Agomelatine: a potential novel approach for the treatment of memory disorder in neurodegenerative disease
Qiang Su , Tian Li , Guo-Wei Liu, Yan-Li Zhang , Jun-Hong Guo , Zhao-Jun Wang Mei-Na Wu Jin-Shun Qi
Abstract Agomelatine is a selective agonist of melatonin receptor 1A/melatonin receptor 1B (MT1/MT2)and antagonist of 5-hydroxytryptamine 2C receptors.It is used clinically to treat major depressive episodes in adults.The pro-chronobiological activity of agomelatine reconstructs sleep-wake rhythms and normalizes circadian disturbances via its agonistic effect of melatonin receptor 1A/melatonin receptor 1B, which work simultaneously to counteract depression and anxiety disorder.Moreover, by antagonizing neocortical postsynaptic 5-hydroxytryptamine 2C receptors, agomelatine enhances the release of dopamine and noradrenaline in the prefrontal cortex, increases the activity of dopamine and noradrenaline, and thereby reduces depression and anxiety disorder.The combination of these two effects means that agomelatine exhibits a unique pharmacological role in the treatment of depression, anxiety, and disturbance of the circadian rhythm.Emotion and sleep are closely related to memory and cognitive function.Memory disorder is defined as any forms of memory abnormality, which is typically evident in a broad range of neurodegenerative diseases, including Alzheimer’s disease.Memory impairment and cognitive impairment are common symptoms of neurodegenerative and psychiatric diseases.Therefore, whether agomelatine can improve memory and cognitive behaviors if used for alleviating depression and circadian-rhythm sleep disorders has become a research “hotspot”.This review presents the latest findings on the effects of agomelatine in the treatment of psychologic and circadian-rhythm sleep disorders in clinical trials and animal experiments.Our review evaluates recent studies on treatment of memory impairment and cognitive impairment in neurodegenerative and psychiatric diseases.
Key Words: agomelatine; antidepressant; anxiety; apathy; circadian-rhythm sleep disorder; cognitive impairment; depression; melatonergic; memory disorder; mood disorder; neurodegenerative disease
Introduction
Agomelatine (AGO) is N-(2-[7-methoxy-1-naphthalenyl]ethyl) acetamide(S20098).It was discovered and developed by the European Pharmaceutical Company Servier Laboratories Limited in 1992 (Yous et al., 1992; Armstrong et al., 1993).As the first melatonergic antidepressant, AGO was approved by the European Medicines Agency in the European Union in 2009 and Therapeutic Goods Administration in Australia in 2010 for the treatment of major depression.AGO alleviates circadian-rhythm sleep disorders in patients suffering from depression with synergistic agonism at melatonin receptor 1A/melatonin receptor 1B (MT/MT) and antagonism at 5-hydroxytryptamine 2C(5-HT) receptors.AGO provides a useful alternative pharmacological strategy to existing antidepressant drugs (Norman and Olver, 2019).
Commonly used drugs for depression are selective serotonin reuptake inhibitors (SSRIs) and selective serotonin-norepinephrine reuptake inhibitors(SSNRIs), which ameliorate depression by increasing the 5-hydroxytryptamine(5-HT) level.However, SSRIs and SSNRIs produce adverse effects, such as withdrawal syndrome, sexual dysfunction, difficulties in sleeping, and agitation (Erdoğan et al., 2020).Compared with SSRIs and SSNRIs, AGO exerts an antidepressant effect by binding directly to 5-HTreceptors in postsynaptic membranes without affecting 5-HT concentrations in synaptic clefts.Furthermore, compared with natural endogenous melatonin, the replacement of an indole ring with a naphthalene ring in AGO (Table 1)enhances the metabolic stability of AGO and prolongs its biological half-life,which leads to more effective correction of circadian-rhythm disorders and alleviation of sleep disorders by activating MTand MT.With synergistic agonism at MT/MTand antagonism at 5-HT receptors, AGO has obvious positive effects on the sleep-wake cycle and depression while lacking serious side effects (including sexual side effects) (Kennedy et al., 2008).
