigated the effect of alloying elements on improving the room temperature ductility of Mg alloys through the molecular dynamics simulation and reported that dislocation binding is an origin for the enhancement of room temperature ductility of Mg alloys.It is clarified that solute atoms have stronger dislocation binding tendency and solid solution strengthening effect on basalslip planes than on non-basal<
It was reported that the materials with the bulk/shear modulus(B/G)ratio above the critical value of 1.75 are considered to be ductile[222].Liu et al.[117]reported all studied Mg52RE2(RE=Sc,Y,Gd-Tm~3.7 at.% RE)solid solutions exhibit better ductility than pure Mg for their higher B/G ratios above 1.93.And from their calculation results,the ductility of the binary Mg-RE solid solutions decreases gradually with the increasing atomic number for the elements in lanthanide series[117].In addition,Liu et al.[80](Liu and Li 2015)studied the generic correlation between the electron work function(EWF)of solutes,which largely reflects their electron behavior,and mechanical properties of solute-added Mg.They predicted that adding solutes with EWFs lower than that of Mg can improve both strength and ductility of Mg,while adding solutes with higher EWFs results in higher strength but lowered ductility,as shown in Fig.13.The result shows that the EWF of solutes is an important parameter for the solute selection.
Curtin’s group had thoroughly studied the origins of high hardening and low ductility of Mg[43],proposed a mechanistic and quantitative theory to enable high ductility as a function of alloy composition[158],as well as the theory and a mathematical model to identify ductile dilute Mg solid solution alloys in terms of solute elements and their concentrations[201].Based on the long-time molecular dynamics simulations with a density-functional-theory-validated interatomic potential,they reported that the key
Recently,Curtin’s group[201]further revisited the theory for solute-accelerated cross-slip[158]with an explicit atomistic derivation and extended it to the theory including very dilute multiple solute concentrations.And the prediction of ductility for a series of Mg alloys based on the revisited theory was in good agreement with the experimental results.Thus,the revisited theory was then applied to predict the composition range for ductility in RE-free dilute Mg alloys and the predicted ductile Mg alloys could be the guidance for the design of new ductile Mg alloys.
4.4.Section summary
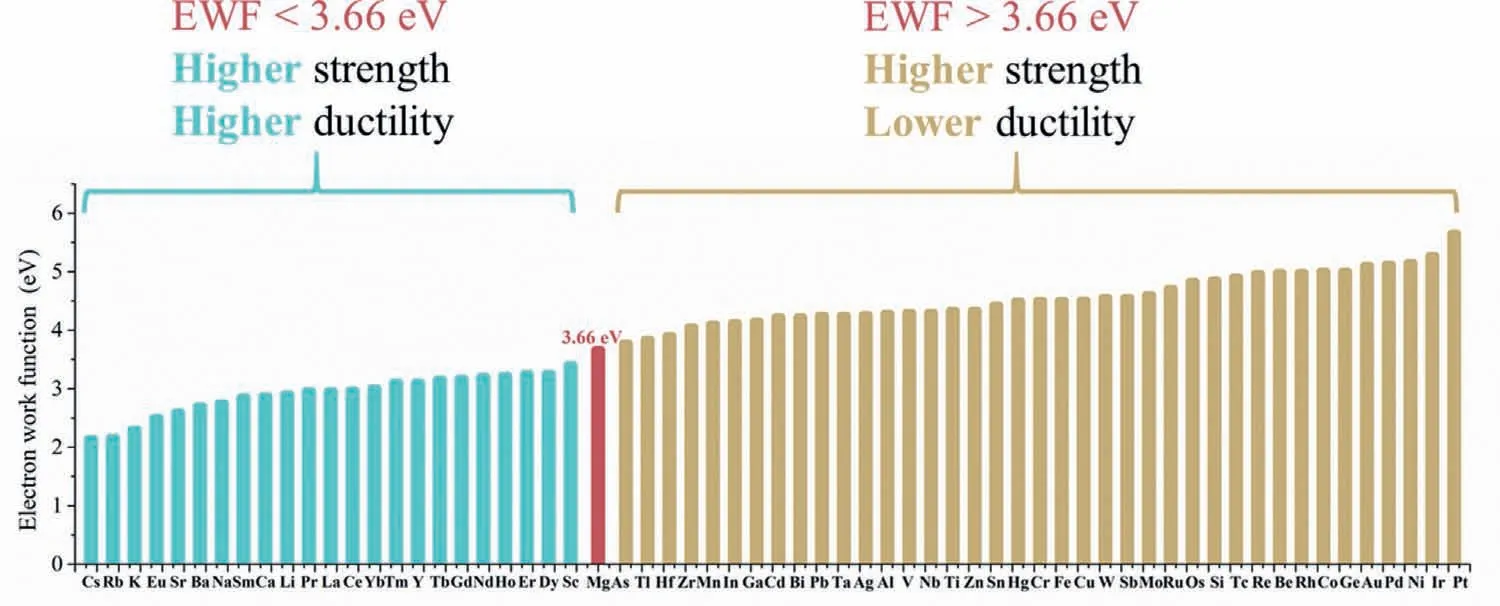
Fig.13.A schematic illustration of effects of elements as solute on the strength and ductility of Mg with respect to their EWF values,redraw from[80].
