Calcium phosphate conversion technique:A versatile route to develop corrosion resistant hydroxyapatite coating over Mg/Mg alloys based implants
G.S.Hikku,C.Arthi,R.B.Jn Robrt,K.Jyasubramanian,R.Murugsan
aMedical Bionanotechnology,Faculty of Allied Health Sciences,Chettinad Hospital and Research Institute,Chettinad Academy of Research and Education,Kelambakkam,Tamilnadu 603103,India
b Nanobiotechnology,Amirta Centre for Nanoscience and Molecular Medicine,Amirtha Vishwa Vidhyapeetham,Ernakulam,Kerala 682041,India
cDepartment of Mechanical Engineering,AAA College of Engineering and Technology,Sivakasi,Tamilnadu 626005,India
d Department of Chemistry,Mepco Schlenk Engineering College,Sivakasi,Tamilnadu 626005,India
e Karpaga Vinayaga Institute of Medical Sciences and Research Centre,Palayanoor,Tamilnadu 603308,India
Abstract Globally,vast research interest is emerging towards the development of biodegradable orthopedic implants as it overcomes the toxicity exerted by non-degradable implants when fixed in the human body for a longer period.In this context,magnesium(Mg)plays a major role in the production of biodegradable implants owing to their characteristic degradation nature under the influence of body fluids.Also,Mg is one of the essential nutrients required to perform various metabolic activities by the human cells,and therefore,the degraded Mg products will be readily absorbed by the nearby tissues.Nevertheless,the higher corrosion rate in the biological environment is the primary downside of using Mg implants that liberate H2 gas resulting in the formation of cavities.Further,in certain cases,Mg undergoes complete degradation before the healing of damaged bone tissue and cannot serve the purpose of providing mechanical support.So,many studies have been focused on the development of different strategies to improve the corrosion-resistant behavior of Mg according to the requirement.In this regard,the present review focused on the limitations of using pure Mg and Mg alloys for the fabrication of medical implants and how the calcium phosphate conversion coating alters the corrosive tendency through the formation of hydroxyapatite protective films for enhanced performance in medical implant applications.
Keywords:Biodegradable implants;Magnesium;Controlled corrosion;Alloying;Hydroxyapatite;Calcium phosphate conversion coating.
1.Overview
Orthopedic surgeries are performed for patients who are having conditions like fractures,joint replacement,bone reformation,dislocation,etc.,where the implants are fixed to deliver mechanical support and enhance the bone healing process[1–5].Conventionally,many types of implants are available in the market that is manufactured using metals and alloys such as titanium,silver,stainless steel,Co-Cr-Ni alloy,etc.[6–9].The orthopedic implants are designed and manufactured with different architectures such as pins,wires,rods,plates,etc.that are implanted to perform various functionalities[10–14].Apart from metals and alloys,ceramic-and polymer-based implants are nowadays used in many cases[15–17].Various types of inorganic and organic materials that are used as medical implants in the human body are provided in Table 1.Most of the implants commercially available are non-biodegradable and therefore while using those implants,they are found intact for a longer period even after the bone tissues are completely healed[18].Further,incertain cases,the implants may cause allergic reactions,irritations,discomfort,etc.Under such circumstances,the implants originally placed have to be removed from the human body which requires additional surgery[19,20].Furthermore,in the presence of biological fluids,the ions released from the conventional implants cause serious biological effects to the nearby tissues such as infections,toxicity,allergic response,etc.[21,22].More insights about the merits and demerits of the conventionally used implants are summarized in Table 2.To alleviate such limitations,the current research interest has been focused on bio-degradable implants.These are normally fabricated using biocompatible materials which are easily digestible in due course under the influence of biological fluids present in the human body.The bio-degraded products will be absorbed or excreted by the human system and therefore,it does not pose any adverse effects too[22,23].Additionally,the use of biodegradable implants cut down the need for the surgical process to remove the implant after patient recovery which considerably reduces the cost for the healthcare procedure[22].

Table 1Materials used as implants for various functionalities.
Among the three categories of materials such as metals,ceramics,and polymers,metal-based implants provide remarkable mechanical strength which is a prerequisite needed for manufacturing an orthopedic implant[62,63].Therefore,extensive research is focused on developing metal-based low cost,biodegradable,low density,high mechanical strength,and highly bioactive implants.
Mg is a near-ideal implant material and is easily degraded in the biological environment.Mg is the lightest of all metals with a density of 1.74 g/cm3[64]which is lighter than commonly used lightweight aluminum(33%)and is much lighter than steel(77%)but its mechanical properties are equivalent to them[14].Moreover,the Mg has a very high damping capability which improves the bone-implant compatibility[65,66].The biodegradability of Mg in the human body was recognized very early and since then Mg and its alloys are widely studied as orthopedic implant material[67,68].Mg is relatively more abundant in the human body which is approximately the 4thabundant metal constituting 0.5 g/kg of a human weight.Besides,the daily requirement of Mg is 375 mg[69,70],which is also considered a co-factor for numerous enzymes that take part in metabolism.
Mg is a highly recommended material as a temporary(short-term)implant since it avoids secondary surgery owing to its digestive nature in the body fluids with respect to time[71].Placing Mg-based implants in the body leads to complete degradation which is useful for patients because of the limited period of their persistence in the body.Further,it enhances the bone healing process by reducing the stressshielding phenomenon.Its antimicrobial activity further helps in avoiding infection during the recovery stage[72,73].The high biocompatibility of Mg does not delay the healing process caused by induced inflammation.Also,Mg ions(Mg2+)are found to be well-known for their tissue-healing capability[74].Moreover,the excess Mg2+does not induce cytotoxicity rather it gets excreted through urine[74].Table 3 emphasizes the most beneficial properties of Mg which make it a good implant material.

Table 2Advantages and drawbacks of commercially used implants.

Table 3Comparison of properties of natural bone and Mg.
Even though,Mg poses many advantages over other implants,a major limitation that hinders its widespread usage is its high degradation rate.Even at room temperature,Mg is easily dissolved in aqueous media either present in the environment or in body fluid resulting in the generation of metal hydroxide(Mg(OH)2),because of its lower corrosion potential value[81].Further,the rapid corrosion process releases H2gas as a by-product which accumulate in the nearby tissues causing clinical issues.When the absorption saturation of H2gas is attained at nearby tissues,the excess gas forms cavities resulting in the development of callus which inhibits the bone regeneration process[82].Also,there are cases where the Mg-based implants are found to undergo complete dissolution before complete healing or regeneration of the fractured bone[83].Due to such drawbacks,the commercial viability of Mg-based implants is declined over time.To overcome such drawbacks,more attention is given to alter the corrosion behavior of Mg by different technologies/methodologies such as alloying and surface coating with different materials.Through proper strategies,the negative impacts of Mg-based implants can be eventually eliminated.
2.Corrosion mechanism associated with Mg
Mg is considered a highly corrosive metal that gets corroded even under the influence of water[84].Before discussing the methods for tuning the corrosion behavior of Mg,this section briefly outlines the mechanism of Mg corrosion.The corrosion of Mg initiates with the formation of anodic and cathodic islands on the substrate’s surface resembling an electrochemical cell[85].Mg dissociates into Mg2+with the liberation of 2e-at the anode which is used up to reduce H2O to liberate H2gas and OH¯ions at the cathode(Fig.1).This process results in a continuous evolution of H2gas on the Mg metal surface and increases the pH due to the generation of OH¯ions.When the corrosion rate is high,the H2evolution at the cathode is easily visualized as microbubbles,preferably through pitting corrosion[82].Pitting is a phenomenon that occurs commonly in almost all metals(except noble metals)due to the localized corrosion influenced by elemental composition,the microstructure of the metallic surface,and the types of electrolytes[86].The Mg2+ions undergo a chemical reaction with OH¯ions and form Mg(OH)2on the metal surface developing an oxide layer[87].But,in an aqueous environment,the Mg(OH)2does not act as a protective layer(unlike oxide layers of Cr and Al)resulting in further corrosion.Thus,the corrosion of Mg is not terminated,but it continues until the complete degradation,causing serious biological hazards.The chemical reactions that take part in the corrosion are shown as Eqns.(1)–(4)[87],
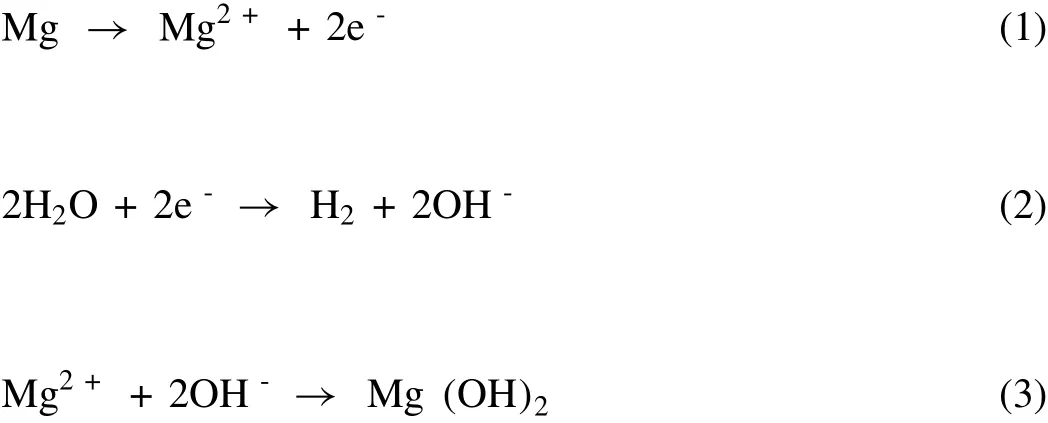
The overall reaction is


