Promising application of a new ulnar nerve compound muscle action potential measurement montage in amyotrophic lateral sclerosis: a prospective crosssectional study
Yi-Xuan Zhang , Jing-Yue Ma , Xiang-Yi Liu Shuo Zhang Zhou Yu Dong-Sheng Fan
Abstract Previous studies have shown that ulnar nerve compound muscle action potential recorded by the conventional “belly-tendon” montage does not accurately and completely reflect the action potential of the ulnar nerve dominating the abductor digiti minimi muscle due to the effects of far-field potentials of intrinsic hand muscles.A new method of ulnar nerve compound muscle action potential measurement was developed in 2020, which adjusts the E2 electrode from the distal tendon of the abductor digitorum to the middle of the back of the proximal wrist.This new method may reduce the influence of the reference electrode and better reflect the actual ulnar nerve compound muscle action potential.In this prospective cross-sectional study, we included 64 patients with amyotrophic lateral sclerosis and 64 age- and sex-matched controls who underwent conventional and novel ulnar nerve compound muscle action potential measurement between April 2020 and May 2021 in Peking University Third Hospital.The compound muscle action potential waveforms recorded by the new montage were unimodal and more uniform than those recorded by traditional montage.In the controls, no significant difference in the compound muscle action potential waveforms was found between the traditional montage and new montage recordings.In amyotrophic lateral sclerosis patients presenting with abductor digiti minimi spontaneous activity and muscular atrophy, the amplitude of compound muscle action potential-pE2 was significantly lower than that of compound muscle action potential-dE2 (P < 0.01).Using the new method, damaged axons were more likely to exhibit more severe amplitude decreases than those measured with the traditional method, in particular for patients in early stage amyotrophic lateral sclerosis.In addition, the decline in compound muscle action potential amplitude measured by the new method was correlated with a decrease in Revised Amyotrophic Lateral Sclerosis Functional Rating Scale scores.These findings suggest that the new ulnar nerve compound muscle action potential measurement montage reduces the effects of the reference electrode through altering the E2 electrode position, and that this method is more suitable for monitoring disease progression than the traditional montage.This method may be useful as a biomarker for longitudinal follow-up and clinical trials in amyotrophic lateral sclerosis.
Key Words: amyotrophic lateral sclerosis; axonal degeneration; biomarker; compound muscle action potential; distal E2 electrode; early diagnosis; far field potential; nerve electrophysiology; prognosis evaluation; proximal E2 electrode; ulnar motor nerve conduction
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease characterized by the degeneration of both upper and lower motor neurons.Motor neurons are highly polarized cells with long axons, and motor axon degeneration is an early event in ALS (Fischer and Glass, 2007;Dadon-Nachum et al., 2011; Tian et al., 2016; Lo and Lee, 2021; Xu and Yuan,2021).The amplitude of the compound muscle action potential (CMAP) is widely used in clinical practice as an important electrophysiological index to detect motor axonal damage (Liu et al., 2009).Studies have reported that the severity of CMAP amplitude decrement in the early stage of ALS is significantly correlated with the severity of the disease (Imai et al., 2020; Yu et al., 2021).Therefore, accurate and sensitive detection of axonal damage by electrophysiological examination is important for the follow-up evaluation of ALS patients.
In motor nerve conduction studies, CMAP is recorded by the “belly-tendon”montage, which was introduced by Harvey and Masland (Harvey and Masland,1941).The CMAP is recorded using three electrodes: the recording (or active)electrode (E1), the reference electrode (E2) and the ground electrode (E0).E0 is used to reduce background noise and interference.E1 is placed over the muscle belly.E2 is always at the distal muscle tendon or even further distal to E1 in order to reduce stimulus artifacts, particularly when motor conduction studies were first performed and recording equipment was more prone to artifacts (Hodes et al., 1948; Nandedkar and Barkhaus, 2020).Potentials are recorded by a differential amplifier.Hence, the displayed signal results from the difference in activity between E1 and E2 (Kincaid et al., 1993).Under ideal conditions, E2 should be electrically quiet or “inactive”; thus, CMAP would primarily represent the electrical activity at E1.However, in actual practice,the E2 electrode records significant voltage from other muscles innervatedby the stimulated nerve (Kincaid et al., 1993; Brashear and Kincaid, 1996;Nandedkar and Barkhaus, 2007), especially in ulnar motor nerve conduction studies recorded from the abductor digiti minimi (ADM) (Sonoo, 2020).