Due to the poor formability of Mg alloys and the few tailoring strategies,the unique synergy strengthening & ductilizing effects by solid solution for Mg alloys have attracted a lot of attentions.Especially,our group has developed more than 20 kinds of new high-performance Mg alloys based on the theory of solid solution strengthening and ductilizing and the control of long-range ordered phase.Among them,16 kinds of Mg alloys have been approved as national standard alloys and 9 kinds of Mg alloys have been approved as international standard alloys[12].Whatever the experimental results or the calculation results reported up to now confirms that the theory of solid solution strengthening and ductilizing is reliable and feasible.However,though a lot of theoretical calculations to describe the synergy effects have been proposed,the calculated values,like the ratio,the calculated SFE,cannot directly quantitively connected with the CRRS,and the elongation values.Additionally,though it is observed the solute can decrease the GSEF of non-basal slip system,the absolute difference between the values of GSFE of non-basal slip system and that of basal slip system is still quite large(200~300 difference compared with baseline~100).Hence,in this case,it is hard to say the real increasing magnitude of the ductility by the solid solution.The in-situ observation of the dislocation slips during the deformation of Mg alloys have also been tried in many groups,but the clear identification for the slip systems is still an elaborated work.In addition,it is reported the solid solution-ductilizing effect is only functional for the very dilute Mg alloy.An atomic scale description of the solid solution ductilizing effect should be developed since the resulted lattice distortion and energy is actually quite small for the dilute alloy.Furthermore,there is still few studies on the multi-solute ductilizing effects.Hence,there is still a lot of work for the revealing of the solid solution ductilizing mechanism,the quantification of the solid solution effects and,the last but not the least,the solid solution design rule for the high ductility Mg alloys.
5.Solid solution corrosion resistance enhancing Mg alloys
5.1.Solid solution corrosion resistance enhancing mechanism
Poor corrosion resistance has always been the shortcoming for the further and wide application of Mg alloys[223–225].Such a shortcoming of Mg and its alloys can be attributed to the special chemical properties of Mg[226],e.g.the low standard electrode potential(-2.37 V vs.Standard hydrogen electrode)and the significantly high chemical activity of Mg.Therefore,the micro-galvanic corrosion resulted by the Mg with low electrode potential as the anode,and other metals,the secondary phases and impurities(with higher electrode potential than that of Mg)exist in the matrix of Mg as the cathode,plays an important role in the corrosion of Mg and its alloys[227–230].
The overall corrosion reaction of Mg or Mg alloys are expressed as[223,224,231,232]:
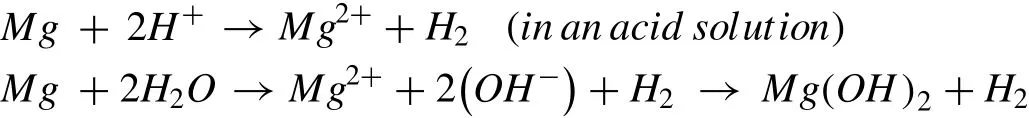

Furthermore,the overall corrosion reaction can also be expressed with separate anodic and cathodic reaction:

The MgO film,which can protect the matrix to some extent,can be formed when the Mg alloys were exposed in air.It is worth noting that the surface MgO film of Mg alloys with the value of Pilling-Bedworth ratio(PBR),which clarified the covering integrity of the passive film on the surface of the alloy(PBR<1,the coverage of the surface film is not sufficient to protect the metal;PBR≥2,the surface film is easy to split and peel off due to the large internal stress;1 Fig.14.The standard electrode potentials of the common alloying elements in Mg alloys in water at 298.15 K[253]. The increase of electrode potential of theα-Mg phase,reduction of anodic dissolution rates,formation of protective films and so on that are beneficial for the corrosion resistance of Mg and its alloys can be realized with the appropriate addition of appropriate alloying elements in Mg[184,226,236–238],and some other methods such as the plastic deformation process[239],heat treatment[240]and surface modification[5,8,241–244]can also effectively improve the corrosion resistance of Mg alloys. As one of the most used methods on the improvement of corrosion resistance,adding alloying elements have attracted increasing interest for their functional prospects.The improvement of the corrosion resistance of Mg alloys with the addition of alloying elements are mainly due to the grain refinement[15,245,246],the protective films formation on the Mg alloys surface[247,248],the electrode potential difference reduction[63,249,250]and the altering of the composition,volume fraction and distribution of secondary phases[251,252].And in this review,the critical effects of the alloying elements in the form of solute atoms on the corrosion resistance of Mg alloys have been discussed. (1)The increase of the electrode potential of the matrix.As shown in Fig.14,the standard electrode potentials of most alloying elements such as Al,Zn Ga,etc.,except RE elements for the similar electrode potentials with Mg,are positive than that of Mg[253].It is presumed,the addition of these alloying elements below their solid solubility limit in Mg alloy can effectively increase the electrode potential of theα-Mg phase,and then decrease the potential difference between the matrix and other cathode phases(secondary phases and impurities),finally suppressing the micro-galvanic corrosion and realizing the improvement of the corrosion resistance of Mg alloys[230,254–257].For example,when Sn is solid solutioned in theα-Mg phase,theEcorrof the matrix increases and the