Sex modulates the outcome of subthalamic nucleus deep brain stimulation in patients with Parkinson’s disease
Tian-Shuo Yuan , Ying-Chuan Chen , De-Feng Liu Ruo-Yu Ma Xin Zhang, Ting-Ting Du, Guan-Yu Zhu , Jian-Guo Zhang ,
Abstract There are many documented sex differences in the clinical course, symptom expression profile, and treatment response of Parkinson’s disease, creating additional challenges for patient management.Although subthalamic nucleus deep brain stimulation is an established therapy for Parkinson’s disease, the effects of sex on treatment outcome are still unclear.The aim of this retrospective observational study, was to examine sex differences in motor symptoms, nonmotor symptoms, and quality of life after subthalamic nucleus deep brain stimulation.Outcome measures were evaluated at 1 and 12 months post-operation in 90 patients with Parkinson’s disease undergoing subthalamic nucleus deep brain stimulation aged 63.00 ± 8.01 years (55 men and 35 women).Outcomes of clinical evaluations were compared between sexes via a Student’s t-test and within sex via a paired-sample t-test, and generalized linear models were established to identify factors associated with treatment efficacy and intensity for each sex.We found that subthalamic nucleus deep brain stimulation could improve motor symptoms in men but not women in the on-medication condition at 1 and 12 months post-operation.Restless legs syndrome was alleviated to a greater extent in men than in women.Women demonstrated poorer quality of life at baseline and achieved less improvement of quality of life than men after subthalamic nucleus deep brain stimulation.Furthermore, Hoehn-Yahr stage was positively correlated with the treatment response in men, while levodopa equivalent dose at 12 months post-operation was negatively correlated with motor improvement in women.In conclusion, women received less benefit from subthalamic nucleus deep brain stimulation than men in terms of motor symptoms, non-motor symptoms, and quality of life.We found sex-specific factors, i.e.,Hoehn-Yahr stage and levodopa equivalent dose, that were related to motor improvements.These findings may help to guide subthalamic nucleus deep brain stimulation patient selection, prognosis, and stimulation programming for optimal therapeutic efficacy in Parkinson’s disease.
Key Words: chronic effect; deep brain stimulation; generalized linear model; initial effect; motor symptoms; non-motor symptoms; Parkinson’s disease; quality of life; sex; subthalamic nucleus
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease, and it can lead to largely irreversible motor and non-motor deficits(Alexander, 2004).Evidence accumulated over the past several decades indicates that PD incidence, clinical course, symptom expression profiles, and treatment responses differ markedly between men and women.For instance,the prevalence of PD is 1.5-2.0 times higher in men than in women (Iwaki et al., 2021), and the age at onset is 2.1 years later in women (Haaxma et al., 2007).After progression to the clinical phase of the disease, women are more likely to present with tremor (Haaxma et al., 2007), whereas men show worse motor performance in the face, neck, and arms (Szewczyk-Krolikowski et al., 2014).Amongst non-motor symptoms, men exhibit more severely impaired cognition (Reekes et al., 2020) and olfaction (Liu et al., 2015).In contrast, anxiety, depression, and sleep problems are reported to occur more frequently in women (Fernandez et al., 2000; Guo et al., 2013).In addition,studies have indicated that women have greater bioavailability of levodopa(Kompoliti et al., 2002) and that they are at greater risk of developing levodopa-induced dyskinesia (Zappia et al., 2005; Accolla et al., 2007).
Subthalamic nucleus deep brain stimulation (STN-DBS) is an established therapy for advanced PD that can relieve motor symptoms and reduce the dose of dopaminergic treatment (Limousin et al., 1998).These benefits are generally sustained, with consistent effects reported even 5 years after implantation of STN-DBS electrodes (Krack et al., 2003).Several studies have found differential effects of STN-DBS in men and women, including a greater improvement in the activities of daily living (ADL) but poorer alleviation of bradykinesia among women (Hariz et al., 2003; Accolla et al., 2007).Women were also reported to achieve a smaller reduction in the levodopa equivalent daily dose (LEDD) compared with men (Chandran et al., 2014).These discrepancies indicate that more detailed analyses of larger populations are required to identify sex-specific STN-DBS responses and achieve greater therapeutic consistency.
In the present study, we investigated the sex differences and sex-specific effects of STN-DBS on motor symptoms, non-motor symptoms, and quality of life in men and women with PD.In addition, we investigated factors related to the efficacy and intensity of STN-DBS for each sex.