Fig.1.Mechanism of Mg corrosion by H2O.
3.Declining the corrosion rate of Mg by alloying
Generally,pure Mg has declined corrosion-resistant property leading to a high degradation rate.However,through the alloying process,the strengthening of the Mg can be achieved through grain refinement,precipitation hardening,and solid solution strengthening mechanism.For being used as a degradable implant,the alloying elements for Mg alloys are selected not only based on the enhancement in the mechanical property but also,in view of the bio-compatibility and degradability[67].Also,the oxide layer formed over the surface of alloys will have oxides of alloying elements along with Mg(OH)2which will have an impact on the passivating nature of the oxide layer.In this regard,elaborate discussions regarding the above statements are provided below.
3.1.Alloying process
The process of alloying is employed to reduce the rate of corrosion by introducing appropriate elements to the parent material[88].The alloying process is performed by two mechanisms via substitutional and interstitial alloying.In these processes,the alloying elements with the same atomic size or in different sizes are being used.In the substitutional alloying process,atoms of relatively similar size as that of the parent atoms are used as an alloying element.Alloying takes place in the metallic crystal where the parent atoms are replaced by the alloying atoms.In contrast,in the interstitial alloying process,the atoms with a smaller size or radius compared to parent atoms are employed as an alloying element where they penetrate the interstitial gaps of the crystal lattice forming alloys[89].
3.2.Consideration for alloying process
Usually,Mg-based implants are alloyed with suitable materials to encourage the natural healing process of the bone during its healing phase and provide mechanical support in the development phase[90].However,while alloying,secondary phases are introduced in the Mg matrix.In general,the alloying elements are selected in such a way that the corrosion potential(Ecorr)of the matrix and the developed secondary phase are close to each other.If the difference inEcorrof matrix and secondary phase is high,then the corrosion current density(icorr)of the alloy is accelerated than the base metal[91].If the secondary phase has higherEcorrthan the matrix metal,the matrix metal will act as an anode and readily dissociates into their respective ions,resulting in highericorr[92].In such cases,alloyed Mg implants show higher corrosion than pure Mg,and therefore,the selection of alloying metal is critical.The alloying element should be added in such a way that itsEcorris less than that of the matrix material.So that the corrosion occurs only in the secondary phase where it acts as a sacrificial anode[93].Hence,for Mg implants,theEcorrof the secondary phase should be less than theEcorrvalue of Mg.But unfortunately,the most alloyed metal i.e.,Aluminium(Al)has higherEcorrthan Mg.Thus,the heterogeneous nature of the secondary phase promotes the anodic behavior of Mg and suffers from corrosion.Further,there are alloying elements that possess low electrode potential than Mg.However,those elements are either not used or introduced in very low concentrations for developing Mg alloys.
3.2.1.Solubility of alloying elements
However,studies revealed the role of the strengthening mechanisms where the corrosion behavior of Mg is improved through alloying that are contrary to the above statements.Here,the solubility of other metals in the Mg matrix comes into the limelight.If alloying metal is added below the solubility range,then strengthening occurs.In contrast,if the metals are added beyond the solubility range,then the secondary phases occur resulting in enhanced corrosion.Hence,to strengthen the matrix efficiently,the alloying element with a higher solubility limit can be chosen.Therefore,before alloying any constituent,one must know the solubility of the alloying component in Mg(Table 4).To understand this concept,the corrosion behavior of Mg alloys can be discussed.Ocal et al.[94]have studied the corrosion behavior of pure Mg and AZ91 in simulated body fluid(SBF).From the potentiodynamic polarization(PDP)studies,theicorrof Mg and AZ91 were elucidated as 0.35 μA/cm2and 0.13 μA/cm2,respectively(Fig.2a).Here,the authors revealed that the corrosion resistance of AZ91 was improved while alloying with metals like Al(9 wt.%),Zn(0.8 wt.%),and Mn(0.2 wt.%).In a similar study made by Wen et al.[95],theicorrof Mg,AZ31,and AZ61 were analyzed as 80.46 μA/cm2,23.19 μA/cm2,and 22.72 μA/cm2after exposure to modified-SBF for 24 days.In these reports,the improvement in the corrosion resistant property may be due to the strengthening mechanisms such as dislocation density,inter-particle distance,grain refinement,thermal expansion,elastic modulus,etc.[96].The solubility of Al,Zn,and Mn in Mg is 12.6 wt.%,6.2 wt.%,and 2.2 wt.%,respectively[96].But,the alloying elements in AZ91,AZ61,and AZ31 are well below the solubility range and do not cause any free standing secondary phase but induce the strengthening of Mg.

Table 4Role and characteristics of alloying metals in commercially available Mg alloys.
3.2.2.Influence of intermetallic phases
However,in a simultaneous study made by Wen et al.[95],the corrosion behavior of AZ91D is compared with pure Mg.Here,theicorrof AZ91D was found to be 100.42 μA/cm2,higher than pure Mg(80.46 μA/cm2).This fact is attributed to the presence of a higher percentage of Fe in the AZ91D composition than AZ31[95],AZ61[95],and AZ91[94](no Fe).Even though the wt.% of Fe(0.00392 wt.%)was far below the solubility,theicorrof AZ91D was higher than pure Mg.Here comes the concept of the intermetallic phase where Fe forms an intermetallic phase;Al3Fe in the presence of Al.Al3Fe is found to be highly cathodic compared to the Mg phase resulting in increased corrosion[135].A similar study was performed by Matsubara et al.[91]where they introduced Fe as an impurity phase into the AM50 and AM60 magnesium alloys and studied their corrosion resistance performance.From the obtained results,it was concluded that the corrosion was preferentially initiated at the site where Fe was present.

Fig.2.(a)PDP curves of Mg and Mg alloys in SBF[94]and(b)Tafel plots for Mg,AZ31 and AZ91 alloys in 3.5% NaCl solution[140].

Fig.3.Corrosion processes occurring on an Mg alloy surface[139].
The concept of the intermetallic phase acting as the cathode phase can be further explained by the study made by Ocal et al.[94]where the WE43 alloy(Y=4 wt.%,Zr=0.5 wt.%,and Nd=6.2–7.2 wt.%)displayed a highericorrvalue(0.77 μA/cm2)than Mg(0.35 μA/cm2)(Fig.2a).Here,the intermetallic phases of Y and Nd rich phases were formed into particles like morphology and were non-uniformly distributed.In such cases,the anodic Mg phase around the intermetallic phases got corroded easily which led to the undermining(fall out)of intermetallic particles.Therefore,non-uniform corrosion occurred over the specimen leading to an enhanced corrosion process.Further,Meng et al.[119]detailed the decremental corrosion effect of alloying Rare Earth(RE)to Mg alloy containing Zn.Here,the intermetallic phase[Mg-Zn-RE]has a higher cathodic behavior than the Mg-Zn phase.Therefore,Mg-Zn-RE acts as the cathode,and Mg-Zn acts as the anode resulting in a higher corrosion rate for the overall system.Similar results were obtained by Cain et al.[136]in which AM60 was alloyed with La and Ce,and they found that the corrosion resistance possessed by AM60 was declined.This is because of the formation of the Al-RE phase in AM60 which acted as a cathode phase and shows higher corrosion than AM60.In this context,the formation of the intermetallic phase plays a major role in the corrosion behavior of Mg alloys where the difference in corrosion potential between intermetallic phases and Mg matrix should be minimized i.e.,close and zero.
However,in the case of AZ91,AZ61,and AZ31,a higher fraction of the intermetallic phase(Mg17Al12)occurs that is equally distributed at the grain boundaries throughout the bulk of the material and increases the strength[137].Also,as the weight fraction of Al increases,the corrosion resistant property increases[92].This is due to the fact that while increasing the Al content,the difference between theEcorrof the matrix phase and intermetallic phase decreases.
3.2.3.Influence of oxide layer formation and electrolyte
Rationally thinking,a small difference in the matrix phase and intermetallic phase should increase the corrosion as per the schematic representation(Fig.3)displaying the corrosion behavior of Mg alloys(containing Al)occurring at the surface.But the corrosion of Mg alloys with Al and Zn content shows declined values.This phenomenon is due to the formation of a passive layer over the substrate surface that hinders the anodic oxidation process.It has been reported that the reduction inicorrvalue is due to the incorporation of Al in the oxidation layer of Mg[138].Therefore,the formation of a passive oxidation layer with better stability is one of the prerequisite factors to inhibit corrosion of Mg alloys i.e.,for AZ91,AZ61,and AZ31 alloys.
However,some reports are found contrary to the above discussions.Singh et al.[140]studied the corrosion behavior of AZ31 and AZ91 alloys and compared them with pure Mg.It was reported that theicorrof Mg alloys(AZ91=25.5 μA/cm2and AZ31=90.0 μA/cm2)was increased compared to pristine Mg(5.5 μA/cm2)which is contradictory to the previous discussions(Fig.2b).Also,it was reported that the formation of a passive layer of Mg(OH)2by pure Mg over the substrate surface hindered the anodic oxidation process more efficiently than the mixed hydroxide layers(inclusion of Al)formed by the AZ91 and AZ31 alloys which is contrary to the discussion made by Unocic et al.[138].To clarify these conflict results,the corrosion behavior of Mg alloys in different electrolytes is summarized in Table 5.In Sl.Nos.1 and 2,AZ91 and AZ31 display highericorrcompared to Mg recorded under 3.5% NaCl electrolyte validating the discussion made by Singh et al.[140].On contrary,from the Sl.Nos.26 and 33,AZ31 and AZ91 have declinedicorrcompared to Mg recorded under modified SBF and SBF electrolyte,respectively as that of the report provided by Ocal et al.[94](where SBF was used as electrolyte).