corrosion driving force decreases,and finally the corrosion tendency of theα-Mg phase itself is inhibited[230] (2)The solid solutioned alloying elements can also make Mg alloys become more passive.The passivation of Mg alloys is caused through the formation of a protective film on the surface,and the substrate alloy can influence the passivation and corrosion behaviors through influencing the composition of the surface film[258].For example,Al[259],Sn[247,260],Sc[61,62]and especially some REs solute atoms[261–263]can form the related oxide layer,and the oxide layer that is insensitivity to the NaCl solutions can act as a passive film to effectively inhibit the anodic dissolution after the deactivation of the outer layer Mg(OH)2.Similarly,the incorporation of solute atoms in the passive film,which is in the form of their oxides or hydroxides[264],can make the film more stable and thus reducing the susceptibility to the dissolution of passive film[263–270].In addition,the accumulated Sn at the interface of substrate Mg and passive film was suggested to effectively improve the corrosion resistance of Mg alloys[271]. (3)Some alloying elements such as Al,Zn,Cd[236],Ga[248,272,273]and Sn[230,272,274]possess high hydrogen overpotential.It is proposed theses alloying elements solid solutioned in theα-Mg phase can inhibit the hydrogen evolution reaction(cathodic reaction shown as Eq.(12)),thus improving the self-corrosion potential and corrosion resistance of the Mg alloys. (4)Alloying elements like Mn[275],Zn[276],Sr[269]and Y[277]were reported to increase the charge transfer resistance of Mg alloys,raising the resistance of corrosion reaction of Mg alloys.Especially,the diffusion resistance of corrosion product film(the resistance of Mg2+diffusing through corrosion product film into bulk solution)can also be enhanced after the trace amount Sr addition[269].And REs were also reported to increase the diffusion resistance of product film and thus inhibiting the film hydration process of Mg alloys,which can finally improve the passivity of corrosion product film[278,279]. Once the content of alloying elements in Mg alloys is higher than the solubility limit,second phase precipitates will form.Normally,the effect of the presence of second phases on the corrosion resistance can be determined by two aspects[224]:(i)the volume fraction and the distribution of second phase.Once the second phase is in large amount and distributes continuously,it can act as barrier to the propagation of corrosion.On the contrary,the second phase can act as microgalvanic cathode and thus accelerating the corrosion.(ii)The cathodic reduction rate of second phase.It is well known that the cathodic process of Mg is mainly dominated by the hydrogen reduction process,and the higher cathodic reduction rate inevitably results in the faster hydrogen evolution rate,therefore accelerating the micro-galvanic corrosion.For example,the second phases MgxXy(X=Al,Zn,Y,Gd…)with few amount and discontinuous distribution can form microgalvanic couples with theα-Mg phase and therefore accelerating the corrosion rate of Mg alloys,finally deteriorating the corrosion resistance[61,102,280–282].Contrarily,the second phases can act as corrosion barrier when they are in large amount and distributes continuously,thus improving the corrosion resistance of Mg alloys[17,252,280,282,283]. Mg-Al-X system:The effects of Al with different content on the corrosion resistance of Mg alloys has been systematically studied.It was suggested that enhanced corrosion resistance can be obtained by the high segregation of Al atoms[223,280,284],which was suggested to efficiently prevent the propagation of corrosion cracks due to the increased electrode potential of Mg by alloying with Al and the formation of continuous barrier to corrosion attack,whereas the low level of Al segregation cannot take the same effect[284]:Zhang et al.[250]have also recently indicated a higher content Al,with a more noble standard electrode potential-0.76 V than that of Mg-2.36 V,in theα-Mg phase can increase the electrode potential of Mg and thus improving the corrosion resistance of theα-Mg phase.It was reported that the corrosion rate of as-cast Mg-6Zn-8.16Y-2.02Mn in the Hank’s solution at 37°C can decrease by 79.5%,from 4.82 to 0.99 mm/y,with 0.3 wt.% Al addition,in which the standard electrode potential enhancement of theα-Mg phase has a contribution[250].In addition,the formation of an Al-rich oxide layer and its incorporation to the surface corrosion product film of Mg alloys can make the film more stable and Mg alloys more corrosion-resistant[259,266,267,270].Though,the asextruded Mg-5Al alloy still shows a relatively high average corrosion rate~8.2 mm/y in 0.6 M NaCl solution[267],the addition of another alloying element such as Mn[267]and Sn[259]can facilitate the formation of an Al-rich oxide layer which is one of the reasons for the enhanced corrosion resistance of Mg alloys[259,266,267,270].In addition,the Al addition less than the limited solubility in theα-Mg phase was also reported to decrease the anodic kinetics of Mg[75]and thus retarding the anodic dissolution. Mg-Zn-X system:The effect of Zn addition on the corrosion resistance Mg alloys have been extensively investigated[102,233,281,285].Normally,the corrosion resistance of Mg alloys can be enhanced with the 0–2 wt.