Methods
Study participants
In this retrospective observational study, we enrolled 90 PD patients (35 women) aged 63.00 ± 8.01 years who agreed to undergo bilateral STN-DBS electrode implantation at Beijing Tiantan Hospital between 2015 and 2019(Additional Figure 1).The inclusion criteria were (1) diagnosis of PD based on the UK Brain Bank criteria (Hughes et al., 1992), (2) implantation of electrodes for DBS in the STN, and (3) clinical assessment at 1 and 12 months after surgery.The exclusion criteria included (1) a history of prior thalamotomy or other cerebral neurosurgery and (2) loss of follow-up at 1 or 12 months.This study was conducted following approval by the Ethics Committee of Beijing Tiantan Hospital of Capital Medical University (KY 2020-030-02, approved on September 14, 2020; Additional file 1) and all protocol were performed in accordance with theDeclaration of Helsinki
.This study was reported according to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement (von Elm et al, 2007).All patients provided written informed consent.Surgical procedures and programming
DBS electrodes (model 3389, Medtronic, Minneapolis, MN, USA, or model L301, Pins Medical, Beijing, China) were implanted under local anesthesia(lidocaine and ropivacaine) using a Leksell micro-stereotactic system (Elekta Instrument AB, Stockholm, Sweden) as described previously (Fan et al.,2020).Intraoperative single unit recordings and stimulation testing were performed to assess the optimal electrode location.A microelectrode(Alpha Omega, Nazareth, Israel) was driven into the brain via a microdrive.Electrophysiological signals were recorded to determine the entrance to and exit from the STN.When a standard STN signal was identified, the neurosurgeons tested the stimulation effectiveness and side effects using a temporary stimulator.If the effects of the temporary stimulation were satisfactory, the electrodes were connected to an implantable pulse generator(Medtronic, Minneapolis, MN, USA or Pins Medical, Beijing, China), which was then implanted in the subclavicular area under general anesthesia (e.g.,propofol, succinylcholine, and remifentanil).Postoperative computerized tomography was conducted to verify the electrode position and the absence of intracranial hemorrhage.The implantable pulse generator was turned on 1 month after surgery, and patients received regular reprograming and medication adjustments until the optimal efficacy was established.All postoperative adjustments of the stimulation settings were conducted in the off-medication condition.
Clinical evaluation
The patients received multiple clinical evaluations.First, motor function was assessed using Unified Parkinson’s Disease Rating Scale III (UPDRS-III) (Cilia et al., 2020) total scores and item subscores for tremor (UPDRS items 20 and 21; range 0-28), rigidity (item 22; score 0-20), bradykinesia (items 23, 24, 25,26, and 31; range 0-36), and axial symptoms (items 18, 27, 28, 29, and 30;range 0-20).In addition, motor subtype classifications were conducted by a commonly used method based on the UPDRS, and these included tremor dominant, postural instability and gait disorders, and mixed type (Jankovic and Kapadia, 2001).Non-motor symptoms were assessed using the Restless Legs Syndrome Rating Scale (Garcia-Borreguero et al., 2021), Hamilton Anxiety Rating Scale (Chen et al., 2021), Hamilton Rating Scale for Depression (Nicolettiet al., 2017), Mini-Mental State Examination (MMSE) (Li et al., 2016), and Montreal Cognitive Assessment (Chen et al., 2016).Finally, quality of life(QOL) was evaluated using the Parkinson’s Disease Questionnaire (39-items)(PDQ-39) (Margolius et al., 2018).Clinical evaluations were conducted at the preoperative visit (M0), 1-month (approximately 4-5 weeks) post-surgery,and approximately 11-13 months post-surgery.The patients returned to the hospital for pulse generator activation at 1 month post-operation and a stimulation parameter adjustment at 12 months post-operation.To establish a stable STN-DBS setting, they were observed for 3-7 days at each followup assessment.This was done under the off-medication condition (Off-med),after a minimum 12-hour withdrawal period from dopaminergic medications.An on-medication (On-med) assessment was conducted after the patients took medication at the evaluation center.Postoperative assessments were conducted in the on-stimulation (On-stim) condition.All motor tests were videotaped after obtaining patient consent.Antiparkinsonian medications and stimulation settings were recorded after each evaluation.