When E2 is placed distally, the CMAP produced by ulnar nerve stimulation shows two distinct peaks.Because of contributions from the interosseous muscles, the unintended peak of E2 coincides with the ulnar nerve CMAP peak used for amplitude measurements (McGill and Lateva, 1999; Nandedkar and Barkhaus, 2020; Sonoo, 2020).Hence, the hypothenar CMAP does not truly reflect the amplitude of the innervated ulnar nerve branch and does not change proportionately with the pathology of the test muscle.Considering this, Nandedkar and Barkhaus (Nandedkar and Barkhaus, 2020)used a proximal E2 site at the center of the dorsal wrist that was just distal to the ulnar styloid process.The proximal E2 position provided high-quality recordings with good separation between the CMAP and stimulus artifacts(Nandedkar and Barkhaus, 2020).Electrophysiological analysis has shown the proximal E2 site to be electrically inactive; thus, greater sensitivity may be achieved (McGill and Lateva, 1999; Nandedkar and Barkhaus, 2020).This study applied the proximal E2 electrode configuration proposed by Nandedkar and Barkhaus (Nandedkar and Barkhaus, 2020) to ALS patients to explore the clinical value of this method in detecting ALS-associated ulnar nerve damage.
Methods
Subjects
This was a prospective cross-sectional study conducted at Peking University Third Hospital.All study procedures were approved by the Ethical Committee of our institution (approval No.223-02) on September 5, 2017 (Additional file 1).The subjects provided their written informed consent (Additional file 2) to participate in this study.This study followed the STrengthening the Reporting of OBservational Studies in Epidemiology (STROBE) guidelines(von Elm et al., 2007; Additional file 3).A sample size of 60 achieves 90%power to detect a mean of paired differences of 0.6 and with a significance level of 0.05 using a two-sided paired t-test (calculated by PASS 15; NCSS,LLC., Kaysville, UT, USA).We included 64 ALS patients who met the revised El Escorial diagnostic criteria (Brooks et al., 2000) and 64 one-to-one ageand sex-matched controls, recruited at Peking University Third Hospital from April 2020 to May 2021.The exclusion criteria for the controls were any comorbidities that may involve motor neurons of the upper limbs, such as ALS, neuromuscular disease, autoimmune disease, tumor or other conditions.The collected characteristics of the patients included demographic details,symptom duration, phenotype and site of onset.The Revised ALS Functional Rating Scale (ALSFRS-R) (Cedarbaum et al., 1999) was used to assess ALS severity at the time of recruitment.ALSFRS-R is a self-reported questionnaire consisting of 12 questions that evaluate the bulbar, motor and respiratory functions of ALS patients.Each question is scored between 0 and 4 (where 4 represents totally intact and 0 represents total loss of function) (Bakker et al.,2017), with higher scores indicating more retained function.
Neurophysiological examinations
Clinical neurophysiologic examinations were performed by an experienced electromyography (EMG) technologist using a Keypoint four-channel EMG evoked potentiometer (Medtronic, Minneapolis, MN, USA).The skin temperature was maintained at > 33°C.In our study, the ulnar-ADM combination was used for CMAP recording.The stimulation intensity was supramaximal, and the stimulus duration was 0.2 ms.The stimulation site was the wrist, and the E1 electrode was placed over the muscle belly.Consistent with the practices of the traditional montage method, we placed E2 at the distal tendon of the ADM, the fifth metacarpophalangeal joint; we referred to this setup as the “traditional montage.” For the “new montage” setup,we placed E2 at the proximal position (at the center of the dorsal wrist; the distance between the stimulation site and E2 was more than 3 cm) according to the methods used in the study by Nandedkar and Barkhaus (2020; Figure 1).The interval between the two methods was at least 5 minutes.