% Zn addition,in which Zn can almost completely dissolve in theα-Mg phase[74,102,281,286].Comprehensive factors have been reported,e.g.,the increase of the electrode potential[63,74,254,287],higher corrosion potential and charge transfer resistance[276]of theα-Mg phase,the formation of more sable passive film[102,265,288],the Zn segregation to grain boundaries acting as cathodic areas related to theα-Mg phase[283]and the possible increase of the hydrogen overpotential of Mg[285].For example,the degradation rates of asextruded Mg-6Zn alloy in the simulated body fluid(SBF)are lower than that of high-purity Mg both for immersion for 3 and 30 days,due to the enhancement of both the corrosion potential and charge transfer resistance of Mg[276].Also,the as-solutioned Mg-5Ga-0.9Zn alloy in 3.5% NaCl solution exhibits a lower average corrosion rate 0.835 mg·cm-2·d-1than that of as-solutioned Mg-5Ga alloy 2.06 mg·cm-2·d-1,in which the standard electrode potential of theα-Mg phase is enhanced and the Zn solute atoms in the alloys can form compact and stable protective layers[63]. Mg-Sc-X system:Sc for its high cost has not been extensively investigated and applied in Mg alloys,while Mg alloys with the solution of Sc in theα-Mg phase exhibit better corrosion resistance[61,257].Li et al.[257]have indicated that the integral self-corrosion potential of ZK21 alloy can be enhanced with the solution of Sc in theα-Mg phase,and the more positiveEcorris mainly due to the enhanced standard electrode potential of theα-Mg phase.Additionally,considering influences such as the texture and the performance of corrosion product film incorporation with Sc-containing oxide,Zhang et al.[61]suggested in Mg-Sc alloys with single phase region,the increasing Sc content can make alloys more corrosion-resistant.And especially in the studied alloys,Mg-0.3Sc exhibits a low corrosion rate with a hydrogen evolution rate as 1.52 mm·y-1and a weight loss rate as 2.17 mm·y-1. Mg-Ca-X system:It was concluded that in binary Mg-Ca system,small addition of<0.35 wt.% Ca is essentially inert,whereas the addition of Ca above its solubility seriously deteriorates the corrosion resistance[75]due to the formation of Mg2Ca phase which exists at the grain boundaries,and acts as the anode and dissolves preferentially toα-Mg during the first hour of immersion in 0.9% NaCl solution[289].Alloying element Ca though shows limited solubility in theα-Mg phase,the solution of Ca can also improve the corrosion resistance of Mg alloys.The enhanced corrosion resistance of Mg alloys could be mainly attributed to the formation of Ca-containing oxide and/or CaCO3film and the stability enhancement of corrosion product film[229,270,290].0.1 at.% Ca addition in the as-cast homogenized Mg-2.4Zn(at.%)alloy was reported to decrease the corrosion rate from 4.67 to 2.96 mm/y in the 3.5%NaCl solution with weight loss method,which is mainly attributed to the formation of CaCO3[290].In addition,the corrosion potential of AZ91 alloy was also reported to be enhanced with the addition of Ca,and the average corrosion rate of the as-cast AZ91 alloy alloyed with 0.5Ca wt.% was decreased by 14.28% as about 400 mg·cm-2·h-1in comparison with that of non-Ca AZ91 alloy[249]. Mg-Ga-X system:Ga also exhibits positive effect on the corrosion resistance of Mg alloys.The electrode potential of theα-Mg phase can be increased with the solid solution of Ga which possesses a higher standard electrode potential than Mg,thus effectively suppressing the micro-galvanic corrosion[63,248,256].Additionally,Mg alloys alloyed with Ga were reported to have high hydrogen overpotentials,and therefore the cathodic reaction of Mg alloys can be inhibited and finally improving the corrosion resistance of Mg alloys[248,272,291].Also,the solid solution of Ga can enhance the stability of the passive film[248].Ren et al.reported the corrosion rate of as-cast Mg-5Sn-0.5Ga alloy can exhibit a lower corrosion rate 1.85 mm/y than that of as-cast Mg-5Sn alloy as 3.5 mm/y in 3.5% NaCl solution[248].And Wang et al.also indicated the as-extruded Mg-5Sn-2Ga alloy has much lower corrosion rate in comparison with that of as-extruded Mg-5Sn alloy,the weight loss rate and hydrogen evolution rate in 3.5% NaCl solution as 1.69 and 0.93 mm/y for Mg-5Sn-2Ga alloy v.s.~7 and~5.5 mm/y for Mg-5Sn alloy,respectively[256]. Mg-Sn-X system:The effect of Sn on the corrosion resistance enhancement of Mg alloys has been extensively studied.As like other alloying elements with higher standard electrode than Mg,theα-Mg phase with Sn solution can exhibit a higher standard electrode potential[230,292].Especially,Zhang et al.[230]indicated the slow diffusion of Sn solutes can refine the grain size,improve the chemical uniformity and finally make Mg alloys more corrosion-resistant.Same as Ga,Sn also exhibits a high hydrogen overpotential and effectively retards the cathodic reaction[230,236,247,274].The corrosion rate of Mg-5Gd-3Y-1Sn-0.5Zr alloy in 3.5% NaCl solution for 24 h is 0.13 mg·cm-2·h-1in comparison with Mg-5Gd-3Y-0.5Zr alloy being 0.52 mg·cm-2·h-1[230].The formation of stannic oxide and its incorporation to the passive film can also make the film more stable and keep the anode from further corrosion[247,259,260,268].Liu et al.[247]indicated that Mg-7Sn alloy solution treated at 540°C,which leads to more Sn solid solutioned in theα-Mg phase,shows the lowest corrosion rate in 3.5% NaCl solution as 0.561 mg·cm-2·d-1for the formation of more stannic oxide than other Mg-7Sn alloys solution treated at lower temperatures.Cain et al.[293]also found the Sn-enrichment on the dissolving Mg surface can decrease the cathodic kinetics of Mg-10Sn alloy that was immersed in 0.6 M aqueous NaCl solution for 24 h,which leads to a reduction in the number of favorable Mg sites for hydrogen evolution and presumably prevents the recombination of adsorbed hydrogen atoms close to Sn atoms on the surface,finally retarding the hydrogen evolution reaction rate on the electrode surface. Mg-Sr-X system:Sr solute atoms were reported to take part in the formation of corrosion film,and therefore the stability of corrosion film can be enhanced with the addition of Sr below its solubility limit[294].The as-cast Mg-2Zn alloy alloyed with 0.2 wt.