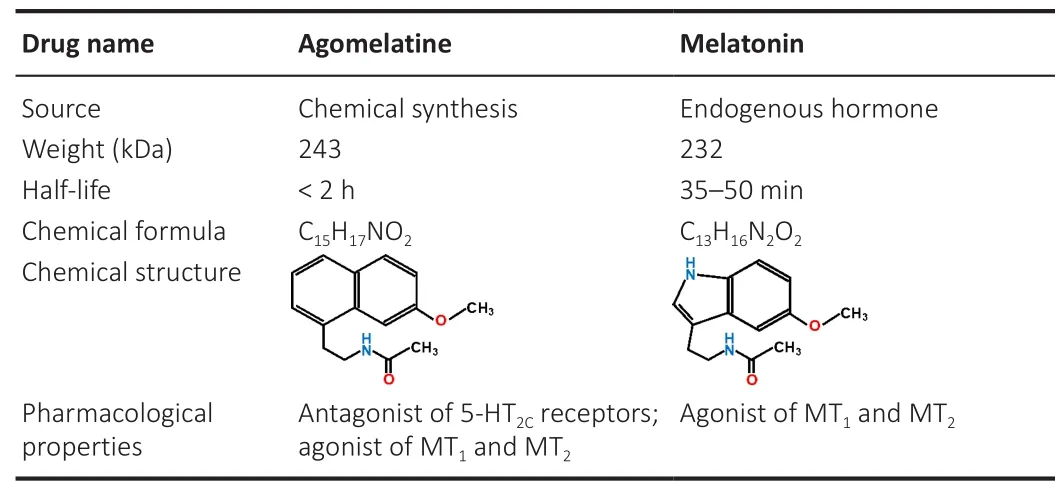
Table 1 |Comparison of drug characteristics between agomelatine and melatonin
Neurodegenerative disease refers to the principal pathology associated with disorders such as Alzheimer’s disease, Huntington’s disease and Parkinson’s disease.The patients with these diseases exhibit diverse patterns of sleep disturbance, memory disorder and cognitive impairment.Many researchers have reported a strong association among sleep disturbance, mood changes,memory complaints, and reduced cognitive performance (Tempesta et al.,2018; Guan et al., 2020; Gutierrez et al., 2021; Xie et al., 2021; Hernandez and Shukla, 2022).Sleep disorders, emotional abnormalities, and cognitive decline are usually present in the same individual and influence each other.An improvement in sleep and mood disorders often alleviates memory deficit and cognitive decline.Therefore, based on the peculiar characteristic of AGOintegrating melatonergic agonism and 5-HT antagonism and the efficacy of AGO in improving sleep and mood, it is intriguing and meaningful to ascertain if AGO can also ameliorate the deficits in memory and cognitive behaviors while attenuating depression and sleep-rhythm disorders.
Here, we summarize the latest research progress of AGO in the treatment of depression, sleep disorders, and cognitive impairments.We aim to provide new insights into the pharmacological action and mechanisms of AGO in the clinical setting.
Search Strategy and Selection Criteria
The studies cited in this narrative review were published from 2000 to 2021.They were searched by QS and TL on 31 December 2021 using the PubMed database and met the inclusion criteria, search terms include at least one of the following the keywords “agomelatine” and “S20098”, and at least one of the following keywords: “mood disorder”, “depression”, “anxiety”, “apathy”,“anhedonia”, “sleep-rhythm disorder”, “memory”, “cognition”, “dementia”,and “neurodegenerative disorder”.
Agomelatine and Mood Disorders
Mood disorders are a group of mental or psychiatric disorders, such as depression, anxiety and apathy, and are characterized by abnormalities of emotional state (Marshall, 2020).These emotional disorders have a negative impact on the physical and mental health of patients, and also impose a heavy burden on their families and society.According to Chisholm et al.(2016),depression and anxiety disorders cost the global economy USD1.15 trillion each year.AGO can improve depression, anxiety, apathy, and other mood disorders, especially major depressive disorder (MDD).
Depression
Depression (also known as depressive disorder) is one of the most common mood disorders, and is characterized by a persistent low mood state.The accompanying symptoms of depression include inactivity, loss of concentration, social withdrawal, sleep disturbances, and cognitive impairments, such as memory deficit (LeMoult and Gotlib, 2019; Price and Duman, 2020).
AGO is used mainly for the treatment of depression, especially MDD.Heun et al.(2013) undertook a study on 222 older patients with MDD.They found that AGO treatment for 8 weeks relieved depressive symptoms efficiently and was well tolerated in older patients suffering from depression (Heun et al.,2013).Similarly, Robillard et al.(2018) reported that AGO reduced depressive symptoms significantly in 24 young adults with depression.Moreover, they found that the timing of dim light melatonin onset (DLMO) was shifted 3.6-hour earlier after treatment with AGO, which indicated a strong correlation between the improvement of depression and the phase shift of DLMO(Robillard et al., 2018).In a network meta-analysis, Cipriani et al.(2018)reported that AGO was more efficacious and acceptable in adult patients with major depression than other antidepressants.Recently, a 1-year multicenter observational study in France showed that AGO improved the quality of life and daily functioning of MDD patients and alleviated depressionrelated functional disability, with good efficacy and tolerability noted during the treatment period (Gorwood et al., 2020).In an open evaluation, AGO treatment for over 14 weeks in 37 patients with acute depression and seasonal affective disorder led to 76% having a response and 70% achieving remission (Pjrek et al., 2007).
