MicroRNAs: protective regulators for neuron growth and development
Zhi-Xuan Ma, Zhen Liu, Hui-Hui Xiong, Zong-Pu Zhou, Li-Si Ouyang, Fu-Kang Xie, Ya-Mei Tang, Zhong-Dao Wu , Ying Feng,
Abstract MicroRNAs (miRNAs) play an important regulatory role in neuronal growth and development.Different miRNAs target different genes to protect neurons in different ways, such as by avoiding apoptosis, preventing degeneration mediated by conditional mediators, preventing neuronal loss,weakening certain neurotoxic mechanisms, avoiding damage to neurons, and reducing inflammatory damage to them.The high expression of miRNAs in the brain has significantly facilitated their development as protective targets for therapy, including neuroprotection and neuronal recovery.miRNA is indispensable to the growth and development of neurons, and in turn, is beneficial for the development of the brain and checking the progression of various diseases of the nervous system.It can thus be used as an important therapeutic target for models of various diseases.This review provides an introduction to the protective effects of miRNA on neurons in case of different diseases or damage models, and then provides reference values and reflections on the relevant treatments for the benefit of future research in the area.
Key Words: brain damage; miRNA; neurodegenerative disorders; neuronal apoptosis; neuronal protection
Introduction
MicroRNAs (miRNAs) are endogenous, 18-22 nucleotide, non-coding ribonucleic acid (RNA) molecules that function as post-transcriptional regulators of gene expression.By binding to mRNAs, specifically at the 3′-untranslated region (3′-UTR) through perfect or imperfect complementation, miRNAs induce either translational repression or RNA degradation in cells (Ambros, 2004; Rana, 2007).The dysregulation of miRNA expression has been observed in many neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis, neurological disorders, and epilepsy.Neurodegeneration and death are important markers of neurodegenerative diseases.The importance of miRNA in the nervous system has been reported in many studies in recent years, and an increasing amount of evidence of miRNA dysregulation in the case of neurological diseases is becoming available.Understanding the expression and activity of these miRNAs may contribute to the development of new therapies (Karnati et al., 2015).During the development of the nervous system, a large number of neurons must undergo apoptosis over a period for them to precisely match their respective target cells (Oppenheim,1991).However, once the corresponding neuron has connected to its specific target cell, its apoptotic program must be strictly controlled because these neurons cannot regenerate, and have limited survival and function in the body(Benn and Woolf, 2004).miRNAs are small, non-coding RNAs that regulate gene expression (Bartel, 2009).Here, we examine whether miRNAs can play a protective role as a key regulator in the growth and development of neurons and whether they can be a target for therapeutic intervention (Figure 1).
Database Search Strategy
The authors used a number of criteria to include research in this review.Studies discussing the effects of miRNAs as protective regulators of neuronal growth and development were considered.The full text of English-language articles published from January 2015 to June 2021 were included in this nonsystematic review.The models of diseases considered pertained to the type of brain injury associated with miRNA.The authors searched the PubMed database to identify the relevant publications.The strategies for literature retrieval were as follows: The terms (1) “miRNA” and (2) “neuron” were combined with (a) “neuron protection” and (b) “neurodegenerative diseases,”such as in “miRNA’s protective effect on neurons i.e., (1) + (a), and “protective effect of miRNA on neurons in the mechanism of neurodegeneration,” i.e.,(1) + (b).We used four queries.We screened the list of references included in each study to identify other studies that might be useful.We first screened the titles and abstracts of papers, and then search their full text for keywords,such as “neuron protection” and “nerve injury,” to find ones that might be appropriate.The process of data extraction focused on information on each type of injury examined and the protective role played by miRNA.
Role of MicroRNAs in Brain Development
The discovery of miRNA-mediated gene regulation has led to a deeper understanding of the regulatory mechanisms of gene expression in the last decade.Several studies have investigated the role and regulation of miRNA in brain development.The potential of miRNAs to regulate individual gene expression provides brain cells, especially neurons, with the ability to control gene expression from their upstream and downstream sites.This is a prerequisite for the formation of the developing brain (Kiecker and Lumsden,2005).miRNAs play significant roles in brain development, iPSCs, stemness,epithelial-to-mesenchymal transformation, and the maturation of different types of cells (Kapranov et al., 2010).Each step of brain development is tightly regulated and requires a specific network of gene regulatory mechanisms.The brain expresses the highest number of unique miRNAs of all organs of the body, which suggests that they have some physiological and metabolic significance (Motti et al., 2012).miRNAs are an important regulatory factor in the basic processes of brain development, such as neuronal apoptosis,neuronal differentiation, and neuronal proliferation (Singh et al., 2014;Jauhari et al., 2017).They also constitute an important regulatory factor in peripheral nerve regeneration (Mahar and Cavalli, 2018).A large number of miRNA analyses and Dicer knockout studies have demonstrated that miRNA expression plays an extremely important role in brain development (Ambros,2004; Bak et al., 2008; Petri et al., 2014; Figure 2).
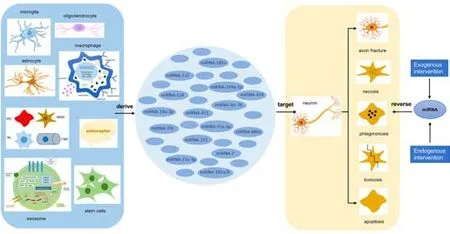
Figure 1 | The effects of miRNAs secreted by different cell sources on neurons.
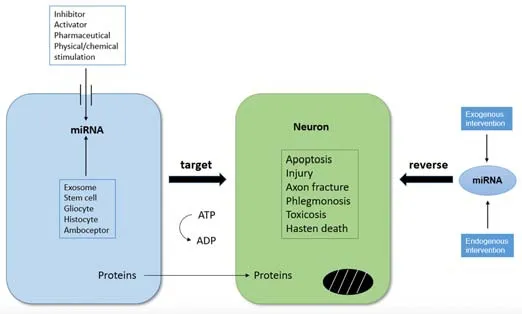
Figure 2| Changes in miRNA expression affect neuronal development and survival.
Role of MicroRNAs in Diseases
Cerebral ischemia/reperfusion injury
Cerebral ischemia/reperfusion injury (CIRI), which is caused by cardiac arrest,shock, stroke, cardiopulmonary bypass during anesthesia, and surgery, is the leading cause of disability and mortality worldwide (Donnan et al., 2008;Pang et al., 2022).The pathophysiological mechanisms of cerebral ischemia/reperfusion injury-induced neuronal damage are complex cellular events involving apoptosis- and oxidative stress-related pathways (Moskowitz et al.,2010).Many studies have elucidated the role and mechanism of miRNAs in cerebral ischemia and related diseases, which makes them a potential target for the diagnosis and treatment of CIRI (Rink and Khanna, 2011; Zhu et al.,2016; Table 1).
However, it has been reported that the inhibition of miR-181 expression in mouse models can reduce the size of the infarct, improve the neural function deficit, and reduce neuronal loss induced by forebrain ischemia(Xu et al., 2015).The injection of the miR-181 antagonist into the brain of a rat upregulates the expression of Bcl-2 and GLT-1, thereby inhibiting neuronal cell death in case of forebrain ischemia (Moon et al., 2013).The overexpression of miR-592 in neurons reduces the level of p75NTR, which is induced by ischemic injury, and attenuates the activation of pro-apoptotic signaling and cell death (Irmady et al., 2014).The injection of miR-124 soon after the onset of stroke results in the M2 polarization of the immune cells,and the modulation of astrocytes and neurons.The early application of miR-124 leads to increased neuronal survival and the functional improvement of neurological deficits.These two findings, of neuroprotection and functional improvement, are strongly correlated with the increased polarization of the microglia/macrophages toward the M2 phenotype (Taj et al., 2016).Li et al.(2019) reported that miR-199a-5p can reduce the volume of the infarct and water content of the brain, improve neurologic function, alleviate neuronal damage, and inhibit neuronal apoptosis and pro-inflammatory cytokines by down-regulating DDR1 in case of cerebral ischemia injury.The reduced miR-134 expression can enhance the expression of the cAMP response elementbinding protein, and the brain-derived neurotrophic factor (BDNF) and Bcl-2 of its downstream genes, thereby alleviating cerebral ischemia injury (Huang et al., 2015).The procedure whereby the down-regulation of endogenous miR-124 protects ischemia/reperfusion (I/R)-induced neuronal death and apoptosis by upregulating its target gene Ku70 has been described in the literature and provides a promising potential therapeutic target for cerebral ischemic injury (Zhu et al., 2014).The upregulation of miR-25 inhibits cerebral I/R injury-induced apoptosis by down-regulating Fas/FasL, which provides a promising therapeutic target (Zhang et al., 2016a).It has been reported that miR-424 reduces the volume of the infarct and inhibits neuronal apoptosis after ischemia/reperfusion by upregulating Nrf2, and protects cells from oxidative stress by upregulating manganese superoxide dismutase (MnSOD)and extracellular superoxide dismutase (Liu et al., 2015).Our observations are in agreement with those of Zhao et al.(2014), whereby miR-23a-3p reduces neuronal cell death and apoptosis as indicated by decreased lactate dehydrogenase leakage and the pro-apoptosis factor protein levels of caspase-3 in neuro-2a cells upon HO-induced oxidative stress.This study indicated that miR-23a-3p suppresses oxidative stress and reduces CIRI.Ginsenoside Rg1 inhibits the expression of miR-144 and promotes the Nrf2/ARE pathway, thereby reducing oxidative stress and protecting neurons after I/R (Chu et al., 2019).Stary et al.(2015) reported that the inhibition of miR-200c and upregulation of Reelin expression may ameliorate acute brain injury and enhance recovery.