Table 5Medical grade Mg alloys and their Ecorr values.
Further,the WE43 alloy displayed declined corrosion resistance than pure Mg in SBF(WE43=0.77 μA/cm2and Mg=0.35 μA/cm2)[94]and 0.1 NaSO4(Mg=132 μA/cm2and WE43=153 μA/cm2)[141]but displays reducedicorrin 0.2 M NaCl(Mg=537 μA/cm2and WE43=158 μA/cm2)[141].It was reported that incorporating Y and Zr in the corrosion protection layer of WE43 alloy facilitates the formation of the thinner and more protective film(0.2 M NaCl).In the present review,the use of Mg alloys as medical implants is being discussed,and therefore,the Mg alloys which possess better corrosion resistance property in SBF i.e.,Al containing Mg alloys(AZ91,AZ61,and AZ31)can be considered for implant fabrication.From these data,it is inferred that the Mg AZ alloys display declined corrosion than Mg in simu-lated body fluid(SBF)and modified SBF but the corrosion increases than Mg when 3.5% NaCl electrolyte is used as a corrosive medium.This is due to the fact that the mixed oxide layer cannot withstand the highly corrosive Cl-ions that damage the oxide layer and reach the underlying Mg substrate where further corrosion process occurs.However,while using SBF as the electrolyte,the presence of Al in the oxide layer effectively acts as a passivating layer.
Table 4 summarizes the alloying elements and their functionality in the commercially available Mg alloys.Further,it is inferred that the alloying metals pose some physiological effects on the human that depends on the nature(toxic/nutrient/allergic)of the alloying metals.From the table,it is clear that most of the alloying elements are found to be toxic or allergen especially Al at a high percentage that can cause neurotoxicity[142].However,from Table 5,it is clear that most of the medical grade Mg alloys have either Al or RE as alloying elements(considered toxic).But Al is introduced to improve the corrosion behavior while RE elements are incorporated to enhance the mechanical properties which are the two pre-requisite parameters of medical implants.Further,the toxicity has occurred only when the dissolution of the elements in the human biological system after reaching their tolerable upper limit(TUL).But reports related to the quantitative analysis of dissociated alloying elements from Mg alloys in SBF are scarce or could not find and therefore,cannot comment on their toxicity effect in real time physiological environment.However,the dissociation of alloying elements occurs with the process of corrosion.While comparing the corrosion behavior of medical grade Mg implants in SBF(Table 5),AZ91 displayed the leasticorrand therefore,the dissociation of alloying elements will be limited in AZ91.Hence,from the consolidated results AZ91 can be used for the fabrication of Mg alloy based medical implants.
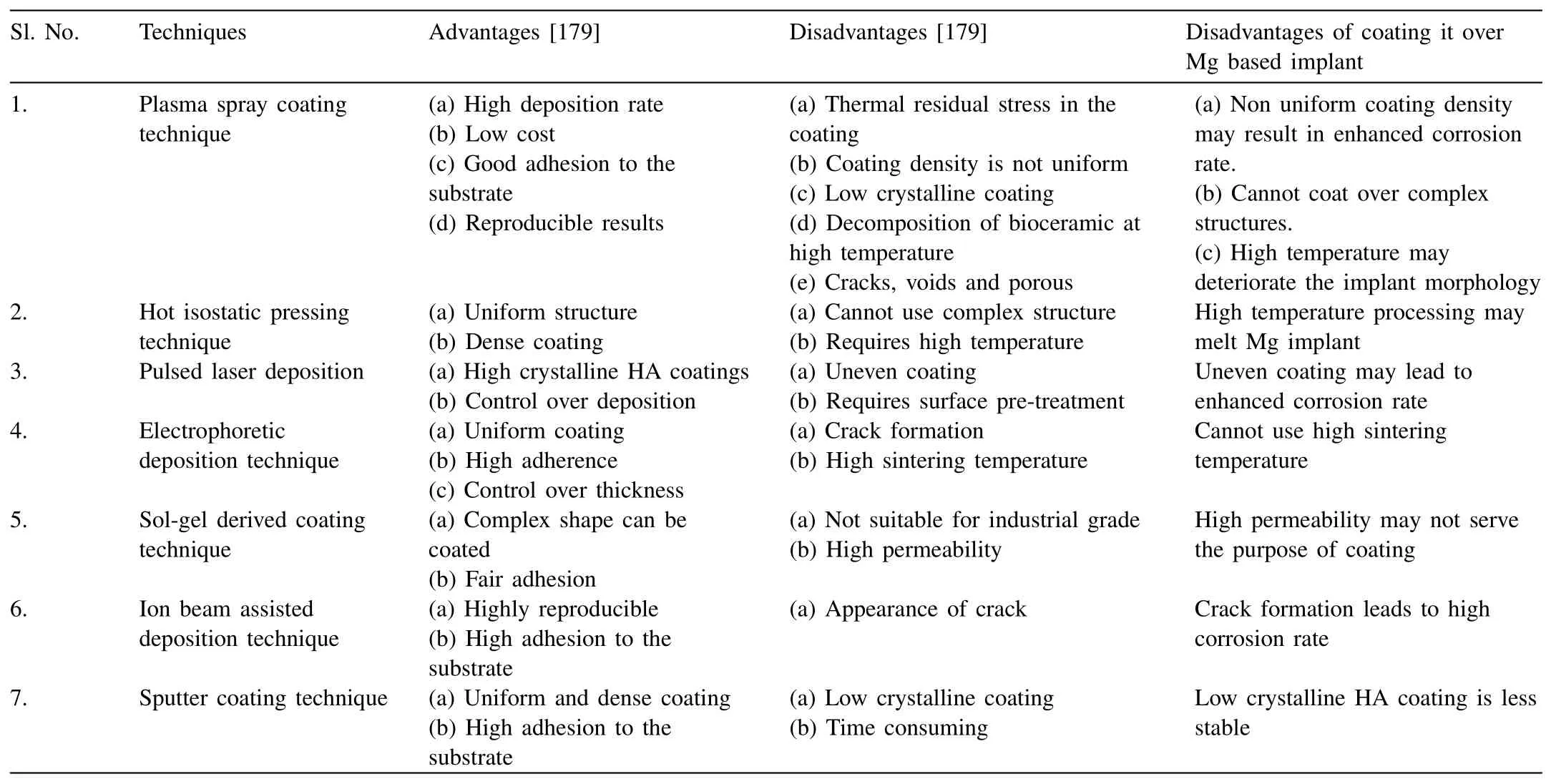
Table 6Advantages and disadvantages of coating techniques for Mg substrate.
3.2.4.Drawbacks
Even though the formation of a stable oxide layer acting as a passive layer for anodic corrosion is a general concept,long term stability has to be taken into consideration.It is known that the naturally occurring oxide films deteriorate after a critical period of exposure facilitating an upsurge in the corrosion behavior through galvanic corrosion.Therefore,after a critical exposure period,the oxide film deteriorates and initiates galvanic corrosion.Enhancing the corrosion resistance behavior of Mg by varying its elemental composition and microstructural characteristics through the alloying process is an easy way.But it is inferred from the previous discussion that the corrosion resistance is not sustainable while increasing the exposure period.Even though AZ alloys display better corrosion resistance in SBF than Mg,they are also reported to have high H2evolution[151].Therefore,further considerations are required to make Mg/Mg alloys an ideal material for developing implants.This can be achieved only by converting the matrix phase inert and hence,the corrosion tendency of the matrix can be declined.But in the present scenario,Mg has high chemical reactivity,and theoretically,to reduce this activity high percentage of inert or passive elements need be alloyed[152].But alloying a high percentage of inert elements contradicts the advantage of biodegradability of Mg implants whereas the passive elements have low solubility in the Mg matrix.Generally,alloying of Mg provides better mechanical properties through solid-solution strengthening,precipitation hardening,and grain refinement[152].However,if the alloying elements are added in a higher percentage,the mechanical properties degrade(passive elements)or improve(inert elements)far beyond what is needed for being an implant material.Hence,overcoming the drawback of the excess liberation of H2gas is limited through the alloying process.
4.Declining the corrosion of Mg by surface modificatio
In the above context,surface modification becomes a convenient option where only the surface of the Mg implant is subjected to treatment and therefore,the mechanical properties constituted from the bulk of the material are not altered[153].As described earlier,corrosion occurs owing to the formation of cathodic and anodic islands over the metal substrate leading to electrochemical reactions.Therefore,if the anodic or cathodic process is restricted,then the reaction rate can be declined.In this context,a proper,chemically inert coating over the metallic substrate can act as a barrier to the occurrence of electrochemical reactions[154].
4.1.Tailoring Mg corrosion by coating
Even though alloying can improve the corrosion behavior of Mg,the corrosion resistance of Mg alloys is still low which may not rectify the problems faced by pristine Mg-based implants.In this regard,developing many surface modification techniques[the implant surfaces are coated with suitable materials]restricts the corrosion behavior which is an active research area.The coating acts as a barrier between the anode(Mg)and electrolyte(body fluids)and thereby,reduces the anodic oxidation effectively[155].Generally,chromium coating is considered as best since it delivers enhanced corrosion resistance to any underlying metal substrate.But,it was reported that the wear debris and ions of Cr from the commercial Co-Cr alloys are found to be cytotoxic and cause allergic reactions[153,156].So,there is a need for alternate suitable coating material that competes with the efficiency of chromium coating.The major class of promising corrosion inhibition coating materials includes metals,metal oxides,polymer composites,and phosphates.The coating should exhibit high adherence to the implant,should be chemically inert,and most importantly should be biocompatible.Further,the coating should not possess any chemical reactivity with the underlying Mg substrate[157].
4.2.Hydroxyapatite coating for tailoring Mg/Mg alloy corrosion
In context to the above,immense research attention is gained by the calcium phosphate coatings which could overcome all the drawbacks.Calcium phosphate is mostly found as the structural component in teeth and bone.Therefore,coating calcium phosphate over Mg/Mg alloys could improve the biocompatibility and also,enhance the corrosion resistance owing to its chemical stability.Calcium phosphate occurs with different phases like dicalcium phosphate dihydrate(DCPD),tricalcium phosphate(TCP),and hydroxyapatite(HA).However,HA is the major constituent present in the human bone(60–70 wt.%)along with other impurity phases[158].So,coating HA over Mg-based implants provides biocompatibility.HA is also known for its chemical inertness,does not dissociate in water,good temperature stability,etc.[159,160].Due to these properties,HA coatings have been investigated as a substitute for chromium-based coatings for medical implant applications[161].Numerous techniques have been employed to attain HA coating over metallic substrates which are briefly discussed.
4.2.1.Plasma spray technique
The plasma spray technique is a thermal spraying procedure where the material is melted using heat energy from plasma and the molten material is accelerated towards the surface of interest as fine droplets[162].The plasma gun consists of a cathode which is usually tungsten and an anode made of copper.The arc discharge is initiated by high frequency where the plasma gas that flows between the electrodes spreads out in the form of the arc at the nozzle reaching a temperature between 10,000 °C and 15,000 °C.The material to be coated is fed to the plasma arc where the material is melted and is accelerated towards the substrate via plasma flame as molten droplets.The fine molted droplets that reach the target surface cool down and solidify instantaneously via heat transfer to the substrate which forms a coating.Livingstone et al.[163]developed HA coating over implant surface through plasma spray coating technique and their process parameters were optimized using full factorial design.The high crystalline film was formed when the input current was high,
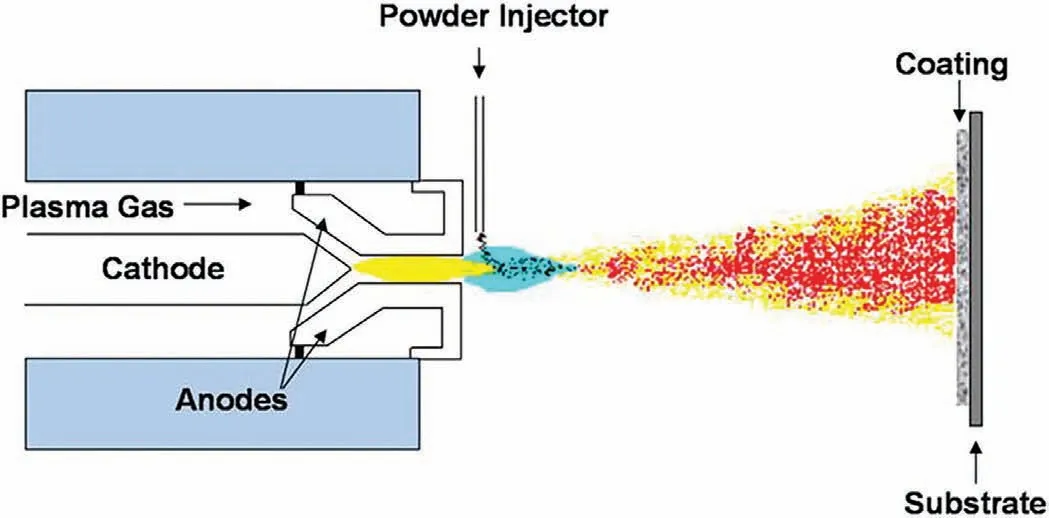
Fig.4.Schematic illustrating the plasma spray coating technique[163].
low spray/substrate distance,and coated with a low plasma gas flow rate substantiating the fact that the HA coating quality depends on the coating parameters(Fig.4).Gao et al.[164]improved the corrosion resistance of AZ91HP Mg alloy in SBF through HA coating via plasma spraying technique.From the obtained results,they have concluded that the HA coated Mg alloys restricted the pitting corrosion where the bare Mg alloys undergo serious pitting corrosion.Further,HA declines the blood coagulation and improves the cell adhering property than bare Mg alloy.Baslayici et al.[165]coated HA over AZ31 and AZ91 Mg alloys via plasma spray technique to improve theirin-vitrocorrosion resistance.The corrosion rate for coated and uncoated samples was estimated using the weight loss technique(ASTM G31–72).After 1 h of exposure to SBF,the corrosion rate of uncoated AZ31 and AZ91 were evaluated as 3.86 mm/y and 3.75 mm/y,respectively.But,after coating HA,the corrosion rate declines to 0.48 mm/y and 0.46 mm/y for AZ31 and AZ91,respectively,after 1 h of exposure.
4.2.2.Hot isostatic pressing technique
Hot isostatic pressing is a technique where the ceramic materials can be coated with reduced porosity and improved density resulting in an enhancement in the mechanical properties of the coating.The HA coatings developed using the plasma spraying technique display porous nature and tend to dissolve easily in the biological system.Since the hot isostatic pressing technique develops highly dense coatings,the drawback possessed by the plasma spray technique can be rectified.Here,high pressure of the gas at high temperature is applied to compact the ceramic materials and subjected to sintering(Fig.5).This results in the enhancement of mechanical properties of the coatings such as Young’s modulus,compressive strength,etc.[166].Onoki and Yamamoto[167]utilize a double layer capsule where the first capsule was made up of polyethylene to accommodate Mg alloy surrounded with calcium hydrogen phosphate dehydrate and calcium hydroxide as a precursor material for HA.The capsule I was again enclosed by the second capsule made up of polyvinyl chloride where alumina powder was placed in-between capsule I and capsule II.The completely sealed double layer capsule was subjected to isostatic pressing under hydrothermal conditions where the pressure and temperature were maintained at 40 MPa and 150 °C,respectively resulting in the formation of HA coated samples.