Bipolar disorder is a chronic episodic mental disorder characterized by intermittent episodes of depressive and manic symptoms (Post, 2005; Tondo et al., 2017).Long-term use of antidepressants can increase the risk of patients with depression suffering mania or hypomania.Hence, a combination of antidepressants and mood stabilizers (e.g., lithium or sodium valproate)is, in general, the main approach for treating bipolar disorder (Grunze et al.,2018).AGO, as an adjunctive treatment with lithium or sodium valproate, was evaluated in bipolar disorder and was found to be efficacious in alleviating the symptoms of the patients after 6-week treatment (Calabrese et al., 2007;Fornaro et al., 2013).
The mechanism of action of AGO in the treatment of depression is focused on the synergistic effects of AGO on activation of MT/MTand antagonism of 5-HTreceptors.It has been reported that 90% of patients suffering from depression showed different degrees of sleep disorders (Tsuno et al., 2005).The quality of sleep and the outcome of depression had a mutually causal relationship.Thus, improvement in sleep quality can directly alleviate the emotional status of patients suffering from depression.Activation of MT/MTby AGO can improve the sleep quality and daytime wakefulness of patients which, in turn, attenuates depressive symptoms.
5-HTreceptors are the only known G protein-coupled receptors subjected to “RNA editing”, a mechanism which generates multiple variants of a particular gene product, thereby producing isoforms of 5-HTreceptors with various properties (i.e., affinity, coupling, and constitutive activity) (Werry et al., 2008; Schmauss et al., 2010).Weissmann et al.(2016) documented a marked increase in RNA editing on 5-HTreceptors in specific brain regions of patients who felt suicidal and depressed.Hence, region-specific changes in editing of the messenger-RNA of 5-HTreceptors and deficient receptor function likely contribute to the etiology of depressive disorder or suicidal ideation (Weissmann et al., 2016).Usually, 5-HTreceptors inhibit downstream release of dopamine and norepinephrine from some brain regions (e.g., prefrontal cortex) involved in regulation of emotion (Alex and Pehek, 2007).Compared with SSRI antidepressants, the antidepressant and anti-anxiety effects of antidepressants are stronger if 5-HTreceptors are antagonized (Demireva et al., 2020).AGO can antagonize 5-HTreceptors effectively without influencing the activity of G protein-coupled receptors, and it can normalize signaling at 5-HTsites by blocking the actions of agonists and inverse agonists, thereby returning activity to a baseline value (Werry et al., 2008).By antagonizing 5-HTreceptors, AGO can also disinhibit release of dopamine and norepinephrine in the prefrontal cortex, thereby contributing to the effects of antidepressants (Millan et al., 2003; Stahl, 2014).In addition,AGO has neuroprotective effects because it has been shown to improve neuronal plasticity in the rat brain (Calabrese et al., 2011; Dagyte et al.,2011), upregulate brain-derived neurotrophic factor (BDNF) expression in the prefrontal cortex and hippocampus (Yucel et al., 2016; Lu et al., 2018), and activate the extracellular signal-regulated kinase-protein kinase B-glycogen synthase kinase 3β (ERK-AKT-GSK3β) signaling pathway (Duda et al., 2020),which can help to ameliorate the depressive disorder.
The development of antidepressants is slow due to the complexity of the etiology and unclear pathogenesis of depression.However, converging evidence from clinical trials and animal experiments has shown that the unique antidepressant effects of AGO breakthrough the conventionaltreatment concept of SSRI and SSNRI antidepressants to provide a new strategy for the treatment of depression.Nonetheless, the antidepressant pharmacological mechanism of AGO has not been elucidated.