Apolipoprotein lipoprotein particles shuttle miRNAs from astrocytes to neurons, leading to the inhibition of cholesterol biosynthesis and an increase in histone acetylation.They also inhibit the expression of HMGCR to block cholesterol synthesis in neurons.The accumulation of the substrate-adduct HMG-CoA increases histone acetylation in neuronal nuclei, upregulates intermediate early genes, and improves learning and memory (Li et al.,2021b).In the relevant procedure, microRNA-126-3p was chosen to protect PC12 cells from apoptosis and increase neurite outgrowth.The evidence suggests that treatment with EC-Exo improves motor function in the model of occlusion/reperfusion of the middle cerebral artery (MCAO/R) by altering neural plasticity in the motor cortex, likely through the transmission of microRNA-126-3p.These studies are important for the knowledge of subsequent interventions at molecular targets in case of ischemic stroke and are useful for promoting neural remodeling for functional recovery (Gao et al., 2020).The elevated expression of miR-381 by dexmedetomidine (Dex)can inhibit inflammation in rats with ischemic brain injury.Dex and miR-381 overexpression or silenced IRF4 improve neurological function and inhibit the apoptosis of neuronal cells in MCAO rats.Moreover, Dex has been found to increase miR-381 expression and reduce IRF4 expression in MCAO rats to reduce interleukin (IL)-9 expression in turn, which suppresses the inflammatory response and cell apoptosis bothin vivo
andin vitro
(Fang et al., 2021).Rong et al.(2020) have claimed that miR-29 inhibits neuronal apoptosis in rats with cerebral infarction by upregulating the Akt signaling pathway, thereby serving as a protector.The overexpression of miR-211 may protect against MCAO injury by targeting PUMA in rats and reduce the volume of the infarct, neurological score, and neuronal apoptosisin vivo
,paving the way for the treatment of CIRI (Liu et al., 2020c).Our observations are in agreement with those of Li et al.(2021a), whereby the overexpression of miR-27a-3p significantly reduces cerebral I/R damage by targeting FOXO1, which provides a new direction for future research on cerebral I/R therapy.A study on the role of miR-193b-3p expression in rats revealed that it has neuroprotective effects against focal CIRI in cultured cells.These neuroprotective effects are likely mediated by the inhibition of 5-LOX.These findings indicate that miR-193b-3p can be used as an agent for the treatment of focal CIRI (Chen et al., 2020).It has been reported that melatonin plays an anti-inflammatory and CIRI-improving protective role by regulating the miR-26a-5p-NRSF axis and the JAK2-STAT3 pathway, which may provide new ideas for the treatment of CIRI-related ischemic stroke and other diseases of the central nervous system (Yang et al., 2020a).In lower motor neurons,vascular endothelial growth factor 2 (VEGFR-2) expression was significantly reduced during maturation in conjunction with an increased level of miR-129-5p.In the sensory dorsal root ganglia, VEGFR-2 expression increased during maturation and was accompanied by the overexpression of miR-130a-3p.While miR-129-5p seems to directly reduce VEGFR-2 expression in the central nervous system, miR-130a-3p might indirectly control VEGFR-2 expression in the peripheral nervous system.This suggests that direct or indirect control of VEGFR-2 expression may improve ischemia or peripheral nerve injury (Glaesel et al., 2020).circ_016719 directly targets miR-29c to play a pro-apoptotic role, thereby regulating the expression and function of Map2k6.This suggests that miR-29C has a direct role in the apoptosis of the nerve cells, and can be used as a therapeutic target for research (Tang et al., 2020).The upregulation of lncRNA ZFAS1 can down-regulate its downstream gene miR-582-3p to play a protective role in case of brain I/R injury, protect neurons from injury,and regulate inflammation, oxidative stress, apoptosis, and nitric oxide levels(Zhang and Zhang, 2020).Ischemic stroke
Ischemic stroke, which results from the obstruction of blood supply, is a devastating disease because of the high incidence of disability associated with it worldwide (Chaturvedi and Kaczmarek, 2014; Alwjwaj et al., 2021).Ischemic brain injury is caused by the insufficient blood supply to the brain, which in turn induces symptoms such as oxidative stress, hypoxia, inflammation,and ultimately cell death, eventually leading to death (Dirnagl et al., 1999;Lo et al., 2003).miRNAs have recently been found to contribute to stroke pathogenesis and to determine vascular endothelial cell angiogenesis (Yin et al., 2015).A stroke can be induced by many types of disease.Those other than the ischemia/reperfusion injury described above are described in subsequent sections (Table 2).
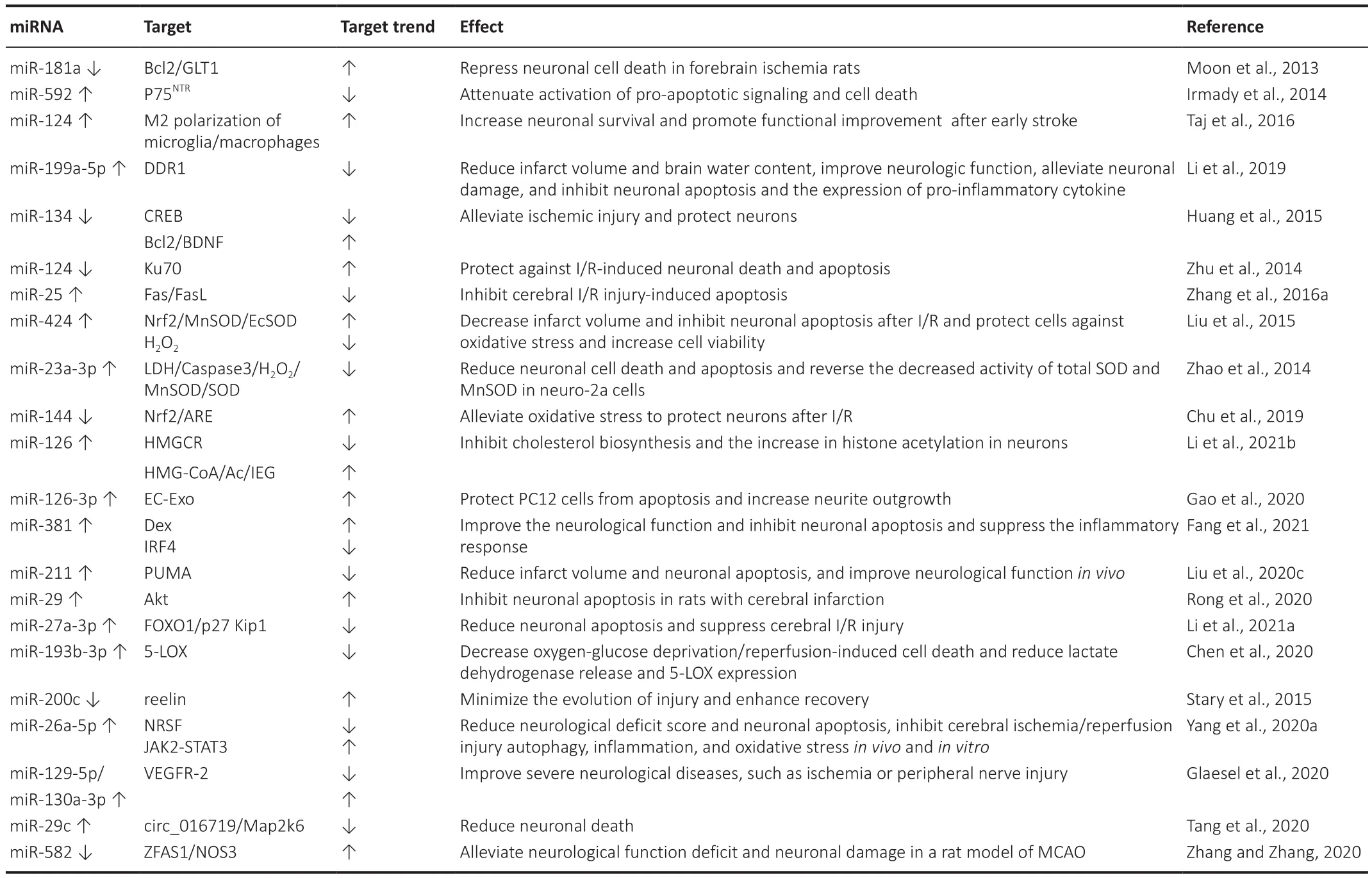
Table 1 |miRNA expression/function and effect during cerebral I/R injury

Table 2|miRNA expression/function and effect during ischemic stroke
Neural cell death caused by an arachidonic acid insult in glutathione-deficient cells in case of neurodegeneration associated with acute ischemic stroke is preceded by a 12-lipoxygenase-dependent loss of miR-29b.The delivery of the miR-29b mimic to blunt this loss has been found to be neuroprotective.miR-29b inhibition potentiates the death of neural cells.12-Lipoxygenase knockdown and inhibitors can attenuate the loss of miR-29b in challenged cells (Khanna et al., 2013).Lv et al.(2019) claimed that the main findings of their study support the concept of the mal-mediated underexpression of miR-150, which directly promotes the concept of protecting the function of the central nervous system by activating the ERK1/2 axis to influence the survival and function of cerebral cortical neurons.In the early stage of focal cerebral ischemia, the level of miR-124-mediated pro-survival p53 signaling protein iASPP decreases.This pathway should be investigated as a new therapeutic approach for promoting neuronal survival in case of stroke and brain injury (Liu et al., 2013).M2 microglia-derived exosomes have been reported to attenuate ischemic brain injury and promote neuronal survival via exosomal miR-124 and its downstream target USP14.M2 microglia-derived exosomes represent a promising avenue for treating ischemic stroke (Song et al., 2019).The upregulation of ADIPOR2 ameliorates miR-19a-3p-induced cerebral ischemia injury, where inhibiting miR-19a-3p effectively alleviates the IR/OGD-induced inhibition of glycolytic enzyme expression, glucose uptake, lactate production,and neuronal apoptosis.Therefore, the inhibition of miR-19a-3p may provide a new therapeutic target for the treatment of ischemic stroke injury (Ge et al., 2019).A study by Nampoothiri and Rajanikant (2019) provides evidence that the inactivation of HDAC4in vitro