% Sr shows lower average corrosion rates both in 0.9% NaCl and Hank’s solution as 2.89 and 1.67 mg·cm-2·d-1than that of as-cast Mg-2Zn alloy as 3.3 and 2.21 mg·cm-2·d-1,respectively,for the corrosion product film with high stability[294].It’s worth noting that,Yu et al.[269]have reported that the charge transfer resistance of Mg alloys and the diffusion resistance of corrosion product film can be simultaneously raised and obtain maximum values with 1 wt.% Sr addition,enhancing the corrosion resistance of Mg alloys. Mg-Mn-X system:For Mg alloys alloyed with Mn,the corrosion resistance increases with the increasing Mn content in theα-Mg phase[111,184,295,296].The retard microgalvanic corrosion for the decrease of the potential difference,resulting by the increase of electrode potential of theα-Mg phase after the solution of Mn,can be one reason for the enhanced corrosion resistance[184].The corrosion resistance of as-cast Mg-3Ni(wt.%)alloy in 3.5% NaCl solution was reported to be significantly improved with Mn addition up to 1 wt.% because the solution of Mn in theα-Mg phase can enhance the electrode potential of theα-Mg phase,and thus reducing the potential difference between the Mg2Ni and theα-Mg phase(Wan,Wang et al.2008).In addition,the formation of Mn-containing oxides and their incorporation to the corrosion product layer can increase the corrosion resistance of the layer,eventually enhancing the corrosion resistance of Mg alloys[275].Cho et al.[275]also indicated the resistance of charge transfer can greatly increase with the Mn addition,which inhibits the corrosion reaction of Mg alloys.It was reported that the corrosion rate of as-cast Mg-4Zn-0.5Ca alloy in Hank’s solution can be greatly improved by alloying with 0.8 wt.% Mn as~0.002 from~0.0435 mg·cm-2·h-1for above reasons[275].Furthermore,Bazhenov et al.[286]also reported that the addition of 1 wt.% Mn in Mg-Zn-Ca alloys can lead to a positive shift of+0.06 V in the corrosion potential and retard the anodic kinetics that represent the dissolution rate of Mg over a wide potential range,therefore reducing the corrosion rate of Mg-Zn-Ca-(Mn)alloys. Mg-Ti-X system:Alloying element Ti was reported to effectively enhance the corrosion resistance of AZ91 Mg alloys.The small addition of 0.1 wt.% Ti was reported to improve the corrosion resistance of AZ91E alloy due to the decrease of cathodic reaction rate even though the anodic activity was enhanced slightly[297].In addition,the corrosion resistance of AZ91 alloy can be enhanced with Ti addition(0–0.5 wt.%),and especially 45 fold enhancement with 0.5 wt.% Ti addition(decreasing from 77 for AZ91 alloy to 1.7 mg·cm-2·d-1for AZ91-0.5Ti alloy),and the solution of Ti and increasing solution Al content in theα-Mg phase have an effect[116]. Mg-RE-X system:RE elements have been also alloyed in Mg alloys to improve corrosion resistance[264,279,291,298]besides the strength and ductility.As shown in Fig.14,the standard electrode potentials of RE elements is equivalent to that of Mg and thus the electrode potential of Mg changes little when alloyed with RE elements.On the other hand,RE elements are known to have strong affinity with oxygen and form related oxides with low chemical activities on the surface of Mg alloys with oxygen[299,300].The oxides are insensitive to the NaCl solution and can act as passive films to protect the substrate.After the dissolution of outer layer Mg(OH)2,the oxide film can also have a relatively strong protective effect[299]. Alloying element Y,for its reasonable price and advantages on the performance enhancement of Mg alloys,has attracted great interest.The charge transfer resistance of AZ91 alloy with Y addition up to 1.2 wt.% was reported to be higher than that of AZ91 alloy[277],which indicates that the Y addition can increase the corrosion reaction resistance of AZ91 alloy.In addition,the formation of Y2O3film on the alloys surface has been widely reported to enhance the stability of surface film,which can protect Mg alloys from further corrosion[263,282,301,302].Wang et al.[263]have reported the corrosion rate of as-extruded Mg-6Zn-0.5Mn-1Al alloy in 3.5% NaCl solution can be decreased from 9.48 to 1.46 mg·cm-2·h-1with 0.5 wt.% Y addition.And the oxidized Y enriched in the passive film was suggested by Miller et al.[301]to be a major contributor to the significantly improved corrosion resistance of Mg-22Y(at.%)solid solution alloy at high pH values(11.6–12)made by magnetron sputter deposition. Similarly,the corrosion product film can become more passive with the incorporation of Gd2O3after Gd addition[262,291].For example,with 3–5 wt.% Gd addition,the corrosion rate of as-cast pure Mg can decrease from~4 mm·y-1to less than 0.5·mm·y-1in 9 g/L NaCl solution[291].Also,the corrosion product film can be made denser and more stable for the incorporation of Nd2O3[262,266,291,298].Takenaka et al.[303]have reported that even the minor addition of 0.1 wt.% Nd can significantly improve the corrosion resistance of pure Mg,and the 0.1–1 wt.%Nd addition can further improve the corrosion resistance of pure Mg,which both show better corrosion resistance than that of AZ31 alloy.And later,Kubasek and Vojtech[291]also reported that the Nd addition up to 4 wt.% can significantly decrease the corrosion resistance of as-cast pure Mg(~4 mm·y-1)to~0.9 mm·y-1in a 9 g/L NaCl solution,which is not so effective on the enhancement of corrosion resistance of Mg alloys as that of Gd:~0.23 mm·y-1for as-cast Mg-3Gd alloy,and they attributed it to the higher solid solubility of Gd in theα-Mg phase.Yang et al.