Anxiety disorder
Anxiety disorder is a common clinical neurosis characterized by excessive and persistent worry, with vegetative symptoms such as headaches and gastrointestinal complaints.Studies have demonstrated that AGO exhibits a good anxiolytic effect and tolerability (Stein et al., 2017; Slee et al., 2019),with a stronger clinical response and earlier improvement of symptoms than those elicited by SSRIs or SSNRIs (Buoli et al., 2017).AGO alleviated social isolation-induced anxiety in rats effectively and reversed increased plasma levels of vasopressin (Harvey et al., 2019).Similar to depression, the pathogenesis of anxiety disorder is complex, and the anxiolytic mechanism of AGO is incompletely understood.Several studies have suggested that the anxiolytic effects of AGO are associated mainly with its antagonism of 5-HTreceptors (Sant’Ana et al., 2019; Demireva et al., 2020).The modulation of glutamate neurotransmission, anti-inflammatory action, antioxidant action,and correction of melatonin rhythms are also involved in the anxiolytic-like effects of AGO (Tchekalarova et al., 2018; Santos et al., 2019).Moreover,anxiety disorder and depression often accompany each other, and a similar pathogenesis may exist between them.Therefore, AGO may relieve anxiety disorder by improving depression, andvice versa
.Other emotional disorders
Apart from its antidepressant and anxiolytic properties, it has been suggested that AGO may exhibit special curative effects on particular neuropsychiatric symptoms, such as apathy, anhedonia, and abulia.Apathy is one of the most prevalent behavioral and psychological symptoms of dementia.Apathy is characterized by an insidious decline in motivation and goal-directed actions, which leads to reduced interest in social, recreational, occupational,and creative pursuits.Callegari and colleagues showed that AGO (but not melatonin) improved apathy in patients with frontotemporal dementia and was well-tolerated (Callegari et al., 2016).Moreover, an analysis of the literature showed that AGO had an obviously positive effect on the treatment of apathy in people suffering from dementia (Harrison et al., 2016).De Berardis et al.(2013) showed that AGO reversed escitalopram-induced apathy markedly in a patient with MDD.Whether used alone or in combination with acetyl-L-carnitine, AGO can alleviate apathy in older patients with mild or moderate depression (Gavrilova et al., 2015).Clinical studies suggest that AGO has potential for the treatment of apathy, but how it improves apathy merits further exploration.
Another prominent symptom of many neuropsychiatric disorders is anhedonia (loss of interest and pleasurable feelings in response to previously rewarding stimuli) (Husain and Roiser, 2018).Anhedonia is most notable in MDD and schizophrenia.A pooled analysis of an open clinical trial showed that AGO could improve depression and anhedonia in a broad range of patients suffering from depression (di Giannantonio et al., 2019).AGO has been shown to reverse anhedonia-like deficits in rats exposed to chronic constant light (Tchekalarova et al., 2018).How AGO improves anhedonia is not well understood, but it has been reported that treatment with AGO or an antagonist of 5-HTreceptors, SB242084, reversed the anhedonia-like state in mice with knockout of glutamate ionotropic receptor NMDA type subunit 2D (Yamamoto et al., 2017).Hence, antagonizing 5-HTreceptors may be a strategy for treating anhedonia.Importantly, anhedonia has also been linked to dysfunctions in the dopamine system, which plays a part in reward prediction, motivational arousal, and responsiveness to conditioned incentive stimuli (Tye et al., 2013).Chenu and collaborators (Chenu et al.,2013) reported that chronic administration of AGO for 14 days increased the number of spontaneously active dopamine neurons, the “burst” activity of dopamine neurons, the firing rate of 5-HT neurons in the dorsal-raphe nucleus, and tonic activation of postsynaptic 5-HTreceptors located in the hippocampus.Those findings suggest that AGO may be involved in modulation of dopamine release in anhedonia.Nevertheless, the therapeutic mechanism of action of AGO in apathy and anhedonia must be elucidated.
Agomelatine and Sleep Disorders
Sleep disorders are a group of conditions in which the normal sleep pattern or sleep behaviors are disturbed.Primary sleep disorders include insomnia,hypersomnia, early waking, circadian-rhythm disorders, parasomnias, sleeprelated movement disorders, and sleep-related breathing disorders (Pavlova and Latreille, 2019).Sleep disorders bring heavy economic burdens to patients’ families and society.Annual insomnia-related expenses have been estimated to be USD150-175 billion worldwide in 2016 (Reynolds and Ebben,2017).Patients with psychiatric disorders and dementia have more severe sleep disorders which, in turn, contribute to earlier onset and more rapid progression of neurodegenerative disorders (Benca et al., 1992; Shi et al.,2018).Frobӧse et al.(2012) showed that a young patient with fatal familial insomnia had improved sleep efficiency, enhanced slow-wave sleep, and fewer awakenings during sleep periods after AGO treatment.In another study, AGO treatment for 6 months decreased the Depression Scale score significantly,and improved limb movements, sleep, and awakening significantly in patients suffering from depression and Parkinson’s disease (Avila et al., 2015).Quera-Salva et al.(2010) demonstrated that AGO ameliorated all aspects of sleepwake abnormalities in patients with depression (particularly falling sleep and the quality of sleep) with an improvement in daytime alertness.The effects of AGO on sleep architecture in MDD have been measured using polysomnography: significant improvements in sleep efficiency, slow-wave sleep, and the distribution of delta activity throughout the night have been documented, but with no change in the amount or latency of rapid eye movement (REM) sleep.Moreover, after AGO treatment, the depressive symptoms of patients suffering from depression were reduced significantly and, on average, the timing of DLMO shifted 3.6-hour earlier, sleep onset was phase-shifted 28-minute earlier, and total sleep time increased by 24 minutes(Robillard et al., 2018).