can upregulate mir-9, and contributes to neuronal survival and regeneration after ischemic stroke.Therefore, miR-9 may serve as a new therapeutic target for ischemic stroke.Research has shown that miR-124 promotes neuronal survival under ischemic conditions via Usp14-dependent REST degradation.Therefore, both factors most likely affect post-ischemic neuroregeneration and sustained neuroprotection(Doeppner et al., 2013).The lncRNA TUG1 sponge microRNA-9 promotes neuronal apoptosis by upregulating Bcl2l11 in case of brain ischemia, possibly providing a new therapeutic target in cases of stroke (Chen et al., 2017).The REST-dependent overexpression of miR-132 reduces ischemia-induced neuronal death and may serve as a novel therapeutic target to ameliorate neurodegeneration and the cognitive deficits associated with ischemic stroke(Hwang et al., 2014).The upregulation of miR-9 levels reduces abnormalities and apoptosis in MCAO mouse modelsin vivo
andin vitro
by specifically inducing the elevation of Bcl2l11 in case of ischemic stroke (Wei et al., 2016).Yang et al.(2017) claimed that modified exosomes, with the glycoprotein of the rabies virus, fused to the exosomal protein lysosome-associated membrane glycoprotein 2b, can efficiently deliver miR-124 to the infarct site.The systematic administration of glycoprotein-exosomes of the rabies virus loaded with miR-124 enables cortical neural progenitors to obtain neuronal identity and protect against ischemic injury by robust cortical neurogenesis.This study suggests that the glycoprotein-exosomes of the rabies virus can be therapeutically utilized for the targeted delivery of gene drugs to the brain,where this has significant potential for clinical applications (Yang et al., 2017).We have seen similar reports to the above in the last 2 years.Cell-derived exosomes from exercise mice can protect neurons from hypoxia-induced apoptosis and axon growth.Overexpression of miR-126 and PI3kin vitro
can also achieve the same function (Wang et al., 2020b).The study by Yasmeen et al.(2019) provides evidence that HCMEC/D3 cells transfected with miR-27a-3p and miR-222-3p mimics can reduce the relative expression of PDE3A protein, and regulating its expression may improve the progression of cerebral ischemia disease.In-vitro
validation experiments have shown that blocking the maternally expressed gene 3 that specifically binds to miR-378 can downregulate the expression of GRB2, and in turn, promotes the activation of the Akt/mTOR pathway.These results suggest that miR-378 may have protective effects on neuronal autophagy and nerve function injury (Luo et al., 2020).The high expression of miR-126 in EPC-EX alleviates acute brain injury and promotes neurological recovery in the case of diabetic ischemic stroke,providing an active strategy to enhance the therapeutic effect of EPC-EX, and thus may lead to a new, cell-free treatment for diabetic stroke (Wang et al.,2020c).miR-137 has a neuroprotective effect on ischemic stroke by weakening oxidation, apoptosis, and inflammation pathways by inhibiting the SRC-dependent MAPK signaling pathway (Tian et al., 2021).The inhibition of miR-668 can inhibit neuronal apoptosis by regulating the mitochondrial function and the NLRP3 signaling pathway, namely, by improving the expressions of the caspase 3, Bax, and Bcl-2 proteins in I/R stroke rats (He et al., 2020).Exosome miR-146b is an important neuroregulatory factor in neurogenesis as it promotes endogenous neural stem cell differentiation in neurons around post-stroke ischemia.Electroacupuncture promotes endogenous neural stem cell differentiation by stimulating the expression of the exosome miR-146b,thus improving nerve injury after ischemic stroke (Zhang et al., 2020d).It has been reported that circ-HECTD1 knockdown inhibits the expression of TRAF3 by targeting miR-133b, thereby attenuating neuronal injury caused by cerebral ischemia (Dai et al., 2021).The inhibition of miR-130a improves neural function in MCAO rats, alleviates nerve injury, increases cerebral angiogenesis, promotes neuronal activity, and inhibits apoptosis by regulating its target gene XIAP (X-linked inhibitor of apoptosis protein) in both animal models and cellular models (Deng et al., 2020).The inhibition of BCL6 may alleviate oxidative stress-induced neuronal injury and reduce the area of cerebral infarction in IS mice by targeting the miR-31/PKD1 axis.This can be achieved by down-regulating PKD1 and inhibiting the activation of the JAK2/STAT3 pathway, thus alleviating OGD-induced cell injury (Wei et al., 2021).The study by Chang et al.provides evidence that miR-195 can downregulate KLF5 and block the JNK signaling pathway, ultimately inhibiting neuronal apoptosis in rats with ischemic stroke (Chang et al., 2020).Hypoxic-ischemic brain damage
Hypoxia-ischemia is thought to be the final, common endpoint for a complex convergence of events.Some of these events are genetically determined and some are triggered by an in-utero (but not necessarily intrapartum) stressor(McLean and Ferrier, 2004; Table 3).
Sun et al.(2018a) reported that the upregulation of miR-592-5p and the suppression of the PGD2/DP signaling pathway can protect hippocampal neurons from hypoxic-ischemic brain damage (HIBD) in neonates.The administration of GW0742 after HI reduced the area of the infarct,attenuated neuronal apoptosis, and improved neurological outcomes.The neuroprotective effects of GW0742 can be mediated via the PPAR-β/δ/miR-17/TXNIP signaling pathway, with current evidence supporting the idea that GW0742 is a promising therapeutic candidate as it can salvage neurons after hypoxic-ischemic encephalopathy (HIE) (Gamdzyk et al., 2018).In a mouse model of hypoxia-induced neuronal apoptosis, the overexpression of the miR-23b and miR-27b clusters inhibited neuronal apoptosis induced by intrauterine hypoxia.For the first time, the researchers discovered that miRNAs regulate the sensitivity of neurons to apoptosis during development and hypoxia-induced brain injuries (Chen et al., 2014).