Figure 1|Diagram of the new proximal E2 montage.
The CMAP amplitudes and waveforms measured by each method were recorded bilaterally.For analysis, data from the left side were selected for controls and patients with only bulbar or lower limb involvement, and data from the more affected side were selected for patients with upper limb involvement.The amplitude results of ALS patients were stratified into four groups based on the levels of decrease relative to the normal range of controls (Table 1): normal (≥ the lower limit of normal, LLN), mild decrease(< LLN but ≥ 50% of LLN), moderate decrease (< 50% but ≥ 30% of LLN), and severe decrease (< 30% of LLN).Furthermore, all ALS patients underwent concentric needle EMG in the tested ADM.Spontaneous activity was recorded with the muscle at rest, and was assessed by fibrillation potentials and positive sharp waves.We defined muscles without fibrillation potentials/positive sharp waves as EMG (-) and muscles with any degree of fibrillation potentials/positive sharp waves as EMG (+).

Table 1 |Stratification criteria of CMAP amplitude (mV)
Statistical analysis
Statistical analyses were performed using SPSS 22.0 (IBM, Armonk, NY, USA).The CMAP amplitudes obtained by both methods were normally distributed(Shapiro-Wilk test for normality,P
> 0.05).For each patient, a pairedt
-test was used to compare the CMAP amplitudes obtained by the two methods.The Wilcoxon test was used to compare the CMAP amplitudes between the four groups stratified by levels of relative decrease.Pearson’s correlation analysis was conducted to detect the correlation between the CMAP amplitude of the traditional montage and the difference of the two montages.Partial correlation analysis was used to detect the relationship between the CMAP amplitude and disease progression.P
< 0.05 was considered statistically significant.Results
Demographic and clinical data of ALS patients and controls
A total of 64 sporadic ALS patients and 64 one-to-one age- and sex-matched controls underwent EMG examination.According to the revised El Escorial criteria (Brooks et al., 2000), we included 9 definite (14%), 15 probable (23%),19 laboratory-supported probable (30%) and 21 possible (23%) ALS patients.The control group consisted of 60 participants who came to our laboratory for electrophysiological examination, including 41 participants with lumbar spondylosis, 15 with peroneal nerve injury, 4 with facial palsy, and 4 healthy controls from our colleagues (Additional Figure 1).The demographics and general clinical characteristics of the patients and controls are summarized in Table 2.

Table 2 |Demographics and general clinical characteristics of the amyotrophic lateral sclerosis (ALS) patients and healthy controls
Ulnar nerve CMAP in the control group CMAP waveform patterns
First, we compared the CMAP waveforms obtained by the traditional montage(CMAP-distal E2, CMAP-dE2) with those obtained by the new montage (CMAP-proximal E2, CMAP-pE2).CMAP-dE2 waveforms could be classified into three patterns: The first was bimodal, with a waveform characterized by a lower peak followed by a posterior higher peak that was used for measurement(“low-high”).This was the most common pattern, accounting for 67% of the cases.The second was also bimodal, with a waveform characterized by ahigher anterior peak that was used for measurement followed by a lower peak(“high-low”).This pattern accounted for 17% of the cases.The third pattern was unimodal, accounting for 16% of the cases.Waveform examples are shown in Figure 2.In contrast, CMAP-pE2 had a uniform unimodal waveform,which was characterized by a negative peak followed by a low amplitude wave(Figure 3).