In adults with autism spectrum disorder with intellectual disability, AGO treatment alleviated circadian-rhythm sleep-wake abnormalities, corrected rhythm disorders of the M5 sleep phase, and had good tolerability (Ballester et al., 2019).O’Neill et al.(2014) revealed AGO administration to result in an immediate and sustained improvement in sleep and the indices of challenging behavior in a man with severe brain damage suffering from a non-24-hour sleep-wake disorder.Armstrong et al.(1993) demonstrated that AGO advanced sleep onset in rats with delayed sleep-phase syndrome,which lays the foundation for further studies of AGO on circadian-rhythm disorders and sleep disorders.Descamps and colleagues showed that AGO could attenuate impairments of sleep-wake architecture, reverse the beta-1 electroencephalogram power band, and improve stress-related rebound of REM sleep in older rats (Descamps et al., 2014).Furthermore, Tchekalarova et al.(2020) demonstrated that AGO could adjust circadian homeostasis of motor activity and the sleep-wake cycle in a rat model of chronic constant light, including enhancing the latency of RME sleep and non-REM sleep and upregulating expression of MTand BDNF protein.The researchers previously demonstrated AGO to be effective in restoring impaired circadian patterns and plasma melatonin levels as well as improving depression and anxiety-like behavior in rats exposed to chronic constant light (Tchekalarova et al., 2018).
In mammals, melatonin is a neuroendocrine hormone.It is synthesized and secreted principally by the pineal gland at night (Lerner et al., 1960).Its primary physiological function is to convey information concerning the daily cycle of light and darkness to body structures, to regulate circadian rhythms, and to synchronize rhythms.Usually, the rhythmic secretion of melatonin is driven by the circadian clock in the suprachiasmatic nucleus of the hypothalamus.However, light can suppress or synchronize melatonin production according to the light schedule, suggesting that melatonin secretion from the pineal gland is closely related to the duration of darkness(Kennaway, 2019).Melatonin acts via melatonin receptors distributed widely in various tissues to respond to periodic changes of light, such as the sleepwake cycle.Exposure to light activates the suprachiasmatic nucleus and suppresses melatonin production, which then transmits the light information from the circadian clock and induces awakening in daytime.At night, the synthesis and secretion of melatonin remain high, which promotes sleep(Zisapel, 2018).Therefore, if the rhythmic secretion of melatonin is disrupted,then circadian rhythms are also disrupted (e.g., poor sleep quality and circadian-rhythm sleep-wake disorders).5-HT and its receptors have been reported to be involved in sleep and wakefulness, as well as cognition and mood.Antagonism of 5-HT/5-HTreceptors prolongs the duration of slow-wave sleep and enhances low-frequency (< 7 Hz) activity in the sleep electroencephalogram (Landolt and Wehrle, 2009).Moreover, antagonism of 5-HTreceptors stimulates dopaminergic and adrenergic pathways and exerts antidepressant and anxiolytic actions in behavioral paradigms, which favors sleep (Millan, 2005).AGO activates MT/MTand antagonizes 5-HTreceptors synchronously, so it has a unique role in regulating the sleep-wake cycle and correcting sleep structure.
Agomelatine and Impairment of Cognition and Memory
A series of studies demonstrated that emotional disorders and sleep disorders can impact the function of memory and cognition negatively (McHutchison et al., 2020; Xu et al., 2020).Neurodegenerative diseases characterized by memory and cognitive impairments (e.g., Alzheimer’s disease and dementia) are characterized by abnormal emotional performance and sleep performance.About 50 million people worldwide have dementia (mainly Alzheimer’s disease), and the number is expected to increase to 152 million by 2050 (Alzheimer’s Disease International and Patterson, 2018).The estimated total global societal cost of dementia is $1.3 trillion, which is expected to surpass $2.8 trillion by 2030 as the number of people living with dementia and care costs increase (World Health Organization, 2021).However,efficacious disease-modifying therapeutics for dementia management are lacking.Interestingly, AGO has been reported to be efficacious in the treatment of emotional disorders, and also in improving cognitive deficits(Bogolepova et al., 2011; Altinyazar and Kiylioglu, 2016; Callegari et al., 2016).