Fig.5.Working chamber of the hot isostatic press[168].
4.2.3.Sol-gel dip coating technique
Through the sol-gel coating technique,the surface properties of metallic substrates such as chemical,mechanical,etc.can be improved[169].In the sol-gel process,the precursor is dissolved uniformly in the organic solvent which is then hydrolyzed to obtain sol.With proper treatment parameters,polycondenzation occurs resulting in the formation of a gel.For developing coating over substrates,the dip coating route is preferred.The substrates are dip coated with the sol-gel forming a layer over the surface of the substrates(Fig.6).Further,the dip coated samples are subjected to high temperature heating to develop ceramic coating over the substrates.It has been reported that the immersion and withdrawal rate,sol viscosity,number of repetition,etc.are the parameters that influence the coating parameters[170].Rojaee et al.[171]used the sol-gel technique to develop HA coating over AZ91 Mg alloy.Here,calcium nitrate and phosphorous pentoxide were taken as precursor materials to develop a solution containing a Ca:P ratio of 1.67:1.Both precursors were dissolved in ethanol and mixed with dropwise addition.The mixture was closed and stirred for 5 h at 400 rpm maintaining 26 °C.The AZ91 samples were dip coated with the above mixture at a speed of 0.1 mm/s.The coated samples were subjected to the aging process at room temperature for 24 h Further,the samples were also kept at 60 °C for 24 h and consequently subjected to calcination at 400 °C to obtain HA coated AZ91 Mg alloy.The corrosion behavior of prepared samples was estimated in SBF where the results suggest that the HA coating declines the biocorrosion and the release of Mg2+.It was also mentioned that the presence of HA coating improves the activity of osteoblast cells which enhances the regeneration of damaged bone.
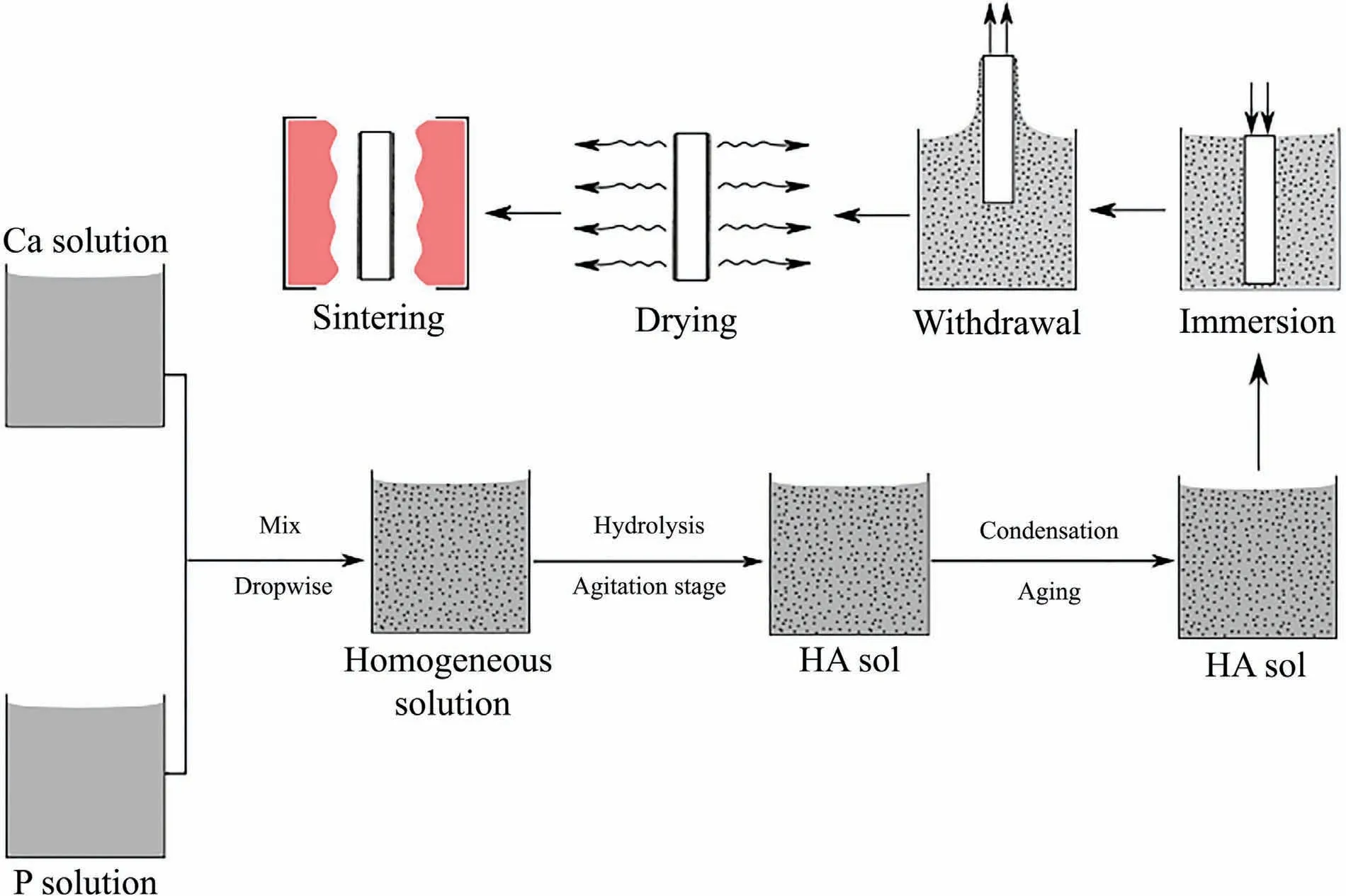
Fig.6.Fundamental stages of sol-gel HA preparation and deposition by dip coating[170].
4.2.4.Pulsed laser deposition technique
Pulsed laser deposition(PLD)is another high-temperature coating technique employed for the growth of thin films.PLD is a physical vapor deposition technique where the high-power pulsed laser is focused toward the target material which develops high temperature and vaporizes the material to form a plasma plume(Fig.7).The plasma plume is made to deposit on the nearby substrate through nucleation and crystal growth[173].Rau et al.[174]coated HA on Mg-Ca alloy via PLD technique to improve its corrosion resistance behavior in thein-vitrocondition.Here,the influence of substrate temperature on the coating efficiency of HA was estimated where the results showed that at 400 °C,the coating starts to decompose,and therefore,the recommended temperature was 200 °C to 300 °C.Further,the corrosion studies displayed that the HA film coated with the substrate temperatures of 200 °C and 300 °C exhibited improved corrosion resistance properties in SBF.
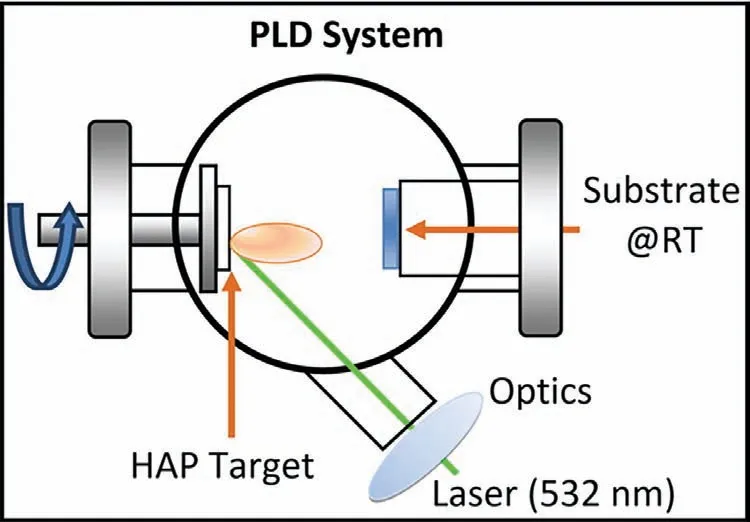
Fig.7.Nd:YAG(532 nm)pulsed laser deposition technique[172].
4.2.5.Electrophoretic deposition technique
In the electrophoretic deposition route,the particles migrate toward the opposite electrode and uniformly deposit on the substrate under the influence of an electric field.As the electric field is responsible for the deposition of particles,by varying the applied field,the film thickness can be tuned.This technique can be classified into two types depending upon the electrode and charged materials.In the cathodic electrophoretic deposition,+ive particles are deposited over the cathode whereas-ive particles are deposited over the anode in the anodic electrophoretic deposition.Even though the electric field is the primary force to perform electrophoretic deposition,the charge and mobility of the particles in the electrolyte also have a major influence on the deposition[175].Kumar et al.[176]developed HA coating over Mg-3Zn alloy using the electrophoretic deposition route.Here,two alloy plates were used as electrodes dipped in the solution prepared by dispersing HA in isopropanol(20 g/L)using the ultrasonicator.The deposition was carried out using 20 V/cm DC power for 10 min.The HA coated samples were collected,aged for 2 h,dried at 60 °C for 2 h,and annealed.The HA coated Mg alloys display 25 times higher corrosion resistance than the bare substrate duringin-vitroexposure.Further,it was
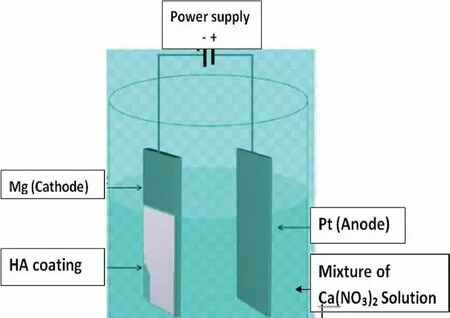
Fig.8.HA coating procedure through electro-deposition technique[177].
also found that the presence of HA coating improved bone cell growth over the Mg alloy.In another study conducted by Jamesh et al.[177],HA was coated over pure Mg from the precursor solution(not from the particles)through the electrodeposition route(Fig.8).Here,the electrolyte was composed of 0.06 M(NH4)3PO4,0.1 M Ca(NO3)2,and 10 mL/L of 30 vol% H2O2coated at 27 °C having a pH of 4.After the electrodeposition process,to obtain crystalline HA,the specimens were treated with 1 M NaOH aqueous solution for 2 h at 80 °C.The HA coated specimen displayed declinedicorrvalue of 1.39×10-3μA/cm2compared to pure Mg(icorr=95×10-3μA/cm2).Further,it was reported that owing to the flaky structures of HA,osteointegration was improved.
4.2.6.Ion beam assisted deposition technique