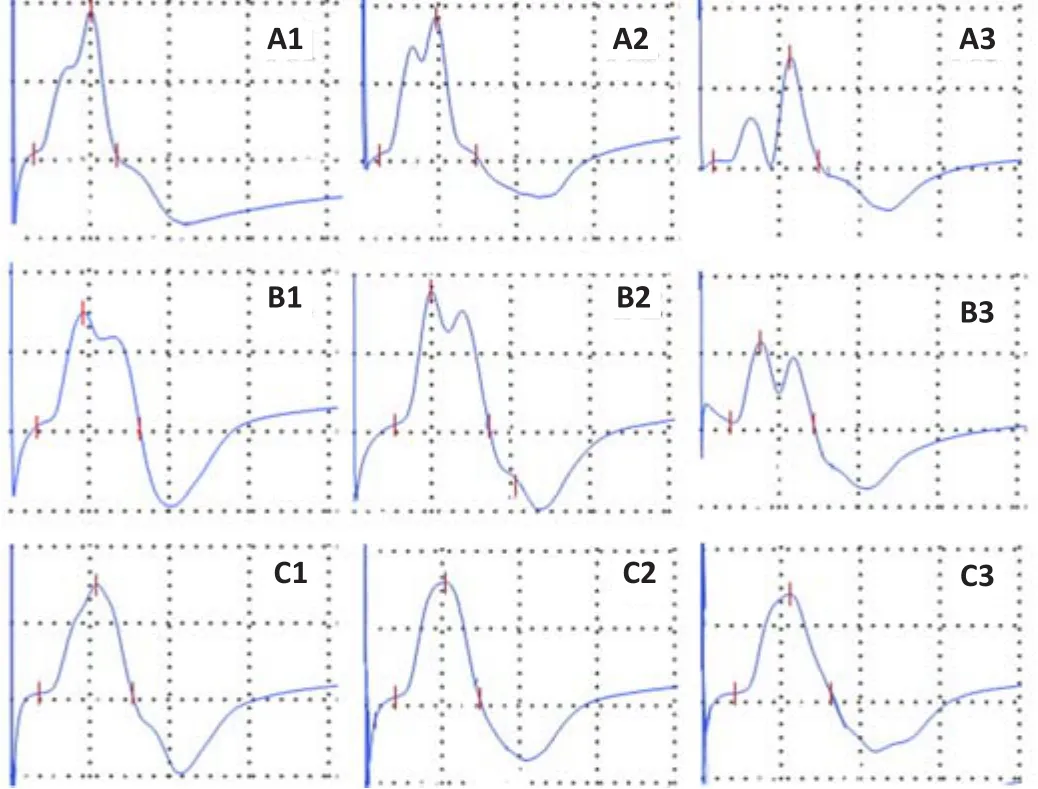
Figure 2 | Waveform patterns of the CMAP obtained by the traditional (CMAP-dE2)montages in controls.

Figure 3|Comparison of waveform patterns of CMAP-pE2 and CMAP-dE2 in healthy controls and amyotrophic lateral sclerosis patients.
Comparison of CMAP amplitudes
In the controls, the CMAP-dE2 amplitude was 7.715 ± 2.00 mV, and the CMAP-pE2 amplitude was 7.719 ± 2.00 mV; no significant difference was observed between the two results (P
= 0.982).We divided the CMAP-dE2 results according to the waveform pattern.The amplitude of the first pattern (bimodal, low-high waveform) was 7.81 ± 2.08 mV, which was significantly higher than that obtained by the new method (7.42± 1.90 mV,P
< 0.01).The amplitude of the second pattern (bimodal, highlow waveform) was 7.06 ± 1.78 mV, which was significantly lower than that obtained by the new method (8.07 ± 2.23 mV,P
= 0.015).However, for the unimodal waveform, there was no significant difference between the values measured by the two methods (P
= 0.397; Table 3).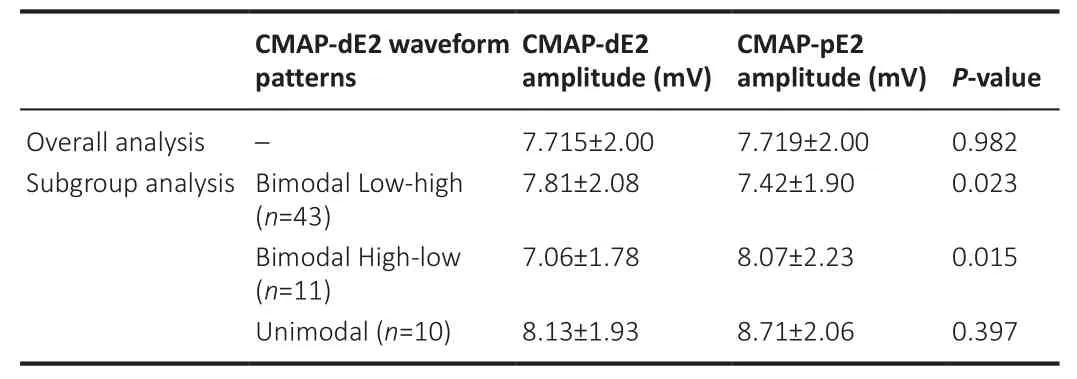
Table 3 |Ulnar nerve CMAP amplitude obtained by the traditional (CMAP-dE2) and new (CMAP-pE2) montages in the healthy controls
Ulnar nerve CMAP in ALS patients CMAP amplitude comparison
CMAP-dE2 became uniform with ulnar nerve injury, and mainly presented a unimodal pattern.However, in CMAP-pE2, the waveform consistently presented a unimodal pattern regardless of the decline in CMAP amplitude(Figure 3).
Next, we compared the CMAP measurements obtained by the traditional and new methods.The amplitude was 4.41 ± 2.53 mV using the traditional method and was 3.85 ± 2.62 mV using the new method, presenting a significant decrease (P