Additional Table 1
summarizes the effects of AGO on memory impairment and cognitive impairment in clinical trials.Most AGO studies have focused on the treatment of patients with emotional disorders, but some studies have also shown that AGO improves cognitive function in patients with Alzheimer’s disease (Altinyazar and Kiylioglu, 2016), frontotemporal dementia(Callegari et al., 2016), stroke (Bogolepova et al., 2011; Antonen et al., 2015),schizophrenia, or severe depression.Through evaluation of cognitive ability,Englisch et al.(2018) and Bruno et al.(2014) found that AGO treatment could efficiently ameliorate the reasoning/problem-solving ability and correct“perseverative errors” in the Wisconsin Card Sorting Test in patients with schizophrenia (Additional Table 1).Moreover, studies have indicated that patients suffering from depression with cognitive impairments (especially those with major depression) had different degrees of improvements in cognitive ability after AGO treatment (Gavrilova et al., 2014; Gorwood et al.,2014, 2015; Kalyn et al., 2015; Cléry-Melin and Gorwood, 2017; Medvedev et al., 2018).Safarova et al.(2018) and Gavrilova et al.(2015) showed that a combination of AGO and carnicetine or AGO and actovegin allowed for a more rapid and pronounced therapeutic effect for cognitive dysfunction in older patients with mild or moderate depression.In a trial involving 20 older patients with mild or moderate depression, AGO reduced anxiety disorder and depressive symptoms effectively as well as improving the health-related quality of life of patients significantly, with a slight increase in the Mini-Mental State Examination score and without pronounced or serious adverse events (Gavrilova et al., 2014).In another trial of 15 patients with primary fibromyalgia, treatment with AGO improved depression, anxiety disorder,and pain significantly in patients; the authors reported a trend towards the improvement of performances in executive/cognitive symptoms (Bruno et al., 2013).Through a double-blind parallel-group design in healthy volunteers, Harmer et al.(2011) observed that AGO (25 mg) decreased the subjective rating of sadness, reduced recognition of sad facial expressions,improved affective memory, and reduced the emotion-potentiated startle response, whereas AGO (50 mg) reduced only the emotion-potentiated startle response without affecting other types of emotional processing.The researchers indicated that AGO is also beneficial for memory improvement in healthy cohorts.Nevertheless, how AGO improves the cognitive dysfunction accompanied by these diseases is not known.
Additional Table 2
summarizes the characteristics of AGO on memory and cognition in healthy animals and pathological animal models.Chronic AGO treatment reduced the error percentage of streptozotocin-treated rats in the eight-arm radial arm maze test, thereby suggesting that AGO could improve the spatial working memory of rats with Alzheimer’s disease (Bergamini et al., 2016; Ilieva et al., 2019).Furthermore, Gupta et al.(2015) demonstrated that AGO treatment reduced the escape latency and residence time in the target quadrant in the Morris water maze test of mice with chronic cerebral hypoperfusion, thereby implying that the long-term learning and memory of mice were improved.Moreover, the cognitive function of rats with renovascular hypertension-induced vascular dementia was ameliorated obviously by AGO treatment (Singh et al., 2015).It has also been demonstrated that AGO significantly attenuates 3-nitropropionic acid-induced learningmemory deficits in rats with Huntington’s disease (Gupta and Sharma,2014), and improves the restraint stress-induced impairments of short-term recognition memory and long-term spatial learning and memory in mice and rats (Conboy et al., 2009; Gumuslu et al., 2014; Lapmanee et al., 2017).Marrocco et al.(2014) found that AGO treatment markedly corrected abnormalities in social-memory performance in adult rats with unstressed or prenatal restraint stress, and Martin et al.(2017) simultaneously observed that effectively alleviated episodic memory deficits of mice with chronic social defeat stress in the novel object recognition test (NORT).Hence,AGO might improve the interactive behaviors of animals by enhancing their recognition memory.In addition, it has been demonstrated that AGO improves the memory deficit induced by scopolamine in mice (İlkaya et al., 2015), and alleviates the short-term memory despair of mice injected with pentylenetetrazol to induce kindling (Azim et al., 2017), but did not correct spatial-memory impairment in rats suffering from epilepsy induced by kainate acid (Tchekalarova et al., 2017).Those findings suggest that the effects of AGO treatment in various animal models differ because of different epilepsy-triggering treatments, which provides a direction for study on the pharmacological mechanism of AGO.In the probabilistic reversal-learning test, Drozd et al.(2019) found that AGO treatment improved the ability of cognitive judgment in pessimistic rats.Notably, single administration of AGO (10 and 40 mg/kg) in the evening or morning improved the recognition memory of normal rats significantly in the NORT (Bertaina-Anglade et al., 2011).Moreover, studies in normal rats revealed that long-term AGO treatment enhanced spatial memory obviously in the Morris water maze test(Demir Özkay et al., 2015), and improved recognition memory in the NORT(Ladurelle et al., 2012).How AGO improves memory and cognitive function isnot known, probably owing to the perplexing causes of memory impairment and cognitive impairment in particular diseases.