A study by Zhou et al.(2020) revealed that lncRNA GAS5 absorbs miRNA-221 to promote neuronal apoptosis by upregulating PUMA/JNK/H2AX signaling under hypoxia.This finding deepens our understanding of the role of GAS5 in the pathogenesis of ischemic stroke, and may also provide a novel candidate for the treatment of stroke (Zhou et al., 2020).It is known that miR-146b-5p overexpression alleviates HIE-induced neuronal injury by inhibiting the IRAK1/TRAF6/TAK1/NF-κB pathway.One study identified a new regulatory axis of miR-146b-5p/IRAK1/TRAF6/TAK1/NF-κB in HIE, which is a promising therapeutic target for neuronal HIE (Yang and Zhao, 2020).The inhibition of miRNA-199a-3p expression in exosomes derived from hypoxia-induced glioma alleviates the peritumor neurons in case of ischemic injury by inhibiting HIF-1α upregulation and promoting the expression of the mTOR pathway (Zhao et al., 2020).TCONS00044054 (Vi4) overexpression and miR-185-5p knockout promote neuronal survival and neurite growth, suppress cell apoptosis, and reduce motor and cognitive dysfunction in rats with HIE,while Igfbp3 intervention has the opposite effect.Vi4-miR-185-5p-Igfbp3 may be a drug target for HIE therapy (Xiong et al., 2020).The study by Xin et al.(2020) showed that miR-21a-5p is transferred to neurons and microglia in the damaged brain by the uptake of the mesenchymal stromal cells-derived extracellular vesicle (MSC-EV), which means that an important component of the neuroprotective properties of these MSC-EV is involved in targeting the Timp3 gene.It has a neuroprotective effect in case of HI injury in newborn mice, which suggests that MSC-EV may provide a new treatment strategy for HIE (Xin et al., 2020).Slit2 is a target gene of miR-200b-3p.Hypoxia/ischemic brain damage in neonatal rats was alleviated by inhibiting miR-200b-3p via Slit2.Therefore, miR-200b-3p may be a potential therapeutic target for HIBD (Zhang et al., 2020c).HIBD in neonatal rats can result in acute and long-term cerebral dysfunction.The overexpression of miR-410-3p can promote neuronal survival and inhibit neuronal apoptosis to alleviate the motor, learning-, and memory-related dysfunctions caused by HIBD.Thus, the overexpression of miR-410-3p may not only provide an effective treatment for neonatal HIBD rats, but may also provide novel insights into the clinical treatment or prevention of HIBD (Xiao et al., 2020).The upregulation of miR-21 can significantly reduce the volume of cerebral infarction in HIBD rats,reduce the degree of brain tissue injury, and improve neurobehavioral ability and memory by down-regulating CCL3, which has a protective effect on the brains of neonatal HIBD rats (Liu et al., 2020a).
OGD/R-induced neuronal injury
It is important to study the mechanism of ischemic neuronal injury to explore new therapeutic targets.The model of neuronal injury induced by OGD/R is a classic model for studying brain injury (Table 4).
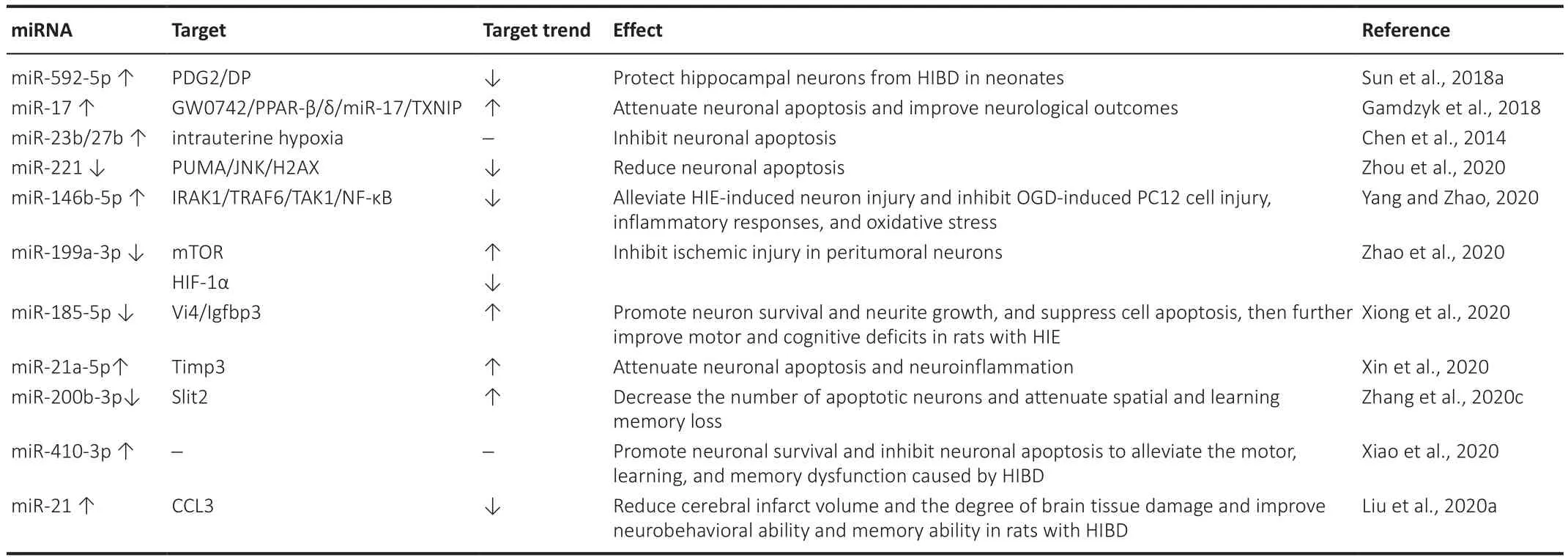
Table 3|miRNA expression/function and effect during HIBD
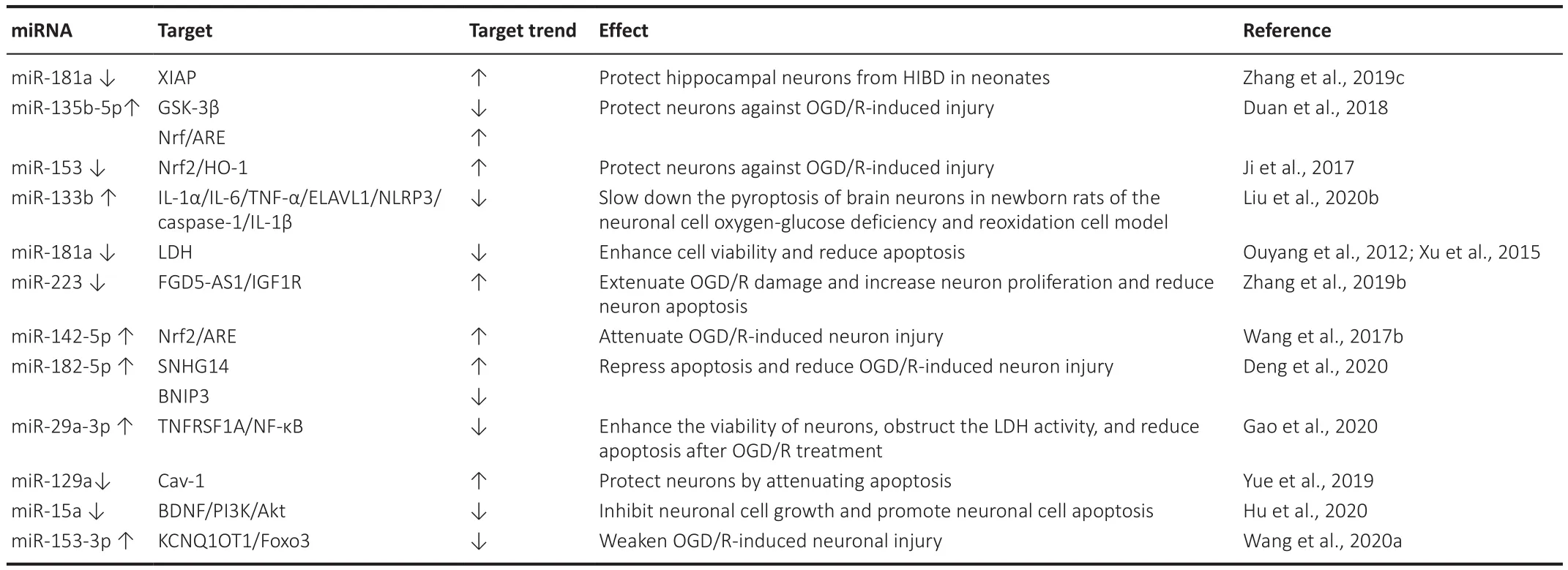
Table 4|miRNA expression/function and effect during OGD/R-induced injury
In the relevant procedure, pretreatment with sevoflurane alleviates miR-181a-induced cellular injury in primary cortical neurons after OGD/R by downregulating miR-181a and upregulating XIAP.This has been identified as a direct target of miR-181a (Zhang et al., 2019c).The study by Duan et al.(2018)suggests that miR-135b-5p protects neurons against OGD/R-induced injury by down-regulating GSK-3β and promoting the Nrf2/ARE signaling pathwaymediated antioxidant responses.The inhibition of miR-153 protects neurons against OGD/R-induced injury by regulating Nrf2/HO-1 signaling, and suggests a potential therapeutic target for CIRI (Ji et al., 2017).Previous studies have reported that miR-181a overexpression promotes lactate dehydrogenase release and apoptosis by reducing cell viability, and promotes damage toprimary cortical neurons after OGD (Ouyang et al., 2012).Our observations are in agreement with those made by Zhang et al.(2019b), whereby FGD5-AS1 might protect neurons against OGD/R injury by acting as a ceRNA for miR-223 to mediate IGF1R expression.This implies the simultaneous downregulation of miR-223 and overexpression of FGD5-AS1.IGF1R also exhibits the additional effects of extending OGD/R damage, increasing neuronal proliferation, and reducing neuronal apoptosis.Wang et al.(2017b) have suggested that miR-142-5p contributes to OGD/R-induced cell injury, and the down-regulation of miR-142-5p attenuates OGD/R-induced neuronal injury by promoting Nrf2/ARE expression.During ischemia, miR-1290 expression decreases while cav-1 expression may be upregulated in neurons, thereby increasing EV intake to protect them (Yue et al., 2019).