Fig.9.Schematic drawing of FIB induced deposition process[181].
Ion beam assisted deposition(IBAD)is a technique where thermal evaporation of target material is achieved by ion bombardment[178](Fig.9).The IBAD is performed in vacuum conditions where the ion bean energy can be tuned as per requirement through altering applied current density,ion species,etc.The deposition process is influenced by many target material characteristics such as elemental composition,chemical nature,structural,and mechanical properties[179].Compared to traditional coating techniques,the coatings developed by the IBAD technique show high adherence to the substrate which is an important parameter to restrict HA dissolution from Mg substrate in the biological system.Yang et al.[180]studied the degradation behavior of magnesium alloy coated with HA using IBAD with post annealing treatment.The results obtained from the accelerated degradation test displayed declined mass loss(one fifth)by the coated sample than the bare sample after 15 days of exposure to the corrosive medium.This is attributed to the efficient hindering of corrosion reaction by HA which could improve the longevity of implants underin-vivoconditions.
4.2.7.Sputter deposition technique
Sputter deposition is one of the best techniques for HA coating over a wide variety of substrates.The target has been bombarded with Argon or Nitrogen gas to generate plasma,coated over substrate placed opposite to the target(Fig.10).The HA coating parameters like topography,Ca/P ratio,density,thickness,etc.can be tuned by varying the sputtering parameters such as atmospheric pressure,applied voltage,distance between substrate and target,time,etc.[182].Surmeneva et al.[183]employed the radio frequency(RF)magnetron sputtering technique to develop HA thin film with thickness ranging between 550 nm and 700 nm over Mg-Ca alloy.Further,thein-vitrocorrosion studies of coated specimens were performed with SBF using electrochemical techniques which displayed~98% reduction inicorrcompared to bare Mg alloy.Surmeneva et al.[184]studied the attachment of bone marrow stromal cells andin-vitrocorrosion of AZ91 coated with HA using RF sputtering.The degradation rate of specimens was indirectly assessed from the concentration of Mg2+in the growth medium and found that the HA coated specimens liberate significantly lesser Mg2+than uncoated AZ91.From the results obtained from thein-vitrodirect culture,HA coated using a substrate bias of-100 V noticeably improves the cell adhesion than uncoated AZ91.