It has been demonstrated that AGO reduced amyloid β-42 (Aβ)accumulation in the frontal cortex and hippocampus of male rats with streptozotocin-induced Alzheimer’s disease (Ilieva et al., 2019), activated melatonin receptors, and prevented Aβ-induced tau phosphorylation by activation of GSK3β and oxidative damage in PC12 cells (Yao et al., 2019).Furthermore, chronic administration of AGO completely prevented the stressinduced increase in glutamate release in the prefrontal/frontal cortex of rats by the synergistic effect of melatonergic and 5-HTreceptors in one study(Tardito et al., 2010).Recently, Ilieva et al.(2021) suggested that chronic treatment with AGO alleviated anxiety disorder and depressive-like behavior and decreased the Aβ level in the hippocampus by enhancing α-secretase and decreasing γ-secretase in a rat model of Alzheimer’s disease.Cankara et al.(2021) showed that AGO attenuated cisplatin-induced neurotoxicity in a mouse hippocampal neuronal cell line (HT22), and improved cisplatin-induced deficits in memory and recognition.In addition, in rat models with cognitive impairments, AGO also reversed neuronal loss in the hippocampus (Can Ö et al., 2018; Ilieva et al., 2021) and caused significant enhancement in the volume of hippocampal CA1-3 subfields and the total number of pyramidal neurons in this region (Demir Özkay et al., 2015), thereby implying that AGO had neuroprotective effects.
BDNF is a neurotrophin distributed widely in the mammalian brain.BDNF has prominent functions in plastic regulation, including control of neuronal and glial development, neuroprotection, and modulation of short- and longlasting synaptic interactions, which are critical for cognition and memory(von Bohlen Und Halbach and von Bohlen Und Halbach, 2018).It has been reported that AGO alleviates depressive symptoms effectively in patients suffering from depression, with restoration of the plasma level of BDNF(Martinotti et al., 2016).AGO also increases the hippocampal BDNF level and the number of BDNF-positive neurons in rats with chronic unpredictable mild stress (Lu et al., 2018).Notably, Ladurelle et al.(2012) demonstrated that chronic administration of AGO increased the level of mature BDNF in the hippocampus significantly, and promoted rapid, sustained, enhanced cognitive activity in normal rats.
Cyclic adenosine monophosphate-responsive element binding protein (CREB)is a transcription factor.CREB regulates expression of several genes involved in the control of neuroplasticity, circadian rhythms, cell survival, and cognition(Carlezon et al., 2005).Moreover, CREB is a pivotal component of the“molecular switch” that converts short-term memory to long-term memory(Lisman et al., 2018).Furthermore, the CREB family is a major regulator of BDNF expression after tropomyosin receptor kinase B (TrkB; a BDNF receptor)signaling (Esvald et al., 2020).Recent evidence suggests that chronic administration of AGO leads to improvements in memory deterioration and upregulation of hippocampal expression of CREB and BDNF in mice exposed to unpredictable, chronic, mild stress (Gumuslu et al., 2014).It has been revealed that activation of MTand MTactivates ERK-90 kDa ribosomal S6 kinase-CREB-BDNF signaling (Sung et al., 2018) and BDNF-TrkB signaling in hippocampal neurons (Li et al., 2018).Moreover, mice with deletion of the 5-HTreceptor show increased levels of the mature form of BDNF in the hippocampus (Hill et al., 2011).In naïve rats, Musazzi et al.(2014) found that chronic administration of AGO—contrary to traditional antidepressants—did not increase CREB phosphorylation, but instead modulated the mitogenactivated protein kinase (MAPK)-ERK1/2 and AKT-GSK3β pathways.ERK1/2 is one of the best-characterized members of the MAPK family.ERK1/2 mediates the proliferation, differentiation, apoptosis, inflammation, and synaptogenesis of cells (Albert-Gascó et al., 2020).
In neurons, an important function of ERK-MAPK signaling is regulation of synaptic plasticity, which relates to learning and memory processes (Alkadhi and Dao, 2019).Several studies have indicated that phosphatidylinositol 3-kinase can activate AKT in brain cells and, by activation of this protein,GSK3β, which inhibits tau hyperphosphorylation (Chen et al., 2004; Endo et al., 2006).AKT shows high expression in some brain areas that are known to regulate cognition and neuroprotection, thereby having a key role in synaptic and neural survival, neuroprotection, and neural plasticity, as well as supporting the growth and survival of neurons (Beaulieu et al., 2009; Kitagishi et al., 2012).Studies have reported that activation of melatonin receptors(MT1 and MT2) can activate MAPK-ERK1/2 and AKT-GSK3 signaling pathways(Werry et al., 2005; Hadj Ayed Tka et al., 2015; Chagraoui et al., 2016; Li et al., 2020).Therefore, AGO may protect hippocampal neurons and improve memory and cognition by regulation of MAPK-ERK and AKT-GSK3β signaling pathways, but this concept has not been demonstrated definitively.