< 0.01).The ALS patients were divided into the EMG(-) and EMG (+) groups.In the EMG (-) group, there were no significant differences in CMAP amplitude between the two methods (6.44 ± 2.67 mVvs.
6.66 ± 2.50 mV,P
= 0.636), but in the EMG (+) group, the amplitude of CMAP-pE2 (2.76 ± 1.67 mV) was significantly lower than that of CMAP-dE2 (3.46± 1.87 mV,P
< 0.01).The ALS patients were also grouped according to their clinical characteristics into those with ADM muscular atrophy (ADM atrophy(+)) and those without ADM muscular atrophy (ADM atrophy (-)).In the ADM atrophy (-) group, the CMAP amplitudes were not significantly different between the two methods (5.77 ± 2.37 mVvs
.5.53 ± 2.53 mV,P
= 0.399),whereas in the ADM atrophy (+) group, the amplitude of CMAP-pE2 (2.18 ±1.32 mV) was significantly lower than that of CMAP-dE2 (3.05 ± 1.89 mV,P
<0.01; Table 4).
Table 4 |Subgroup comparison of CMAP amplitude (mV) in amyotrophic lateral sclerosis patients
CMAP amplitude stratification
In EMG (+) and ADM atrophy (+) patients, CMAP-pE2 amplitudes were more likely to be stratified as severely decreased relative to controls compared with CMAP-dE2 amplitudes (P
< 0.01; Table 5).Notably, the normal and mildly decreased stratifications exhibited the largest differences between the two methods (Table 5).Furthermore, the amplitude differences between CMAP-dE2 and CMAP-pE2 correlated significantly (r
= 0.39,P
< 0.01) with the CMAP-dE2 amplitudes that were below the normal high value (< 5.87 mV; Figure 4).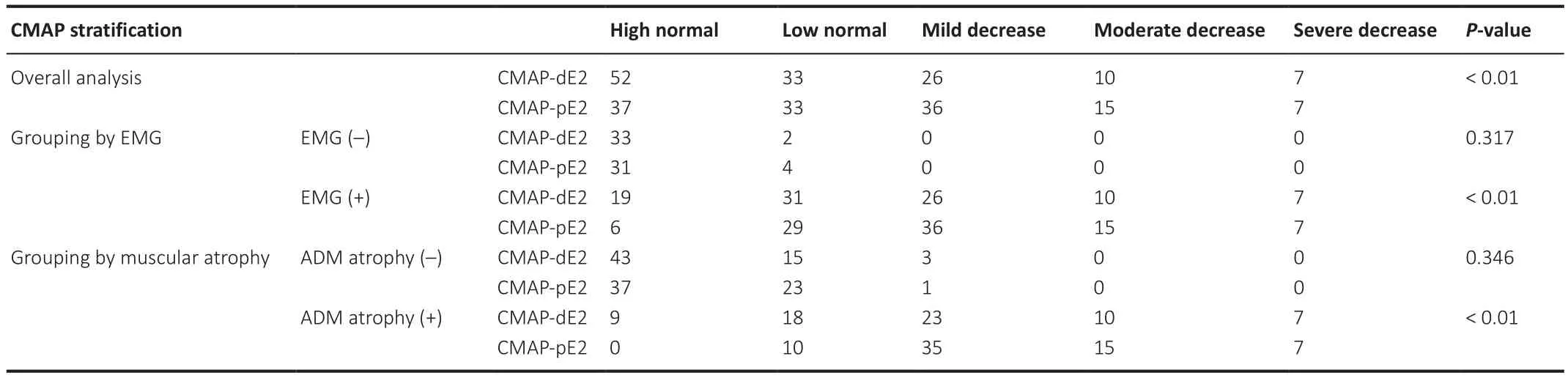
Table 5 |Subgroup comparison of CMAP stratification levels in amyotrophic lateral sclerosis patients
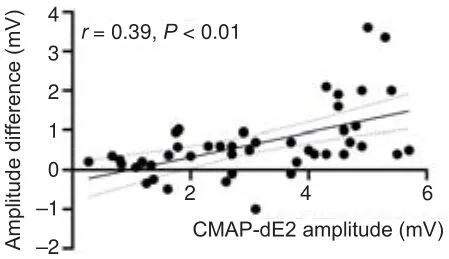