It has been reported that miR-133b down-regulates the expressions of IL-1α, IL-6, the tumor necrosis factor α, ELAVL1, NL-RP3, caspase-1, and IL-1β proteins to slow down the pyroptosis of neurons in newborn rats in a neuron model of OGD/R (Liu et al., 2020b).The study by Gao et al.(2020) illustrated that miR-29a-3p can enhance the viability of neuronal cells, obstruct lactate dehydrogenase activity, and reduce apoptosis after OGD/R treatment by negatively regulating the expression of TNFRSF1A through the inhibition of the nuclear factor-κB (NF-κB) signaling pathway.These findings provide insights into alleviating OGD/R-induced injury (Gao et al., 2020).It is known that lncRNA SNHG14 induces hypermitosis through the miR-182-5p/BINP3 axis in the hippocampal neurons of HT22 mice, thereby promoting OGD/R-induced neuronal injury.This may be a valuable target for cerebral I/R injury treatment (Deng et al., 2020).Neuronal cell growth is inhibited and neuronal cell apoptosis is promoted in the OGD/R model by reducing BDNF and attenuating the PI3K/Akt pathway.These findings contribute to uncovering the novel pathogenesis of ischemic brain injury (Hu et al., 2020).As a ceRNA,KCNQ1OT1 can reduce OGD/R-induced neuronal injury by preventing miR-153-3p from competing with Foxo3.These findings provide insights into the molecular mechanism of CIRI.Targeting KCNQ1OT1 to regulate Foxo3 may be a useful strategy to treat brain I/R injury (Wang et al., 2020a).
Intracerebral hemorrhage
Intracerebral hemorrhage (ICH), which accounts for 10-15% of all cases of stroke, is the most devastating type of stroke that is highly associated with morbidity and mortality (Qureshi et al., 2001; Table 5).Post-ICH edema formation can lead to intracranial hypertension and herniation, and contributes to ICH-induced neurologic deficits and even fatality (Xi et al.,2002; Gong et al., 2004).The restoration of miR-27a-3p reduces brain edema,maintains the permeability of the blood-brain barrier, inhibits neuronal loss,and alleviates neurological deficits in rats with ICH.The protective effect of miR-27a-3p may be mediated by the inhibition of AQP11 in the endothelium of the capillaries of the brain (Xi et al., 2018).The overexpression of miR-124 regulates the polarization of microglia to the M2 phenotype by reducing the level of C/EBP-α in the brains of rats with intracerebral hemorrhage.M2-polarized microglia have a protective effect on neuronal injury, thus improving the inflammatory injury induced by ICH.The regulatory mechanism of miR-124 may also be a new therapeutic strategy for treating cerebral hemorrhage(Yu et al., 2017).One recent study illustrated that miR-146a-5p-enriched BMSCs-Exos can offer neuroprotection and functional improvements after ICH by reducing the rates of neuronal apoptosis and inflammation associated with the inhibition of microglial M1 polarization by down-regulating the expressions of IRAK1 and NFAT5 (Duan et al., 2020).
Alzheimer’s disease
Alzheimer’s disease (AD) is a progressive neurodegenerative disease characterized by the aggregation and deposition of Aβ peptides.Clinically, it is characterized by memory impairment, aphasia, apraxia, agnosia, impairment of visuospatial skills, executive dysfunction, and personality and behavioral changes, and has an unknown etiology (Table 6).

Table 5 |miRNA expression/function and effect during intracerebral hemorrhage

Table 6 |miRNA expression/function and effect during Alzheimer's disease
A study by Zhang et al.(2016b) demonstrated that miR-135b plays a neuroprotective role through the direct targeting of BACE1, and thus may be used for the treatment of AD.Melatonin can protect primary neurons against amyloid-β (Aβ)-induced neurotoxicity in case with AD via the miR-132/PTEN/AKT/FOXO3a pathway, elevate the expression of miR-132, suppress the expressions of PTEN and FOXO3a during Ab2535 exposure, increase the level of p-Akt, and block the nuclear translocation of FOXO3a.The inhibition of the PI3K-Akt pathway can block the protective effects of melatonin, and either the overexpression of miR-132 or the inhibition of PTEN can counteract Aβinduced neurotoxicity (Zhao et al., 2018).miR-200c plays a corresponding role in the intracellular fixation of neurons in patients with AD, mainly supporting the survival and differentiation of neurons.In the early stage of Aβ injury, the ER stress-induced upregulation of miR-200c inhibits PTEN expression and protects neurons from β toxicity (Wu et al., 2016).In the relevant procedure,miR-132 is a major protective regulator of the growth and development of nerve cells, suggesting that miR-132 supplementation may play a protective regulatory role in the treatment of Tau-related neurodegenerative diseases.miR-132 protects primitive mouse and human wild-type neurons as well as more vulnerable Tau mutant neurons from Aβ and glutamate excitatory toxicity (El Fatimy et al., 2018).A study has shown that GRg1 + AGR suppresses the apoptosis of neuronal cells by upregulating the expression of miR-873-5p and downregulating that of HMOX1/Aβin the case of AD (Shi et al., 2018).
A study by Li et al.(2020a) revealed that miR-338-5p is a protective regulator of the development and progression of AD, and can reduce neuronal apoptosis in APP/PS1 mice.The deposition of amyloid plaque and cognitive dysfunction were reduced in APP/PS1 mice by the intrahippocampal injection of the lentiviral overexpression of miR-338-5p, which may be related to the negative regulation of BCL2L11 by miR-338-5p (Li et al., 2020a).SOX21-AS1 inhibition attenuates Aβ-induced neuronal damage by sponging miR-107,which provides a strategy for the treatment of AD (Xu et al., 2020).Berberine can inhibit caspase-3 activity and apoptosis and is thus an effective drug for the treatment of AD patients.The protective effect of berberine by inhibiting neuronal apoptosis is realized by promoting cell viability through the miR-188/NOS1 pathway (Chen et al., 2020).miR-143-3p inhibition promotes neuronal survival in anin vitro
cell model by targeting NRG1, and the miR-143-3p/NRG1 axis is a potential therapeutic target and promising biomarker for the treatment of AD (Sun et al., 2020a).Tian et al.(2021) showed that miR-20b-5p can disturb the progression of AD by regulating cell apoptosis,cleaved caspase-3 expression, and cell viability by targeting the RhoC gene.This indicates that miR-20b-5p might be an underlying curative target for AD.However, the disadvantage of this study was that only PC12 cells were used to examine the mechanism.More adequate experiments on animals can be performed, along with research involving several other cell lines and clinical samples (Tian et al., 2021).Dex also stimulates pro-apoptotic signaling,although it suppresses the Aβ-induced apoptosis of neuronal cells.miRNA-151-3p enhances the neuroprotective effect of Dex against Aβ by targeting DAPK-1 and TP53 (Guo et al., 2021).Sun et al.(2020b) showed that the miR-129/YAP1/JAG1 axis may be the protective mechanism of dexmedetomidine against cognitive dysfunction in AD mice.Dex can enhance the expression of miR-129 in Aβmice, and can thus affect the target gene YAP1 that cannot interact with the downstream gene JAG1.This reduces the apoptosis of hippocampal neurons in mice injected with Aβand, thus, cognitive dysfunction.Epilepsy
Epilepsy is a common disease of the nervous system, the pathogenesis of which is mainly related to the abnormal synchronization of neuronal discharges in the brain (De et al., 2016).The recurrent seizures of epilepsy cause great harm to the physical and mental health of patients.However,the pathogenesis of epilepsy is not fully understood and may be related to the structural and functional damage to the hippocampus and limbic system caused by pathological changes, such as neuronal apoptosis, mossy fiber germination, and synaptic plasticity (Peng et al., 2015).Epileptic seizures can lead to neuronal apoptosis, the mechanism of which can be attributed to the production of a large number of free radicals and the activation of protease related to cell death after epilepsy (Schrӧder et al., 2014; Table 7).

Table 7 |miRNA expression/function and effect during epilepsy
Anti-miRNA-141 protects against epilepsy-induced apoptosis by upregulating the expression of the SIRT1 protein and suppressing that of the p53 protein (Liu et al., 2019a).Morris et al.(2018) revealed that cholesterollabeled antibodies targeting miRNA-134 protected against epilepsy by reducing interference with the properties of hippocampal neuronal or the network function.The upregulation of the long non-coding RNA H19 can induce neuronal apoptosis during the latency of epilepsy, and it acts mainly through competition with sponge miRNA let-7b as endogenous RNA to regulate apoptosis.It can be concluded that maintaining a balance between H19 and the sponge miRNA let-7b can help regulate apoptosis and play a corresponding protective role (Han et al., 2018).