It has been shown that 5-HTreceptors mediate Carelease from endoplasmic reticula via the phospholipase C-inositol 1,4,5-trisphosphate-Capathway (Watson et al., 1995; Wada et al., 2006).5-HTreceptors show high expression and are upregulated in the brains of mice with acute infusion of Aβ (Bonn et al., 2013) and in rats with pilocarpine-induced epilepsy with memory impairment (Krishnakumar et al., 2009).Those studies indicate that intracellular Cadysregulation might (at least in part) result from 5-HToverexpression.Cadysregulation leads to neuronal apoptosis and cell death, which result in memory impairment and cognitive impairment (Kumar,2020).Scholars have applied various sequencing methods and databases and analyzed “omics” data to discover new uses for existing drugs.Such studies have demonstrated AGO to also be involved in regulation of axon development, glutamatergic activity, netrin signaling, synaptic long-term potentiation, and Rho-GTPases-related pathways (an important regulator of morphological neuroplasticity) in hippocampal neurons (Patrício et al., 2015),as well as a neurotrophin signaling pathway and insulin signaling pathway in patients suffering from depression (Dmitrzak-Weglarz et al., 2021), which are closely related to memory and cognitive function.
Although the findings mentioned above are not entirely consistent (possibly caused by different animal models and interventions), they show that AGO improves memory and cognitive function by activating multiple signal-transduction pathways (Figure 1).Clinical and basic-science studies investigating the effect of AGO on memory and cognition suggest that AGO might be a novel strategy for the treatment of memory impairment and cognitive impairment.With the increase in age of populations worldwide,the dementia observed in degenerative diseases has become a growing public-health problem.However, prevention of the memory impairment and cognitive impairment of patients with dementia is not possible, and few drugs can be used for treatment.With its unique pharmacological effects, AGO provides a new idea for the treatment of dementia.

Figure 1 | Pathways involving the neuroprotective effects of AGO (schematic).
Limitations of Agomelatine
Although AGO possesses some distinct advantages, it also has some disadvantages.The commonly reported adverse events in clinical treatment using AGO are headache, nausea and fatigue, which are of mild-to-moderate severity (Stein et al., 2018).Moreover, due to its propensity to increase the level of liver enzymes, AGO is contraindicated in patients with impaired liver function (Štuhec, 2013; Friedrich et al., 2016).Hence, monitoring of liver function is recommended before AGO initiation and periodically during treatment.Nevertheless, developing AGO analogs with low toxicity and few side effects for the treatment of chronic neurodegenerative diseases is important.
Conclusions
The most remarkable feature of AGO is a synergistic action between its agonism at MT/MTand antagonism at 5-HTreceptors.Given its innovative mechanism of action and favorable safety profile, AGO is beneficial for the treatment of mood disorders and sleep disorders, and most reviews have focused on these aspects.Unlike those reviews, in light of the neuroprotective effects of AGO, we have summarized research progress regarding the improvement of memory and cognitive function using AGO.However, the few studies undertaken so far on the clinical treatment of cognition and dementia by AGO (particularly on the specific treatment of neurodegenerative diseases)need to be bolstered with additional studies.Nevertheless, clinical studies and animal experiments have strongly suggested that AGO could be a promising treatment option for improving memory, sleep, and mental activity simultaneously.
Author contributions:
Review design: QS, TL, and JSQ; manuscript writing:QS and TL; data search: GWL and ZJW; data collection: YLZ and MNW;manuscript correction: JHG and JSQ.All authors approved the final version of
the manuscript.
Conflicts of interest:
The authors declare no conflicts of interest.
Availability of data and materials:
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:
Melinda Barkhuizen, Philip Morris International,Netherlands; Nemil N.Bhatt, The University of Texas, USA.
Additional files:
Characteristics of AGO for the treatment of memory and cognitive impairments in clinical studies.
Characteristics of AGO for the treatment of memory and cognitive impairments in animal studies.
- 中国神经再生研究(英文版)的其它文章
- Neural and Müller glial adaptation of the retina to photoreceptor degeneration
- MicroRNAs: protective regulators for neuron growth and development
- In vivo astrocyte-to-neuron reprogramming for central nervous system regeneration: a narrative review
- Intranasal nerve growth factor for prevention and recovery of the outcomes of traumatic brain injury
- Altered O-GlcNAcylation and mitochondrial dysfunction,a molecular link between brain glucose dysregulation and sporadic Alzheimer’s disease
- Signaling interactions among neurons impact cell fitness and death in Alzheimer’s disease