Figure 4 | Amplitude differences between CMAP-dE2 and CMAP-pE2 correlated significantly (r = 0.39, P < 0.01) with the CMAP-dE2 amplitudes that were below the normal high value (< 5.87 mV).
Correlation between CMAP amplitudes and disease severity
Partial correlation analysis adjusting for confounding effects of sex, age and onset site confirmed a significant inverse correlation between CMAP-pE2 amplitude and disease duration (r
= -0.31,P
= 0.016).CMAP-pE2 amplitude also declined significantly with ALSFRS-R (r
= 0.42,P
= 0.014).CMAP-dE2 amplitude did not show any significant correlation between ALSFRS-R (P
=0.117) or disease duration (P
= 0.416; Figure 5).
Figure 5 | Partial correlation analysis between CMAP amplitudes and disease severity.
Discussion
In this study, we compared the CMAP waveforms of the ulnar nerve obtained by the traditional method and a new method in ALS patients.Previous studies demonstrated that the CMAP amplitudes obtained by the traditional muscletendon montage were not sensitive enough to detect ulnar nerve damage in early phase ALS (Fang et al., 2016; Wang et al., 2020; Gunes et al., 2021).This study was the first to apply the proximal E2 montage proposed by Nandedkar and Barkhaus (2020) to ALS patients.We found that, compared with those of the traditional method, the waveforms of CMAP-pE2 were more uniform.In early stage ALS, before involvement of the ulnar nerve, the CMAP amplitudes obtained by the two methods were consistent.However, compared with CMAP-dE2, CMAP-pE2 detected early-stage axonal injury more sensitively and reflected the degree of degeneration more accurately in accordance with disease severity in ALS patients.