[304]indicated the Dy-rich regions for Dy segregation showed better corrosion resistance than that of theα-Mg phase with less content of Dy in the as-cast Mg-Dy binary alloys.And the corrosion resistance of Mg-Dy binary alloys can also be improved with the increasing solid solution Dy content in theα-Mg phase after the heat treatment at 520°C for 24 h,in which the Dy in solid solution has no effect on the cathodic reaction kinetics.It was reported the formation of more corrosion-resistant Dy-rich film that can retard the anodic reaction can explain the enhancement of Mg-Dy alloys[304]. Though the addition of RE alloying elements can improve the corrosion resistance,it is noted the amount of addition should be strictly controlled.Takenaka et al.[303]indicated the optimum Ce addition to improve the corrosion resistance of Mg is 0.3 wt.%because the further addition could be harmful to the corrosion resistance of Mg.And the improvement of corrosion resistance of AZ91E alloy can be realized by the appropriate addition of Ce below its solid solubility limit[297].Takenaka et al.[303]have also suggested that the optimum RE(La and Nd)content 0.5 wt.% is beneficial for the enhancement of corrosion resistance of Mg.And Liu et al.[264]indicated the optimal addition of Sc,Pr or Sm is below 3 wt.%.Furthermore,Mg-RE alloys with light RE elements were suggested to generally show better corrosion resistance than those with heavy RE elements,as shown in Fig.15,in which the incorporation of RE elements(in the form of their oxides or hydroxides)into corrosion products can effectively improve the corrosion resistance[264].Also,Nordlien et al.[305]have early reported that the presence of RE elements can lead to a significant reduction in the hydration of the films and thus improving the surface passivity. It should be noted that Cao et al.[67]and Shi et al.[68]have early reported that for some binary alloys,like Mg-1Mn,Mg-5Sn and Mg-5Zn[67];Mg-5Gd,Mg-0.7La and Mg-0.9Ce alloys[68]all in wt.%,the as-solutioned state alloys show lower corrosion rates than that of as-cast state alloys,in which the improvement of corrosion resistance for solute atoms in Mg alloys is well reflected.However,for some other studied binary alloys,the corrosion rates of as-solutioned state are higher than those of as-cast state alloys like Mg-6Al,Mg-0.3Ca and Mg-0.1Sr alloy[67];Mg-0.6Nd and Mg-5Y alloy[68]all in wt.%.The higher corrosion rates of these assolutioned state alloys were attributed to the existed particles(which contain a high content of alloying elements,oxygen,silicon)and some impurity metals,which can form the microgalvanic corrosion with theα-Mg phase and therefor accelerating the corrosion of Mg alloys[67,68].On the other hand,all the alloying element contents in their studied Mg-X binary alloys are either 5 wt.% or close to the maximum solid solubility in theα-Mg phase at the solution heat-treatment temperature.As mentioned above,the amount of alloying element addition should be strictly controlled to effectively improve the corrosion resistance of Mg alloys.Therefore,another reason for the higher corrosion rates of as-solutioned state Mg alloys could be that the studied alloying element contents by Cao et al.and Shi et al.[67,68]might not be the optimum contents for the solid solution improving corrosion resistance though the solid solution of these alloying elements are promising to improve the corrosion resistance of Mg alloys.Additionally,the intra-granular galvanic corrosion is still possible between the Mg and the solutes,which indicates that the more solid solution content of alloying elements does not necessarily mean that the corrosion resistance of Mg alloys can be better improved. Recently,it is reported that the combination of two beneficial alloying elements can more effectively improve the corrosion resistance of Mg alloys[62,63,69].For example,the as-solutioned Mg-4Sc-1Y(wt.%)alloy was reported to have a much lower corrosion rate as 0.21 mg/cm2/d than that of as-solutioned Mg-4Sc(wt.%)alloy as 2.83 mg·cm-2·d-1,in Fig.15.pH values of Hank’s solution after immersion for(a)72 h(b)360 h.The corrosion rates of Mg-RE model alloys and pure Mg after immersion for(c)72 h(d)360 h which is calculated by weight loss,copied from[264]. which the protective film is further improved by Y2O3in addition to Sc2O3and the driving force for micro-galvanic corrosion is decreased because the Y segregation in Sc-depleted areas can help to reduce the work function difference between Sc-rich and Sc-depleted zones[62].However,with further Y addition from 1 to 2.5 Y(wt.%),though the corrosion rates of these alloys are still lower than that of Mg-4Sc(wt.%)alloy,but higher than that of Mg-4Sc-1Y(wt.%)alloy,due to the increased number of micro-galvanic couples[62].Therefore,selecting beneficial alloying elements and strictly controlling their content,as well as exploring the mechanism of interaction between alloying elements,can effectively guide the design of corrosion-resistant Mg alloys.Fig.16 presents the corrosion rate of Mg alloys in different solutions against the total alloying element content,which further shows that the beneficial alloying elements and their combinations with appropriate contents can effectively improve the corrosion resistance of Mg alloys. The surface energy and work function have been reported to be the important roles on the corrosion behaviors of Mg alloys[237].Surface energy,which represents the energy that can split an infinite crystal into two,is a fundamental parameter of material surface,and the decrease of surface energy is favorable to the stability of the surface.The work function reflects the energy of gaining and losing electrons on the surface,and the high work function indicates that it is difficult to lose electrons and possess high surface stability.Ma et al.