Research has shown that the down-regulation of miR-142-5p through the targeting Miro1 inhibits neuronal death and mitochondrial dysfunction,which in turn attenuates the pilocarpine-induced SE.This suggests the potential involvement of miR-142-5p in the pathogenesis of temporal lobe epilepsy (Zhang et al., 2020a).miR-183 has been found to act as a protective regulator in the process of hippocampal neuron injury and the progression of epilepsy.Inhibited miR-183 can upregulate Foxp1, render the Jak/Stat signaling pathway inactive, promote the proliferation of neurons,and inhibit the apoptosis of hippocampal neurons in epileptic rats (Feng et al., 2019).Knocking down circ_0003170 ameliorates injury to neurons of the human hippocampal that are free of Mgby mediating the miR-421/CCL2 axis (Chen et al., 2021).A study by Li et al.(2020b) revealed that miR-15a-5p was downregulated in children with temporal lobe epilepsy, and the overexpression of miR-15a-5p promoted the viability and inhibited the apoptosis of hippocampal neurons.miR-15a-5p might thus be a promising biomarker for the diagnosis of temporal lobe epilepsy in children.
Parkinson’s disease
Parkinson’s disease (PD), which is the second most common progressive neurodegenerative disease worldwide, is characterized by the aggregation of α-synuclein neuronal inclusions and a massive loss of dopaminergic(DA) neurons (Drui et al., 2014; Nussbaum et al., 2017; Chen et al., 2022).The programmed death of dopamine neurons is the main mechanism of neurodegenerative diseases such as PD (Vila and Przedborski, 2003).In some neurodegenerative diseases including PD, the selective loss of neurons in the dense region of the substantia nigra can negatively affect dopaminergic(mDA) neurons in the ventral tegmental region (Moore et al., 2005).A recent study has demonstrated that miRNAs play a protective role in mDA neuronal differentiation and PD (Harraz et al., 2011; Table 8).
Zhang et al.(2019a) claimed that the inhibition of lncRNA SNHG14 expression can upregulate a and reduce the accumulation of α-synuclein to alleviate dopaminergic neuronal damage, thereby improving the pathological state of PD, while rotenone may reverse the above-mentioned state by upregulating SNHG14 through SP-1.The extracellular matrix protein laminin-511 (LM511)binds to Integrina3B1 and activates the transcription cofactor YAP to promote the survival and differentiation of mDA neurons.The LM511-YAP signaling pathway enhances cell survival by inducing the expression of miR-130a,which can inhibit the synthesis of PTEN while increasing the expressions of LMX1A and PITX3, and preventing the loss of mDA neurons in oxidative stress response (Zhang et al., 2017).miR-212-5p is a neuroprotective regulator in PD, where the overexpression of miR-212-5p can reduce dopaminergic neuronal loss and DAT reduction by targeting SIRT2 (Sun et al., 2018b).The ectopic expression of miR-124-3p was found to attenuate MPP-induced injury by upregulating STAT3 in a PD modelin vitro
by suppressing neurotoxicity,neuronal apoptosis, neuroinflammation, and oxidative stress (Geng et al.,2017).The up-regulation of miR-132 expression in the midbrain of rats resulted in a significant decrease in Nurr1 and BDNF levels.It is possible that dopaminergic neurons, which respond to global stress due to the accumulation of alpha-synuclein, activate miR-132 to shut down Nurr1 and reduce BDNF, a major regulator of neuronal survival.These data highlight that miR-132 is a promising potential biomarker and target for neuroprotective therapy in the case of PD.The development of drugs designed to reduce miR-132 activity may provide a novel strategy for treating PD and other synucleinopathies (Lungu et al., 2013).In the pathogenesis of PD, the regulation of miR-101-3p expression may play a corresponding role in disease progression.The overexpression of lncRNA-T199678 reverses the neuronal damage caused by α-synuclein through the down-regulation of miR-101-3p, which can contribute to improving the pathology of PD (Bu et al., 2020).Astaxanthin suppresses ER-induced stress and protects against PD-induced neuronal damage by targeting the miR-7/SNCA axis, suggesting that astaxanthin is a potentially effective therapeutic agent in the treatment of PD (Shen et al., 2021).Bax overexpression reverses the effects of miR-216a on neural cells, and downstream factors are involved in the miR-216a regulation of MPP-triggered neuronal apoptosis.miR-216a regulates the progression of PD by regulating Bax and may be a target for the treatment of PD (Yang et al., 2020b).
Peripheral nervous system injury
Unlike the central nervous system, the peripheral nervous system has a high regenerative capacity after injury, can restore sensory and motor functions, and remains relatively stable throughout a person’s life (Mahar and Cavalli, 2018; Table 9).Increasing miR-21-5p expression in exosomes,which are important modulators during peripheral nerve repair derived from sensory neurons, supports the transformation of macrophages into a pro-inflammatory phenotype.These pro-inflammatory macrophages are particularly important for clearing cell debris after nerve injury and providing a suitable microenvironment for tissue repair (Liu et al., 2019b).Neurons have been shown to secrete an exome containing miR-132 to endothelial cells,which may promote the angiogenesis of the peripheral nerve and improve vascular integrity (Xu et al., 2017).López-Leal et al.(2020) proved that miR-21 is upregulated in exosomes derived from repaired Schwann cells to a greater extent than differentiated Schwann cells while regulating the growth of neurites by down-regulating PTEN and activating PI3K.The expression of miR-340 was negatively correlated with the plasminogen activator of its target gene tissue after sciatic nerve injury.The overexpression of miR-340 promotes fibrinolysis, axon regeneration, and the clearance of cell debris (Li et al., 2017).
Role of MiRNAs in Pathology
Neuronal damage caused by chemical/physical factors
Some endogenous and exogenous factors can have positive or negative effects on neurons (Table 10).According to the literature, the reduced expression of sevoflurane in the brain may protect against ischemic neuronal injuryin vitro
(Zitta et al., 2010) andin vivo