More uniform waveforms obtained by CMAP-pE2
In healthy controls, the CMAP-dE2 exhibited three patterns: low-high, highlow and unimodal waveforms, which is consistent with previous studies (van Dijk et al., 1999; Sonoo, 2020).However, these waveforms were all unimodal when measured by the new method, showing a large negative peak followed by a low amplitude wave contributed by the reference electrode, which is consistent with Nandedkar’s study (Nandedkar and Barkhaus, 2020).The ulnar nerve innervates the hypothenar and interosseous muscles, which both become stimulated when attempting to record the ADM CMAP.The distal E2 potential of the ulnar nerve was not electrically inactive but registered a triphasic waveform composed of an initial negative (N1), a steep positive(P1) and a bimodal N2 peak, which is consistent with the results of previous studies (Nandedkar and Barkhaus, 2007, 2020; Higashihara et al., 2010; Sonoo et al., 2011; Sonoo, 2020).These reference potentials are far-field potentials produced by interosseous (or other deep motor branch-innervated) muscles:N1 is generated by the second and third dorsal interosseous muscles; P1 by the second and third palmar interosseous muscles, and by the third and fourth dorsal interosseous muscles to a lesser extent; early N2 by the third palmar interosseous muscle and late N2 by the ADM (Nandedkar and Barkhaus, 2007; Higashihara et al., 2010; Sonoo et al., 2011; Sonoo, 2020).Because the E1 electrode has a shorter onset latency than that of the distal E2 (McGill and Lateva, 1999), this time difference results in the bimodal CMAP in the traditional montage.However, the latencies of the N1 and P1 peaks would decrease with the flexion of the second, third and fourth fingers (McGill and Lateva, 1999).When the latencies of E1 and E2 approach each other,the waveform becomes unimodal.In ALS patients, the distal E2 potential decreased with the neurogenic injury to the interosseous muscles; thus, the waveform became unimodal as the disease progressed.In contrast, the proximal E2 recorded a lower volume-conducted signal and yielded a CMAP that was more representative of where the E1 electrode was placed.Therefore, in both healthy controls and ALS patients, CMAP-pE2 was unimodal.
Ulnar nerve degeneration was more accurately monitored by CMAP-pE2
In ALS patients with ADM spontaneous activity or muscular atrophy, the amplitudes of CMAP-pE2 were significantly lower than those of CMAP-dE2 and tended to be stratified into more advanced severity levels.Furthermore,the decline in CMAP-pE2 amplitude was significantly correlated with ALS disease progression.
Nandedkar and Barkhaus (2020) reported that the second peak of bimodal CMAP-dE2 coincided with the positive peak (P1) of the signal recorded by the distal E2 electrode.This indicated that the initial negative E2 signal (N1)cancelled out the first negative potential from the E1 electrode, and that the steep P1 added to the negativity of the E1 signal through differential amplification.In the low-high bimodal waveform, we measured the posterior high peak that overlaid the P1 amplitude from E2.In the high-low bimodal waveform, the first high peak subtracted the N1 amplitude from E2.However,the E1 potential was the main contribution to the CMAP amplitude measured by the new method.Thus, the amplitude of the low-high CMAP was higher than that of the new method, but the high-low CMAP was lower.
When the ulnar nerve was mildly impaired in ALS patients, the interosseous muscles innervated by deep motor branches were also involved.In this state,the N1 and N2 peaks decreased in the distal E2 signal, presenting a one-way wave dominated by the P1 peak (Higashihara et al., 2010), which concealed the early-stage decline in E1 potential, and generated a pseudonormalized amplitude.In our cohort, 27 ALS patients had developed ADM atrophy, but their CMAP-dE2 amplitudes were still within the normal range.However,when measured by the pE2 montage, their amplitudes were stratified into the decreased groups.As the disease progressed, atrophy of the interosseous muscles led to a further decline in the E2 potential, and its interference with E1 was also reduced.Therefore, in the severely decreased group, the main component of CMAP was the E1 potential in both methods, and the stratification of CMAP damage was consistent.
The potential clinical significance of CMAP-pE2
The CMAP of hypothenar muscles has received considerable attentionin ALS research.In ALS, hand muscle wasting preferentially affects the thenar (lateral)hand, with relative sparing of the hypothenar muscles (ADM).This peculiar pattern was termed ‘split hand’ by Wilbourn (2000), which is rarely seen in diseases other than ALS.The later involvement of the ADM has generated considerable interest because of its potential clinical significance.