Fig.10.Schematic illustration of RF-magnetron sputtering system[185].
From the above discussions,it is clear that there are many techniques for the development of HA coating over Mg/Mg alloys.However,each technique has its own advantages and limits which are listed in Table 6.
From Table 6,the conventional coating techniques cannot be used to coat over the Mg-based medical implants because of the complex shape of the implant,uneven coating,high-temperature processing,etc.For example,in the case of the plasma spray coating technique,the temperature generated by the plasma is very high compared to the melting point of Mg.This leads to the deformation of the implant shape.Likewise,for the electrophoretic deposition technique,a high sintering temperature is required for the formation of HA phase and its efficient adherence over Mg.Also,higher end equipment is required that enhances the cost during industrial manufacturing.Further,the pulsed laser deposition,sol-gel coating,and ion beam deposition techniques resulted in high permeable HA coating.The high permeability induces galvanic corrosion where HA behave cathodic and Mg matrix acts as anodic causing serious localized corrosion.Furthermore,the implants are manufactured in complex shapes and the coating techniques such as sputtering and hot isostatic pressing do not possess the design to coat over complex structures.
4.3.Calcium phosphate conversion HA coating
In contrast,the phosphate conversion coating technique can overcome all such drawbacks,HA can be coated over any trivial shape with controlled thickness,can govern deposition rate,low-temperature processing,can achieve the desired degree of adherence,etc.[186–189].The phosphate conversion technique involves three steps.(i)Surface treatment–for cleaning the Mg/Mg alloy surface before the coating process.(ii)Coating process–where the phosphate coatings are formed from the bivalent metal and phosphate precursor solution and(iii)Post-treatment–like thermal and alkaline treatment to improve the property of coatings.
4.3.1.Principle behind calcium phosphate conversion coating
Generally,the precursor solution consists of diluted phosphoric acid(H3PO4)and bivalent cations(M).On immersing Mg/Mg alloys into the precursor bath,the surface of the substrate starts reacting as per the(Eqn.5)given below,
3M+6H++2PO43-→3M2++2PO43-+3H2↑ (5)
The reduction of H+ions in the reaction results in an increase in pH at the surface of Mg.Subsequently,the M2+reacts with phosphate present in the mixture to form M3(PO4)2(Eqn.6).
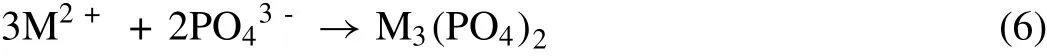
As the surface pH is high,the M3(PO4)2becomes insoluble and gets deposited as a coating over the Mg substrate.However,along with the formation of M3(PO4)2,there are some impurity phases such as Mg3(PO4)2,MgHPO4,and Mg(OH)2are also formed due to the interference of dissociated Mg2+ions[190].Here,the liberation of H2gas influences negatively on the deposition process.The liberated H2gas is hooked over the Mg surface and does not allow the phosphate to deposit uniformly.Therefore,an adequate quantity of oxidizing agents is also introduced to reduce the liberation of H2.In presence of an oxidizing agent,the H2is oxidized to H2O which facilitates uniform and uninterrupted phosphate deposition over the implant surface.
For coating Ca-P over Mg substrate,the bivalent calcium ions are chosen as metallic moiety and the resulting coating will constitute Ca3(PO4)2i.e.,TCP phase.However,altering reaction conditions such as Ca2+concentration,PO43-concentration,pH of the solution,etc.promotes the formation of HA phase(Ca10(PO4)6(OH)2)over Mg/Mg alloys.Owing to the flexibility of the phosphate conversion technique,many studies have employed this technique to develop HA coatings over Mg/Mg substrates to improve their corrosion resistance.
4.3.2.Corrosion behavior of HA coated Mg/Mg alloys
Chen et al.[191]coated pure Mg samples with HA films using a two-step phosphate conversion technique.In the first step,the Mg specimens were submerged in a reaction mixture having Ca(NO3)2·4H2O(Ca2+source),Na3PO4(PO43-source),and HNO3(stabilizer).The ratio of Ca2+source and PO43-source was fixed at 1:1 and the solution pH was adjusted to 4.5 using HNO3.The composition of the bath solution was carefully chosen using the equilibrium predominance illustration where the Ca2+is found to be soluble at pH 4.5 Fig.10a(region A)).However,when Mg samples were immersed in the bath solution,the electrochemical reaction takes place with the formation of OH-ions leading to the increase in pH at the vicinity of the Mg surface.From Fig.10a,with an increase in pH,the HA formation is facilitated(Eqn.(7)),resulting in the deposition of HA over the Mg surface(primary coating).