(Chen et al., 2015).In one study, miRNA-132 was downregulated in rats exposed to sevoflurane, and this caused neuronal apoptosis via the suppression of the PI3K/AKT/FOXO3a pathway.This means that the upregulation of miRNA-132 can relieve neuronal apoptosis induced by sevoflurane by undoing the inhibition of the PI3K/AKT/FOXO3a pathway(Dong et al., 2018).The exogenous inhibition of miR-764 regulates the lentivirus-mediated overexpression of NINJ2, the expression of which is elevated after nerve injury to promote neurite outgrowth as an adhesion molecule that is expressed in neurons and glial cells (Araki and Milbrandt,2000; Wang et al., 2017a).It also protects neuronal cells from HO-induced cell death and apoptosis (Ding et al., 2018).The upregulation of miR-29b can prevent the apoptosis of mature neurons by directly inhibiting the key step of BH3-only protein induction, which means that reducing the loss of miR-29b expression may have a protective effect on neurons and reverse the occurrence of neurodegeneration (Kole et al., 2011).The upregulation of miR-153 can promote the differentiation of the hippocampal HT-22 cells in mice.It was reported that mouse hippocampal HT-22 cells were cloned from HT4 cells that had no prominently distinct processes or branches (Liu et al., 2009),and protected neurons through the upregulation of the neuronal marker γ-enolase, neuronal nuclei, and the functional proteins SnaP23, SnaP25, and PrX5.The main mechanism of differentiation of HT-22 cells induced by the overexpression of miR-153 was one whereby the numbers of cell processes and branches increased, the distribution of the s-phase of the cell cycle decreased, and the rate of cell proliferation decreased (Xu et al., 2019).The major injuries caused by mechanical trauma include tissue tearing, cell rupture, and bleeding, and can lead to serious secondary injuries including edema, hematoma, inflammation, and apoptotic responses.ARC therapy inhibits NF-κB signaling activity through the upregulation of miRNA-16,miRNA-199a, and IL-10, reduces the release of pro-inflammatory cytokines(IL-6 and the tumor necrosis factor α) as well as the number of apoptotic cells,and ultimately prevents secondary injury and promotes healing (Song et al.,2016).Ferroptosisis is a recently discovered form of iron-dependent regulated cell death associated with traumatic brain injury (TBI).In the relevant procedure, the overexpression of miR-212-5p protects against ferroptotic neuronal death in controlled cortical impact in mice, partially by inhibiting the target gene Ptgs2, which is induced by ferroptosis (Xiao et al., 2019).The level of miR-124-3p in microglial exosomes increases from the acute phase to the chronic phase of TBI.The increased miR-124-3p in microglia inhibits neuronal inflammation and contributes to neurite outgrowth by transferring into neurons through exosomes.It can also improve neurological outcomes and inhibit neuroinflammation in TBI mice.These effects of miR-124-3p are realized through the targeting of PDE4B, which inhibits mTOR signaling.Therefore, miR-124-3p is a promising therapeutic target for the management of neuronal inflammation after TBI (Huang et al., 2018).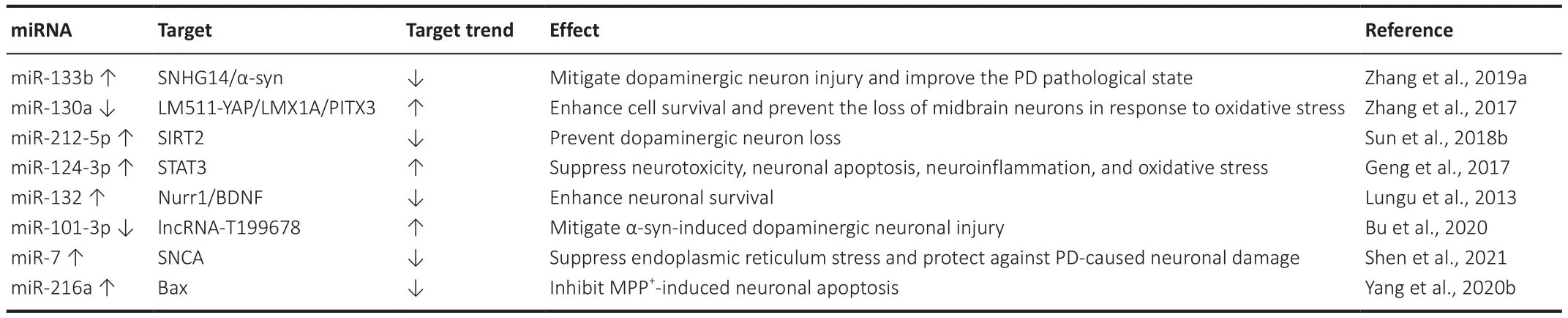
Table 8 | miRNA expression/function and effect during PD
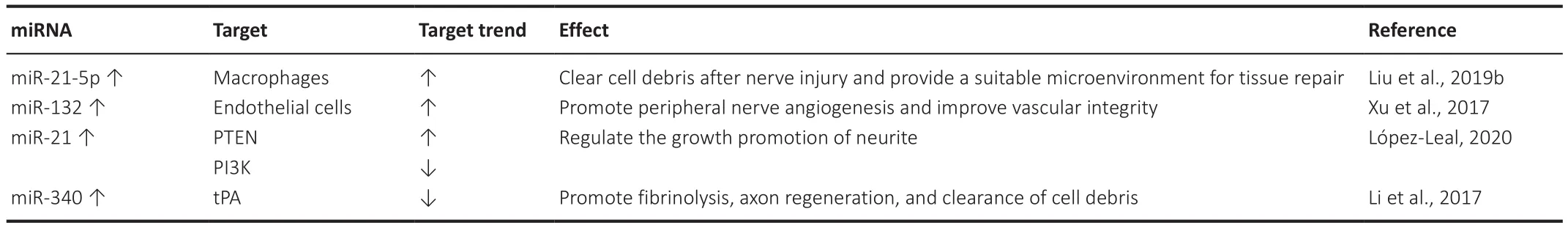
Table 9 |miRNA expression/function and effect during peripheral nervous system injury
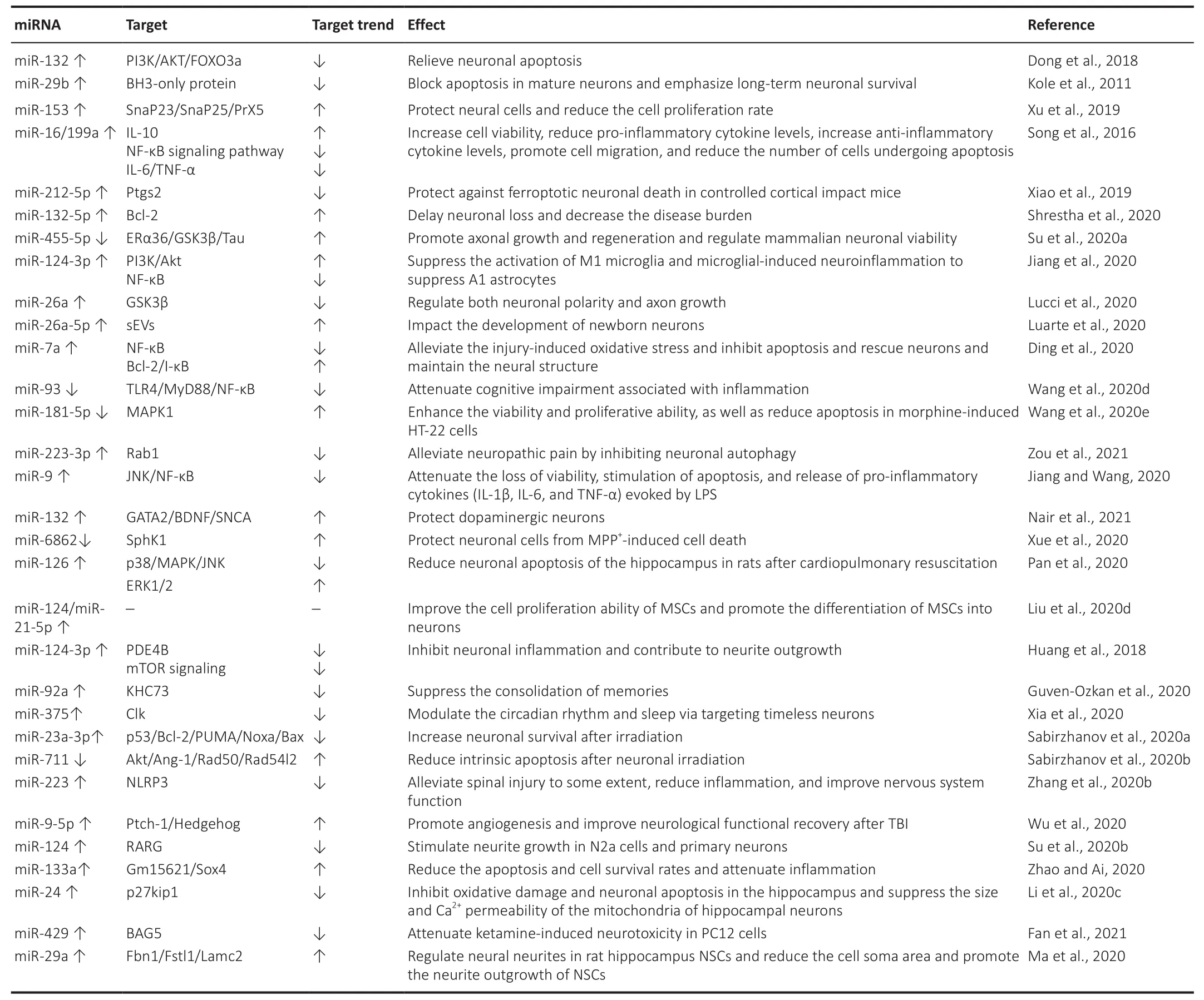
Table 10 |miRNA expression/function and effect during neuronal damage caused by chemical/physical factors
Research has shown that the activation of the nicotinic acetylcholine receptor with nicotine facilitates cell survival by upregulating miR-132-5p, which in turn upregulates the anti-apoptotic protein Bcl-2.These results indicate that miR-132-5p is a potential therapeutic target for neuroprotection that acts by stimulating the nicotinic acetylcholine receptors (Shrestha et al., 2020).The study by Su et al.(2020a) demonstrated that the miR-455-5p suppression of ERα36 stimulates the phosphorylation of the GSK3β(Tyr216)-Tau axis, hinders cell proliferation, and promotes apoptosis in SH-SY5Y neuroblastoma cells.Moreover, the miR-455-5p suppression of ERα36 negatively regulates axonal growth in a manner that is dependent on the activity of the GSK3 kinase.Further research will be beneficial for validating the findings and developing therapeutic strategies targeting the miR-455-5p/ERα36 axis to support neuronal viability and axonal regeneration (Su et al., 2020a).Jiang et al.(2020)highlighted that acellular exosomes from neurons promote functional behavioral recovery by shuttling miR-124-3p in mouse spinal cord injury (SCI).The enriched levels of exosomal miR-124-3p improved therapeutic potential by suppressing the activation of M1 microglia, thus reducing neuroinflammation to suppress A1 astrocytes, and by the MYH9/PI3K/AKT/NF-κB signaling pathway.A combination of miRNAs and neuron-derived exosomes may be a promising and minimally invasive approach to the treatment of spinal cord injuries (Jiang et al., 2020).miR-26a, at a junction of regulatory mechanisms, impinges on neuronal polarity and axon development via the control of GSK3β levels.In this context, the relatively high levels of miR-26a expression in mature neuronal cultures and the central nervous system raise questions about its role in the adult brain.These results demonstrate how axonal miR-26a can regulate local protein translation in the axon to facilitate retrograde communication to the soma, and to amplify neuronal responses in a mechanism that influences axon development (Lucci et al., 2020).Luarte et al.(2020) supported a novel and complex level of astrocyte-to-neuron communication that is mediated by astrocyte-derived small EVs and the activity of their miRNA content.Their study suggests that astrocytes can regulate the dendritic development of neurons by modifying the miRNA cargo of small EVs derived from them (Luarte et al., 2020).The upregulation of miR-7a alleviates injury-induced oxidative stress and inhibits apoptosis by down-regulating the NF-κB pathway in rats with spinal cord injury.In addition, the upregulation of miR-7a can rescue neurons and maintain their neural structure (Ding et al., 2020).Acupuncture attenuates cognitive impairment associated with inflammation by inhibiting the miR-93-mediated TLR4/MyD88/NF-κB signaling pathway in the case of vascular dementia.Acupuncture serves as a promising alternative therapy, and may be an underlying TLR4 inhibitor for the treatment of vascular dementia.Recent work provides a new perspective on the anti-inflammatory mechanism of acupuncture and identifies it as a potential complementary therapy for cognitive dysfunction (Wang et al., 2020d).Morphine induces the apoptosis of hippocampal HT-22 neurons by upregulating miR-181-5p to suppress the level of MAPK1.MiR-181-5p may be a therapeutic target in the future (Wang et al., 2020e).By increasing miR-223-3p expression while targeting Rab1,electroacupuncture reduces neuronal apoptosis (neuronal autophagy as the form of death) and inflammation, and increases the threshold of rats with PHN for mechanical pain (Zou et al., 2021).The neuroprotective effect of matrine may protect PC12 cellsin vitro