[237]have suggested that within the same cathodic hydrogen evolution reaction,the alloying elements that can improve the corrosion resistance of the Mg alloys should lower the corrosion current density and enhance the corrosion potential,which can be realized when the following two conditions are satisfied:i)the surface energy density(Esurf/ρ)is lower than 0.067 eV/atom and ii)a comparable work function(Φ)with valued of 3.674 eV.Therefore,as shown in Fig.17,alloying elements Ga,Cd,Hg,In,As,and Cr,withEsurf/ρlower than 0.067 eV/atom and a comparableΦwith 3.674 eV,can effectively decrease the anodic dissolution rate and thus improving the corrosion resistance of Mg alloys.Nevertheless,the most commonly used alloying elements Al and Zn in Mg alloys have slightly higher surface energy density and work functions than those of pure Mg and therefore increasing the anodic dissolution rate to some extent[237].That is to say,alloying elements with lower surface energy and higher work function are beneficial to reduce the corrosion rate of Mg alloys when they are solid solutioned in theα-Mg phase;while the alloying elements with higher surface energy and lower work function can lead to a decrease in the corrosion potential(the corrosion potential decreases with the decrease of work function[309]),thereby accelerating the corrosion rate when they are solid solutioned in theα-Mg phase. Most recently,Ma et al.[310]have also investigated the effect of alloying elements on the cathodic hydrogen evolution rate of surface(0001)of Mg,and reported that almost all the calculated alloying elements,except for Ag,have been experimentally studied.The experimental results also show these alloying elements can reduce the free energy of the adsorbed hydrogen atom and therefore accelerating the cathodic hydrogen evolution reaction rate,where the negative effect of Zn and Sn was also confirmed. Fig.16.(a)The corrosion rate against total alloying element content of Mg alloys[61,69,74,102,111,248,254,257,264,267,288,290–292,306,307](With one horizontal line drawn at the intrinsic corrosion rate as measured by weight loss of high-purity(HP)Mg of 0.3 mm/y[308],and another line with a corrosion rate of 1 mm/y)and(b)the corrosion rate of Mg-1X(wt.%)alloys. Currently,increasing the potential of theα-Mg phase,decreasing the possible structural potential difference and altering the surface film is the main work objects for the improving corrosion resistance of Mg alloys.Particularly,the solid solution of alloying elements in Mg alloys take the main effects in these three aspects.It is suggested,Mg alloy composed by one homogenized single phase should be promising for the“stainless”since there is no potential difference and a compact surface film is possible to be formed if there is one suitable alloying element or alloying elements’combination.However,there are still some doubts for this design concept.Firstly,the intra-granular galvanic corrosion is still possible between the Mg and the solutes,though much slower.Secondly,since the thermodynamic equilibrium solubility of most alloys elements in Mg alloy are quite limited,the possibility for a dramatic change of the composition and property of the surface protective film is limited.However,the change of the native formed surface film is still the fundamental strategy for the“stainless”Mg alloy design.In addition,the corrosion property should be defined according to the medium where Fig.17.DFT-derived(a)surface energies(Esurf),(b)surface energy densities(Esurf/ρ),and(c)work functions(Φ)of the M-alloying(0001)surface as a function of various M-alloying metal elements for 3d,4d,5d,and some selected p metals,copied from[237]. the alloy is in service.For example,the corrosion rate of Mg and its alloys in the medium atmosphere is much lower than that in the medium 3 wt.% NaCl solution(in atmosphere:the corrosion rate of Mg,AZ31B,AM60 and AZ91 alloy is 0.2,0.04,0.03 and 0.02 mm/y[311],respectively;in 3 wt.%NaCl solution:the corrosion rate of Mg,AZ31B,AM60 and AZ91 alloy is 2.7,2.3,14 and 8 mm/y[312,313]. Within the last three-four decades,the research and application of Mg alloys have been significantly developed.The high cost of resource Mg materials,compared to aluminum alloys,is still high and the grade-scale application of Mg alloy is still limited,while this situation is promising to be improved or solved by the development of low-energyconsuming and low-pollution electrolytic smelting production of abundance resource Mg from the saline lake and the development of low-cost manufacturing of Mg alloy products with the improved formability of Mg alloys.Additionally,the acceptance and application of Mg alloys will be greatly increased or even boosted if the corrosion-resistance Mg alloys are significantly improved. Alloying method is still the most fundamental method to improve the basic properties of alloys.While most works are focused on the strong precipitation-strengthening effect,here,this work has reviewed the possible synergy effects of solid solution on the comprehensive properties of Mg alloys,and some development prospects have been provided as listed: (1)Solid solution in theα-Mg phase:The atoms solid solutioned in theα-Mg phase can bring the effects on the properties of alloys and the multi-solute in theα-Mg phase may bring the“cocktail-effects”.However,the thermodynamic and kinetic information of multi-solute in theα-Mg phase is still