Fig.10.(a)Equilibrium predominance diagram of various calcium phosphates calculated using MEDUSA,for a concentration of 10 mM PO43-,(b)Potentiodynamic polarization curves in MEM for(a)uncoated,(b)as primary coated,and(c)secondary-coated Mg,and(c)Surface morphology of pure Mg specimens immersed in MEM(37 °C,5% CO2)for 48h Left:uncoated Mg;right:secondary-coated Mg[191].
Even though HA was formed,there might be a chance of forming CaHPO4·2H2O i.e.,the DCPD phase as an intermediate which is represented in the B region of Fig.10a.Therefore,to completely convert the film to the HA phase,the precursor-treated Mg specimen was immersed in a higher pH solution(10 g/L of NaOH)as a second step(secondary coating).The XRD measurement revealed that after primary coating,DCPD might be formed over the Mg surface.But,it does not show any peak corresponding to DCPD substantiating the dehydrated amorphous state of DCPD[192].But,upon post alkaline treatment with NaOH solution,the DCPD gets converted to HA.This phenomenon is explained in Fig.10a where the influence of the pH of the solution comes into consideration.At low pH,the phosphate conversion technique facilitates the formation of the DCPD phase.However,at high pH,the growth of HA is favored.Here,the Mg was first treated at a low pH of 4.5 inducing the formation of the DCPD.But,during post-treatment with NaOH(10 g/L)solution,the phase transformation occurred resulting in highly crystalline and dense HA coating.
The potentiodynamic polarization(PDP)analysis of unmodified Mg,Mg surface modified with primary coating and secondary coating carried out in minimal essential medium(MEM)(Fig.10b).From the figure,the pristine Mg shows a highericorrof 600 μA/cm2due to the high biodegradability of Mg in MEM.However,after primary treatment,the plot represents declinedicorrof 37 μA/cm2owing to the substantial decrement in the rate of anodic reaction.The decrease in the anodic current was enabled by the HA/DCPD phase formed over the Mg surface acting as a passive layer.But,after secondary treatment,the impurity DCPD phase was completely altered to the HA phase resulting better reduction inicorrof 2.7 μA/cm2.This phenomenon was understood by the breakdown potential(-1.32 V)found in the PDP curve of Mg modified with a secondary coating which substantiates the passive nature of the secondary coating.Further,theEcorrvalue of secondary coating was found positively shifted than the uncoated Mg and Mg modified with primary coating.This validates less anodic dissolution occurred in the HA phase(secondary coating)than the HA phase with DCPD impurity(primary coating).Further,weight loss studies were evaluated for HA coated and pristine Mg in MEM,in that,uncoated Mg displayed a high degree of dissolution.In contrast,HA coated sample showed deprived dissolution with a low degree of pitting which was not deep and the specimen retained its original dimension(Fig.10c).This fact is attributable to the stable and crystalline nature of the HA films than the DCPD films in the coated Mg formed due to the alkaline treatment.
However,it has been reported that the higher alkaline content also deteriorates the HA coating performance.Zaludin et al.[193]coated Ca-P over 99.9% Mg by phosphate conversion technique using the precursor bath solution containing Ca(NO3)2·4H2O and Na3PO4in the ratio of 0.32 M/0.19 M The pH of the solution was adjusted to 2 by adding HNO3stabilizer.The pre-treated Mg samples were immersed in the bath solution for 60 min at 40 °C.After the coating process,the samples were subjected to post-treatment with 1 M NaOH solution for 60 min at 80°C where the HA phase was formed.The results obtained from PDP analysis of the uncoated Mg were compared with the coated Mg(after coating with primary and secondary coating)(Fig.11a).The coated Mg samples display better corrosion resistance confirmed throughicorrvalues.The uncoated Mg displaysicorrof 186 μA/cm2,while the primary and secondary coated samples displayicorrof 25 μA/cm2and 121 μA/cm2,respectively.However,while comparing the PDP values among primary coated and secondary coated Mg,primary coated samples display better corrosion resistance behavior than the secondary coated Mg samples.Such a contrast result from the previous literature data was attributable to the formation of porosity in the HA film during 1 M NaOH treatment.During the secondary treatment to convert the DCPD phase to the HA phase,cracking,and micro-pores were formed which facilitated the electrolyte to reach the underlying Mg substrate resulting in enhanced corrosion.This phenomenon was further elucidated by evaluating the porosity of primary and secondary coating where HA film showed 22.6% porosity but DCPD film showed only 0.3% porosity.Further,DCPD films were found to have more thickness than the HA film which was due to the dissolution of the HA film at high NaOH content.Despite,HA being said to be stable owing to high crystallinity,the HA-coated Mg(SEC)resulted in low corrosion resistance than DCPD coated Mg(PRI)in SBF(Fig.11b).Even though the reaction conditions such as temperature and period for the secondary coating were the same as that of Chen et al.[191],the quantity of NaOH was almost 1/4th of the quantity used by Zaludin et al.[193].Therefore,it is inferred that during secondary treatment in an alkaline solution,the quantity or concentration of the alkaline content dictates the quality of final HA films over the Mg.
Chen et al.[194]studied the influence of[Ca2+]and[PO43-]concentration in the precursor solution on the development of Ca-P coatings over AZ91D.The Ca(NO3)2·4H2O and Na3PO4were used as the Ca2+and PO43-source and the pH was adjusted to 3 by adding HNO3.Fig.11b represents the PDP curves of uncoated and coated Mg alloy(AZ91D)where the coatings were performed in different concentrations of Ca2+and PO43-.Here,when the coating was performed without the presence of PO43-,theicorr(~30 μA/cm2)was increased than the uncoated Mg alloy(~10 μA/cm2).As there was insufficient PO43-,only Ca was deposited over the surface resulting in a more anodic reaction(since Ca is more cathodic than Mg)leading to an increasedicorr.However,while adding both Ca2+and PO43-,theicorrstarted declining than the uncoated Mg alloy.In particular,Ca2+and PO43-sources with 10 mM and 1 mM concentration,respectively display the lowesticorr(~0.2 μA/cm2)with the occurrence of passivating behavior.Here,the HA phase was visualized as a secondary phase along with the DCPD phase without post alkaline treatment.This study discloses the ratio of Ca2+and PO43-ions in the bath solution is also an important factor in the anti-corrosive performance of the Ca-P coating via single-step conversion.Kim et al.[195]coated pure Mg with HA through a single step calcium phosphate conversion process where the pH of the bath solution was adjusted to 8.9 by adding NaOH.The bath solution constituted 0.05 M Ca-EDTA and 0.05 M KH2PO4salt.After coating for a period of 2 h at 90 °C,HA films were formed over the Mg surface which was confirmed by the XRD pattern.Therefore,even by single-step coating,the HA films can be obtained by adjusting the pH of the solution to a high value.The anti-corrosive property of the HA modified Mg in SBF was evaluated through the PDP measurements.The HA modified Mg displayed a very lowicorrvalue(3 μA/cm2)compared to pristine Mg(6831 μA/cm2).Also,theEcorrvalue was shifted towards the noble side substantiating the anti-corrosive behavior of HA coating through a single-step coating procedure.Further,they have evaluated the quantity of H2gas liberated from the uncoated and HA-coated samples in SBF.27 mL of H2gas was evolved in the case of the uncoated sample whereas the coated sample liberated only 1.5 mL of H2gas after 6 days.H2liberation can be thus drastically reduced by coating HA over Mg/Mg alloys.

Fig.11.(a)Potentiodynamic polarization curves of uncoated(UC),DCPD coated(PRI),and HA coated(SEC)coated Mg samples inside SBF at 37 °C[193],(b)selected polarization curves showing untreated,activated and coated performance.Polarization tests were conducted in 0.1 M NaCl at a sweep rate of 1 mV/s[194],and(c)XRD patterns of the substrate and the coatings:(a)uncoated ZK60 alloy;(b)as-deposited coating;and(c)heat-treated coating[196].
Apart from the post alkaline treatment technique for phase conversion from DCPD to HA,the thermal treatment route has also been investigated.Wang et al.[196]coated Ca-P over ZK60 alloys by phosphate conversion process resulting in the formation of films with DCPD.However,in this study,the Ca-P coated ZK60 was heated at 350 °C for 3 h converting the Ca-P films to the HA phase,as revealed in the XRD pattern(Fig.11c).The PDP measurements carried out in SBF depict corrosion resistance behavior of DCPD and HA coated ZK60 than its pristine form.Theicorrvalues were also declined in 1 and 2.5 order magnitude for DCPD and HA coated ZK60,respectively than uncoated ZK60.The uncoated ZK60 displayed anicorrvalue of 35.39 μA/cm2while DCPD and HA coated ZK60 displayedicorrvalues of 3.56 μA/cm2and 0.14 μA/cm2,respectively.Likewise,theEcorrof DCPD and HA coated ZK60 shifted+100 mV and+148 mV,respectively than the uncoated ZK60.These results substantiate the fact that the DCPD was converted to HA during thermal treatment and provides better corrosion resistance property to the underlying substrate.Further,7 days immersion test was also carried out and the results validated that the HA films were found intact and protected the ZK60 alloy efficiently.
Table 7 summarizes various studies reported on the corrosion resistance behavior of HA-coated Mg/Mg alloys by calcium phosphate conversion technique and reveals that there is a vast interest in the development of this technique to improve the corrosion resistance of Mg/Mg alloys during the past 15 years.Sherds of evidence have started emerging to show that HA coating over Mg/Mg alloys potentially minimizes the corrosion rate.
Recent reports suggest that by surface pre-activation,the phosphate conversion coating can be made more efficient.During the pre-activation process,the natural oxide layer over the Mg alloys is peeled off and hence,more sites will present for the nucleation and growth of HA.Li et al.[202]studied the effect of pre-activation on phosphate conversion coating over ZTM630 Mg alloy.The surface of the Mg alloy was pre-activated using colloidal Ti,oxalic acid,and phosphoric acid.After pre-activation,phosphate conversion coating was performed for all the specimens.From the topographical studies,it was evident that the developed phosphate crystals were smaller in size and densely packed for samples pre-activated with phosphoric acid compared to surfaces pre-activated by oxalic acid.Also,it was reported that in all three cases,the composition of the phosphate coating was the same but the growth rate differed.From the PDP measurements,it was confirmed that the Mg alloys coated with phosphate crystals after pre-activation by phosphoric acid displayed lowericorrthan other samples(no treatment=38 μA/cm2,colloidal Ti=0.3 μA/cm2,oxalic acid=0.16 μA/cm2,and phosphoric acid=0.1 μA/cm2).This fact is attributed to the effective removal of oxide layer by the phosphoric acid leading to the formation of uniform dense phosphate crystals during phosphate conversion reaction.
In-vivostudies in small animal models are needed to validate the effectiveness of HA coated Mg/Mg alloys through the calcium phosphate conversion technique.In this context,Kim et al.[195]studied the efficiency of the HA-coated Mg to be used as implant material throughin-vivoconditions.The obtained results validate the constructive evidence for the passivating nature of HA coating towards biocorrosion.In that,HA coated,and uncoated samples were inserted into bonepericranium pouches on calvaria(Fig.12a).
After the implantation period of 2 to 12 weeks,the implants were removed,and their photographs showed that the uncoated Mg samples biodegraded harshly owing to excessive biocorrosion(Fig.12b).They even became worse with the increase in the implantation period.In contrast,the HAcoated Mg samples displayed a lower corrosion rate validat-

Fig.12.(a)Optical images of the specimens that were implanted into rat bone-pericranium pouches.The inset shows the geometry and dimensions of the specimens for tensile tests,(b)optical images of the bare Mg and HA-coated Mg specimens after 2,4,6,8,and 12 weeks of implantation,and(c)reconstructed l-CT images of the bare(left pictures)and HA coated Mg specimens(right pictures)after 6 weeks(upper images)and 12 weeks(lower images)[195].
?