from LPS-induced injury by regulating miR-9 expression, and targeting its downstream JNK and NF-κB pathways(Jiang and Wang, 2020).The innate ability in humans to appreciate music induces a corresponding miRNA response.miR-132 and Dicer are upregulated when one listens to music, followed by the targeting of the music predisposition regulator GATA2, and BDNF and SNCA are then activated.They are also the preferred candidate genes for musical traits, thereby protecting dopaminergic neurons that are important for maintaining striatum dopamine levels (Nair et al., 2021).Pan et al.(2020) reported that miR-126 can significantly reduce neuronal apoptosis in the hippocampus and improve the neurological function in rats after cardiopulmonary resuscitation, where this may be involved in the regulation of the p38MAPK pathway.The inhibition of miR-6862 upregulates its target gene SphK1 and then protects nerve cells from MPP-induced damage, thereby protecting DA neurons from oxidative damage (Xue et al., 2020).miR-124 and miR-21-5p are considered to be promising tools for improving the efficiency of transplantation in case of nerve injury in that they can functionally regulate the migration, proliferation, and neuronal differentiation of MSCs (Liu et al., 2020).The inhibition of miR-92a expression enhances the expression of a specific kinesin molecule, the kinesin heavy chain 73 (khc73), in neurons of the brain of drosophila to enhance memory consolidation (Guven-Ozkan et al., 2020).Clk disruption abolishes the normal rhythmic expression of miR-375 and leads to the functional regulation of l-LNv neurons, where miR-375 modulates the circadian rhythm and sleep by targeting the timeless neurons.The relevant study provided the first global view of miRNA regulation in circadian rhythms (Xia et al., 2020).Brain irradiationin vivo
occurs extensively in the cerebral cortex and hippocampus, resulting in the down-regulation of miR-23a-3p and the elevation of molecules of the pro-apoptotic Bcl2 family, including PUMA,Noxa, and Bax.The overexpression of miR-23a-3p mimics the reversed neuronal apoptosis induced by the above regulatory pathways (Sabirzhanov et al., 2020a).The use of miR-711 inhibitors blocks the development of these regulated neurodegenerative pathways.The inhibition of miR-711 attenuates the degradation of Akt and ANG-1 mRNA, where ANG-1 has a neuroprotective effect after neuronal irradiation to reduce neuronal innate apoptosis.The inhibition of miR-711 can rescue the expressions of Rad50 and Rad54l2 after neuronal irradiation to enhance DNA repair, and reduce the p53-dependent apoptosis and aging pathways (Sabirzhanov et al., 2020b).The overexpression of microRNA-223 alleviates inflammation and improves neuronal function.NLRP3 is the downstream target of microRNA-223, and its overexpression leads to severe inflammation in the microglia (Zhang et al., 2020b).The upregulation of miRNA-9-5p can be used as a therapeutic target to promote angiogenesis and neurological recovery after traumatic brain injury.It acts mainly through the activation of the Hedgehog pathway to increase the phosphorylation of Akt, thereby promoting the expressions of Cyclin D1,MMP-9, and VEGF in brain microvascular endothelial cells (Wu et al., 2020).RARG knockdown partially eliminated outgrowth defects in neurites caused by the inhibitor of miR-124, while the overexpression of RARG can reverse the neurite-outgrowth-enhancing effect of the upregulation of miR-124.Collectively, these data reveal that the miR-124/RARG axis is critical for neurite outgrowth.RARG has emerged as a target regulated by miR-124 that modulates neurite outgrowth, providing a novel context in which these two molecules function (Su et al., 2020b).Gm15621/miR-133a/Sox4 axis plays an important role in improving cognitive disorders by upregulating the Gm15621/miR-133a/Sox4 axis to reduce the apoptosis and cell survival rates and attenuate inflammation while LncRNA Gm15621 improves sevofluraneinduced neurotoxicity (Zhao and Ai, 2020).Li et al.(2020c) revealed that miR-24 can attenuate isoflurane-induced neurotoxicity in the hippocampus of rats via its anti-oxidative stress function and the inhibition of p27kip1 expression.miR-429 attenuates ketamine-induced neurotoxicity in PC12 cells by downregulating BAG5 (Fan et al., 2021).Ma et al.(2020) demonstrated that miR-29a can promote neurite outgrowth by targeting extracellular matrix-related genes: namely, Fibrillin 1 (Fbn1), follistatin-like 1 (Fstl1), and laminin subunit gamma 2 (Lamc2).These findings may provide a novel role for miR-29a in the regulation of neurite outgrowth and the development of neural stem cells.They have also offered a possible theoretical basis for the migration mechanism of neural stem cells in brain development and damage repair (Ma et al., 2020).Discussion
A number of studies have investigated the role of miRNAs in the growth and development of the nervous system and the pathogenesis of neurodegenerative diseases.Typical neurodegenerative diseases, such as AD, PD, amyotrophic lateral sclerosis, and Huntington’s disease, are the most extensively investigated.Research on them has shown the potential role of miRNAs in neuronal development and function (O’Brien and Wong,2011; Qiu et al., 2015; Leggio et al., 2017).The brain has specific miRNA expression profiles, and each miRNA may perform a specific function to maintain its integrity (Sempere et al., 2004; Bak et al., 2008).miRNAs are being identified increasingly commonly as major regulators involved in a variety of brain pathologies, from neurodevelopmental disorders to brain tumors and neurodegenerative diseases, where they determine cell fate (Idda et al., 2018).This review discussed the importance of miRNA as a protective regulator in neuronal growth and development.miRNA plays an important role in promoting cell repair and cell survival in various neurological disease models mentioned in this review.With the development of new technologies,regulating the expression of specific miRNAs in specific cells and tissues will become an effective means of treating diseases in the future.These findings equip us with many reference targets for drug treatment to provide better treatment strategies for various neurological diseases.However, this review mainly detailed the protective effects of miRNA on neurons in the model of diseases of the central nervous system, and only a brief explanation was provided of the protective effects of miRNA on neurons in models of diseases of the peripheral nervous system.In future work, we plan to discuss the application of models of diseases of the peripheral nervous system in detail.It is important to explore miRNA as a therapeutic target for neuronal injury(Figure 3).
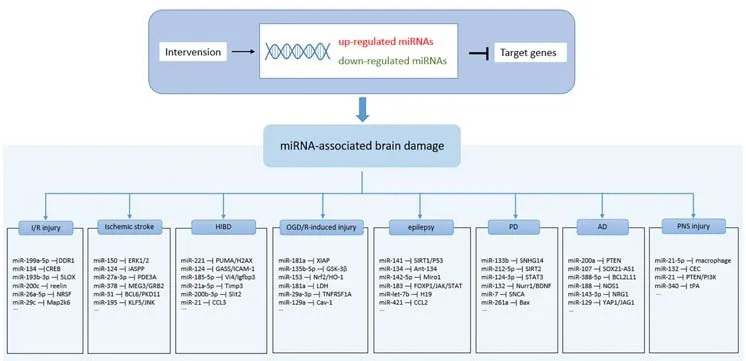
Figure 3|miRNA-associated brain damage.
Author contributions:
ZXM wrote the manuscript.ZXM and YF were responsible for preparing figures.YF and ZDW reviewed and editing the manuscript.All authors participated in the conception of this manuscript and approved the final version of this manuscript.
Conflicts of interest:
The authors declare no competing financial interests.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Neural and Müller glial adaptation of the retina to photoreceptor degeneration
- Agomelatine: a potential novel approach for the treatment of memory disorder in neurodegenerative disease
- In vivo astrocyte-to-neuron reprogramming for central nervous system regeneration: a narrative review
- Intranasal nerve growth factor for prevention and recovery of the outcomes of traumatic brain injury
- Altered O-GlcNAcylation and mitochondrial dysfunction,a molecular link between brain glucose dysregulation and sporadic Alzheimer’s disease
- Signaling interactions among neurons impact cell fitness and death in Alzheimer’s disease