The split-hand index (SI) is derived by multiplying the abductor pollicis brevis(APB) and first dorsal interosseous (FDI) CMAP amplitudes, then dividing the product by the ADM CMAP amplitude according to the following formula:(SICMAP = APBCMAP × FDICMAP/ADMCMAP) (Menon et al., 2013).SI has been suggested as a diagnostic (Corcia et al., 2021; Hu et al., 2021)and outcome (Hannaford et al., 2021) biomarker in ALS.The CMAP-pE2 amplitude may provide a more objective measurement of dissociated axonal dysfunction; however, multicenter studies are required to assess the cut-offvalue, sensitivity and specificity using the new setup.
More importantly, the relative sparing of the ADM may offer pathophysiological insights into ALS, including a lower degree of cortical excitability and corticomotoneuronal projections (Weber et al., 2000; Eisen et al., 2017) and a lower extent of axonal excitability (Shibuya et al., 2013).Nevertheless, pathophysiology questions remain open and subject to intense debate.The measurement of CMAP-pE2 amplitude could shed light on earlier stage degeneration of the ulnar nerve and serve as a more accurate longitudinal biomarker in follow-up studies and clinical trials.
This study had several limitations.First, the patient sample size was limited,which might have led to selection bias.A large-sample multicenter validation study is still needed.Second, although we controlled for sex, age and site of onset when detecting correlation between the ulnar nerve CMAP andALSFRS-R, this study is still a cross-sectional study.A follow-up evaluation will be more convincing.Third, this study focused on the clinical application of the new ulnar nerve examination method, but lacked further study on the underlying electrophysiological mechanism.A combination of surface motor unit potentials and motor unit number estimation is suggested to provide a more comprehensive assessment of mechanisms underlying the far-field potential in ALS patients.
In conclusion, the traditional montage method does not accurately reflect the decrease in the CMAP amplitude of the ulnar nerve in early-stage ALS patients due to electrical activity in distal E2.In some ALS patients who have presented with ADM weakness and muscular atrophy, their ulnar nerve CMAP amplitudes were still in the normal range.However, the new method reduced the reference potential by changing the position of E2, resulting in a more accurate measurement of the ulnar nerve CMAP.The new method was more suitable for the evaluation of the disease progression, and may serve as a longitudinal biomarker in ALS studies and clinical trials in the future.
Author contributions:
Study design: JYM, YXZ, DSF; patient recruitment and evaluation: JYM, ZY; imaging data acquisition: SZ; data analysis: JYM, YXZ,XYL; manuscript drafting: YXZ, JYM.All of the authors read the draft, made contributions, and approved the final manuscript.
Conflicts of interest:
The authors declare that they have no competing interests.
Author statement:
This paper has been posted as a preprint on Research Square with doi: https://doi.org/10.21203/rs.3.rs-1185620/v1, which is available from: https://assets.researchsquare.com/files/rs-1185620/v1/ca56f643-056e-42b3-9b8f-b71e763de81f.pdf?c=1643581609.
Availability of data and materials:
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Hospital ethics approval (Chinese).
Informed consent form (Chinese).
STROBE checklist.
Flowchart of the study.
- 中国神经再生研究(英文版)的其它文章
- Neural and Müller glial adaptation of the retina to photoreceptor degeneration
- Agomelatine: a potential novel approach for the treatment of memory disorder in neurodegenerative disease
- MicroRNAs: protective regulators for neuron growth and development
- In vivo astrocyte-to-neuron reprogramming for central nervous system regeneration: a narrative review
- Intranasal nerve growth factor for prevention and recovery of the outcomes of traumatic brain injury
- Altered O-GlcNAcylation and mitochondrial dysfunction,a molecular link between brain glucose dysregulation and sporadic Alzheimer’s disease