?

Fig.13.Principle of bioactivity and osteoconduction of CaP coatings on titanium implants.Following implantation,the partial dissolution of HA coating leads to the release of Ca2+and HPO42-,precipitation of biological apatite,colonization of osteogenic cells producing the bone extracellular matrix resulting in a direct bone tissue apposition on titanium[203].
ing the significance of HA coating.Further,a model implant was fabricated(screw)using pristine Mg and HA-coated Mg.The model implants were implanted into the tibial defects of rabbits.After the healing process period(6 weeks and 12 weeks),the morphologies and changes in volume were analyzed through microcomputed tomography.As inferred from thein-vivostudies,the screw thread made from uncoated Mg was corroded severely whereas the screw was found somewhat intact in HA coated Mg.The volume reduction observed in HA was 5.28% while for Mg based screw,it was 20% after 6 weeks(Fig.12c).In 12 weeks,the volume reduction of HA in coated Mg was estimated as 0.29% per day showing better performance than uncoated Mg(0.4% per day).From all these reported results,it can be inferred that the corrosion rate can be tuned and thus,H2gas evolution and rapid degradation of Mg/Mg alloys can be declined in the biological environment through calcium phosphate conversion coating.Further,it has been reported that coating HA over implants improves the osteointegration process[203].As HA has a similar composition as that of bone minerals,the Ca2+and PO43-ions dissociated to the peri–implant area leading to the saturation of body fluids.In this context,biological apatite gets precipitated over the implant’s surface(Fig.13).The biological apatite consisting of endogenous proteins facilitates the attachment and growth of osteogenic cells.The osteogenic cells create extracellular matrix promoting appositional growth of bone tissue over the HA coated implant’s surface.
4.3.3.Comparing the corrosion resistance behavior of alloying and HA coating
Comparing the corrosion behavior of Mg alloys and HA coated Mg/Mg from different reports is a tedious task as the testing parameters in each report vary which may lead to false conclusions.Therefore,theicorrvalues of the samples evaluated using the same parameters such as preparation of SBF,pH,and reaction temperature are taken into consideration.In this regard,only limited data can be compiled and is listed in Table 8.Here,the preparation of SBF as followed by[204],pH of 7.4,and temperature of 37 °C are considered for comparing the corrosion results.From the summarized values,it is clear that Mg alloys have declinedicorrthan pure Mg.However,in the case of HA coated Mg,theicorris drastically reduced than Mg alloys substantiating the fact that HA coating inhibits corrosion far better than alloying.

Table 8Comparison of icorr values of Mg,Mg alloys,and HA-coated MG at same reaction conditions.
4.4.Drawbacks
Even though there are a lot of advantages of using HA coating over Mg/Mg alloys through calcium phosphate conversion coating,there are some limitations that need to be addressed.Galvanic corrosion occurs for the specimens owing to the damage caused to the HA coating where the HA coating acts as the cathode and the Mg matrix acts as anode leading to pitting/localized corrosion.However,Hiromoto[187]reported that the hydroxyapatite coated Mg and AZ31 display self-healing activity.For validation,the surface of the HA coated Mg and AZ31 were scratched and immersed in a 0.9% NaCl aqueous solution.Rationally thinking,the scratched specimen should dissociate Mg+ions very easily but the ion release rate was not accelerated.This was due to the fact that at the scratched area,the Mg+ions reacted with the HPO42-(dissociated from HA coating)and precipitated over the scratched surface,thus enabling self-healing activity(Fig.14).
Although it was reported that the HA coating can decline the galvanic corrosion by forming an inorganic layer in the damaged regions[205],the implants are prone to stress and wear in practical conditions while implanted in the human physiological system.Owing to these conditions,the coating undergoes continuous wear/damage which leads to the formation of cracks facilitating galvanic corrosion.The onset of galvanic corrosion can lead to excess H2generation causing health concerns like in the case of Mg/Mg alloys.Also,corrosive wear,stress corrosion,and corrosion fatigue are the other processes that decline the mechanical integrity of the Mg/Mg alloys.However,there are no substantial reports regarding its study on HA coated Mg/Mg alloys through calcium phosphate conversion.But limited literature data is obtained for HA coated through other techniques.Kannan and Orr[206]coated HA over AZ91 through the electrochemical route by applying cathodic voltage.The specimens were subjected to SBF for 5 days and the mechanical integrity of coated and uncoated specimens was analyzed.From the obtained results,the uncoated specimen lost 40% mechanical strength while the coated specimen displays 20% higher strength than the uncoated specimen.Further,from the scanning electron mi-croscopic images it was revealed that the presence of HA declines localized corrosion leading to better mechanical integrity.Furthermore,another study conducted by Mohajernia et al.[207]substantiated the fact that the HA coated AZ31 alloy through plasma spray technique declines the stress corrosion cracking susceptibility where the elongation percentage loss reduces from 52.9%(uncoated)to 26.8%(HA coated)after exposed to slow strain rate tensile in SBF.Even though there are scarce reports suggesting the improvement in the mechanical integrity through HA coating,long term andinvivostudies are not performed to validate their claim which needs to be focused on.

Fig.14.Hydroxyapatite coatings onto the Mg implants[187].
5.Scope for future work
·Hydroxyapatite coating over Mg/Mg alloys by calcium phosphate conversion coating is found to be a potential process to improve the anti-corrosive properties of Mg/Mg alloys for implant applications.However,limited studies are found with regard to the same and therefore,further studies are warranted to confirm the efficacy of this technique.
·There are no optimization studies with respect to the coating parameters such as pH,the concentration of Ca2+and PO43-source,coating duration,bath temperature,the concentration of alkaline content,etc.with proper optimization tools.Such studies can provide guidelines to perform the coating more efficiently to improve the corrosion behavior of Mg/Mg alloys.·In-vivostudies in small animal models are needed to validate the effectiveness of hydroxyapatite coated Mg/Mg alloys through the calcium phosphate conversion technique.Research related toin-vivostudies can provide insights into how the modified implant material will perform in real time biological environment,but studies related to it are scarce.
·Although corrosion studies have been reported for the HA coated Mg through calcium phosphate conversion coating,studies related to long term corrosion behavior employing volumetric and gravimetric analysis are very limited.
·Likewise,the long term mechanical integrity studies concerning stress corrosion cracking,corrosive wear,corrosion fatigue,etc.of the Mg/Mg alloy implants coated with HA through calcium phosphate conversion coating need to be addressed.·Even though there are studies dealing with the corrosion behavior of hydroxyapatite modified Mg/Mg alloys,there are no studies investigating the fate of these modified implants.There is no answer to questions such as how long the coating will be intact with the surface of implants?If the coating is damaged,does it enhance the corrosion of Mg/Mg alloys over a period?If the corrosion rate is enhanced,does it serve the purpose for which it has been used?Without answers to these questions,it is difficult to conclude that hydroxyapatite coating over Mg/Mg alloys by calcium phosphate conversion coating can overcome the drawbacks of pristine Mg/Mg alloys[Scheme 1].Therefore,further intensive research towards the identified gaps is very important to improve the commercial viability of the coating process.

Scheme 1.Scope for future research work.
Acknowledgment
Authors thank Chettinad Academy of Research and Education for providing adequate facilities to complete the present review work.
 Journal of Magnesium and Alloys2022年7期
Journal of Magnesium and Alloys2022年7期
- Journal of Magnesium and Alloys的其它文章
- High-performance magnesium-based thermoelectric materials:Progress and challenges
- Effects of heat treatment on the corrosion behavior and mechanical properties of biodegradable Mg alloys
- Multi-solute solid solution behavior and its effect on the properties of magnesium alloys
- The role of solutes in grain refinement of hypoeutectic magnesium and aluminum alloys
- Characterization on the formation of porosity and tensile properties prediction in die casting Mg alloys
- Biomass-derived carbon doping to enhance the current carrying capacity and flux pinning of an isotopic Mg11B2 superconductor