quite limited and characterization of these solid solution behavior is also complex for the high activities of Mg alloys. (2)Strength and hardness:Generally,all alloying elements solid solutioned in theα-Mg phase can have the strengthening effect.The strengthening map shows that the bigger atoms have the stronger strengthening effects.Particularly,the RE elements show the strong solutestrengthening effects and many high-strength Mg-RE alloys have been developed recently,where,of course,the solute-strengthening effect may be the small part.And we are still expecting the breakthrough of the strength of Mg alloys.While for the grade-scale application of Mg alloys,the currently dilute-solution of Mg-RE alloys and the non-RE contained Mg alloys still dominate the application filed. (3)Ductility:Actually,the attractive point of solid-solution of Mg is attributed to the synergy effects of solidsolution strengthening & ductilizing.The ductility can be improved by the dilute-solution.The most surprising point is that the RE elements,e.g.,Gd,Y,show the strong effects both on the strengthening and softening,and plus the strong refining effect and the stabilizing effect.The development of good balanced mechanical properties of dilute-solution of RE elements have been the mainstream development direction for the high-performance Mg alloys. (4)Corrosion-resistance:The poor corrosion properties and the low ignition point of Mg alloys are the key aspects that prohibit the general acceptance of the civil application.The intrinsic low electrochemical potential of Mg limited the development of the corrosion-resistance of Mg alloys and the surface-treatment is still necessary for the final application of the Mg alloy products.While the corrosion-resistance property is still greatly improved recently,firstly,by the high purification of the alloys(removal the high-electrochemical potential elements,e.g.,Fe,Ni),secondly,by solid solution elements in theα-Mg phase and finally having a passive film on the surface.For the second point,the alkali earth metal and RE elements show good effects on the improvements of the corrosion-resistance and ignition property of Mg alloys.However,there is still great space to improve the corrosion-resistance and the multi-solution of elements in theα-Mg phase may bring new possibilities.Additionally,the solid solution of alloying elements e.g.,Al,Zn and Ga,with high hydrogen overpotential,can effectively retard the hydrogen evolution reaction,which is helpful for the bio-application of Mg alloys. In conclusion,the solid solution of some RE elements,e.g.Gd,Y and Ce,can simultaneously improve the strength,ductility and corrosion resistance of Mg alloys,which thus explaining their wide attraction and application in Mg alloys.Especially,Mg-RE alloys containing light RE elements show better corrosion resistance than alloys containing heavy RE elements.Considering the cost and density,the dilute-solution of RE containing Mg alloys are the main development trend currently. It’s worth noting that,the research at present on the effects of solid solution on the properties of Mg alloys is mainly focused on one single element effect,and there are few reports on the interaction theory and multi-element solid solution enhancing studies.In addition,the properties of base alloys can give the genetic characteristics of the final properties of materials,and that is,the corresponding properties of the original alloy can be further improved with the solid solution of target alloying elements with the original good properties retained.Therefore,it is necessary to clarify the mechanism of effects of solid solution on the property enhancement of Mg alloys and to develop the theory and basic research of multicomponent solid solution alloys to expand the dimension and scope of the design of Mg alloys.Within this respect,in-situ high-resolution characterization,systematic calculation of the effects of solid solution on the properties of Mg alloys,establishments of empirical/semi-empirical models,construction of the thermodynamic descriptions for multi-component Mg alloy systems,etc.can effectively help the prediction of the properties and the design of Mg alloys,providing fundamental for the design of Mg alloys with target performances. Declaration of Competing Interest There is no conflict of interest. Acknowledgments This work is financially supported by National Natural Science Foundation of China(52171100,51971044,U20A20234 and U1910213),the National Key R&D Program of China(2021YFB3701100)and the Natural Science Foundation of Chongqing(cstc2019yszx-jcyjX0004). Data availability The raw/processed data required to reproduce these findings are available on request. Supplementary materials Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.simpat.2017.03.014.
5.2.Alloying element effects

5.3.Calculations and predictions

5.4.Section summary

6.Summaries and prospects
 Journal of Magnesium and Alloys2022年7期
Journal of Magnesium and Alloys2022年7期
- Journal of Magnesium and Alloys的其它文章
- High-performance magnesium-based thermoelectric materials:Progress and challenges
- Effects of heat treatment on the corrosion behavior and mechanical properties of biodegradable Mg alloys
- Calcium phosphate conversion technique:A versatile route to develop corrosion resistant hydroxyapatite coating over Mg/Mg alloys based implants
- The role of solutes in grain refinement of hypoeutectic magnesium and aluminum alloys
- Characterization on the formation of porosity and tensile properties prediction in die casting Mg alloys
- Biomass-derived carbon doping to enhance the current carrying capacity and flux pinning of an isotopic Mg11B2 superconductor