Outcome measures
The primary outcome measures of motor function were changes in UPDRSIII total and motor-item subscores at 1 month post-operationversus
baseline(M0) to assess initial effects (Chen et al., 2022), and at 12 months postoperation to assess chronic effects.Motor symptom responses to levodopa were calculated as (UPDRS
-UPDRS
)/UPDRS
× 100%, which can be considered a measure of the changes in motor symptoms between the on-medication and off-medication conditions (on-off change).The stimulation effect was calculated by the formula (UPDRS
-UPDRS
)/UPDRS
× 100%.To compare general improvement in daily life between sexes, we calculated the effect of brain stimulation plus medication as (UPDRS
-UPDRS
)/UPDRS
× 100%.Some scale scores had 0 as the denominator, so score changes were calculated instead of the percentage of improvement for comparison.We also compared the value changes in the non-motor symptoms and the quality of life at the 12-month followup assessment using the PDQ-39, which covers eight domains: mobility(items 1-10), ADL (items 11-16), emotional well-being (items 17-22),stigma (items 23-26), social support (items 27-29), cognition (items 30-33),communication (items 34-36), and bodily discomfort (items 37-39).Each domain score was calculated asPDQ
/PDQ
× 100%.A PDQ-39 summary index (PDQ-39 SI) score was also calculated as the sum of the eight domain scores divided by eight multiplied by 100% (Bjerknes et al., 2018).The total electrical energy delivered (TEED) was computed asTEED
=(voltage
×pulse width
×frequency
)/impedance
(Rodríguez Cruz et al.,2016).The total antiparkinsonian medication doses were converted to the LEDD for each patient (Schade et al., 2020).Statistical analysis
In the present study, the patients had received treatment at Beijing Tiantan Hospital, and were enrolled retrospectively.All patients who met our inclusion criteria were included in the study.Hence, we did not conduct a sample size calculation or blinded assessment.The data are presented as the mean ±standard deviation.We compared the scale scores at equivalent time points and percentage improvements (or score changes) in scale items between men and women using an independent samplest
-test.The categorical variables are expressed as the number or frequency, and these were compared between men and women using a Chi-squared test.The preoperative scores were compared with the postoperative scores for each sex using a paired-samplet
-test.We used generalized linear models (GLMs) to identify factors related to the efficacy of STN-DBS in terms of motor and non-motor symptoms and factors associated with the intensity of treatment (TEED or LEDD) for each sex.If a factor was mainly significant for one sex but not the other, it was considered sex specific.Pairwise deletion was used to analyze scales that were incomplete because of patient fatigue during the follow-up assessment.For scales with sub-items (UPDRS-III and PDQ-39), the Bonferroni test was used for correction.Statistical analyses were performed using MATLAB 2021a(Mathworks, Natick, MA, USA).AP
< 0.05 was considered significant for all tests.Results
Patient characteristics
The study sample comprised 55 men and 35 women.Age at onset, duration of disease, Hoehn-Yahr (H-Y) stage, and ratios of the three motor subtypes did not differ between the sexes, while the education level was higher in men than in women (P
= 0.0006).There was no difference in the LEDD between sexes before and after surgery, and both sexes achieved a significant LEDD reduction at the 12-month follow-up assessment (Table 1).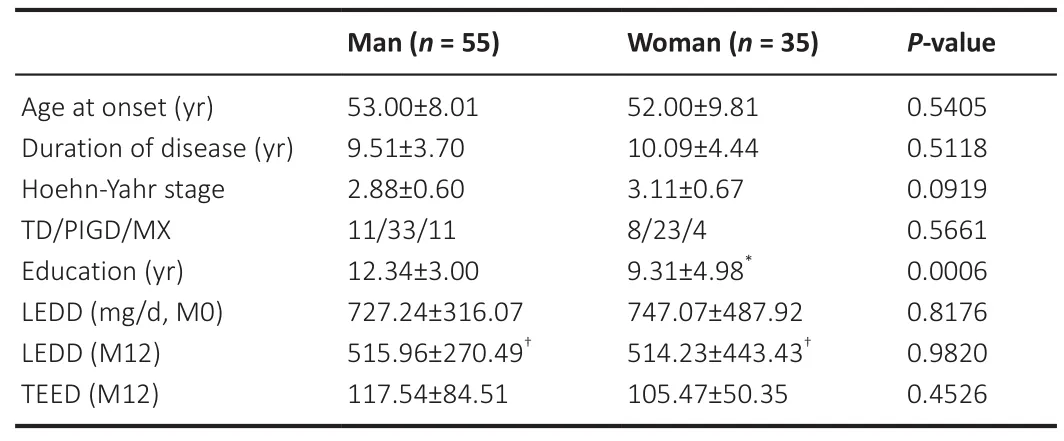
Table 1 |Characteristics of the included patients
Effects of sex on motor symptoms
At the baseline (M0), global UPDRS-III scores and motor sub-scores did not differ between the sexes.At the 1-month follow-up, men showed significant alleviation of motor symptoms compared with baseline, including global motor performance (P
< 0.0001) and four sub-items (P
< 0.005 for every item)in the On-med and Off-med conditions.In women, motor symptoms were improved significantly in the Off-med condition (P
< 0.001 for every item) but similar to preoperative levels in the On-med condition.The analogous results were consistent at the 12-month follow-up assessment (Figure 1).Both sexes achieved motor amelioration in the Off-med condition.In the On-med condition, men exhibited significant improvements in tremor (P
= 0.0099),rigidity (P
= 0.0014), and bradykinesia (P
= 0.0295), and a significant reduction in the on-off change in bradykinesia (P
< 0.0001) and axial symptoms (P
<0.0001) compared with the baseline.In contrast, no differences were found among the women under the On-med condition, compared with the baseline(Table 2).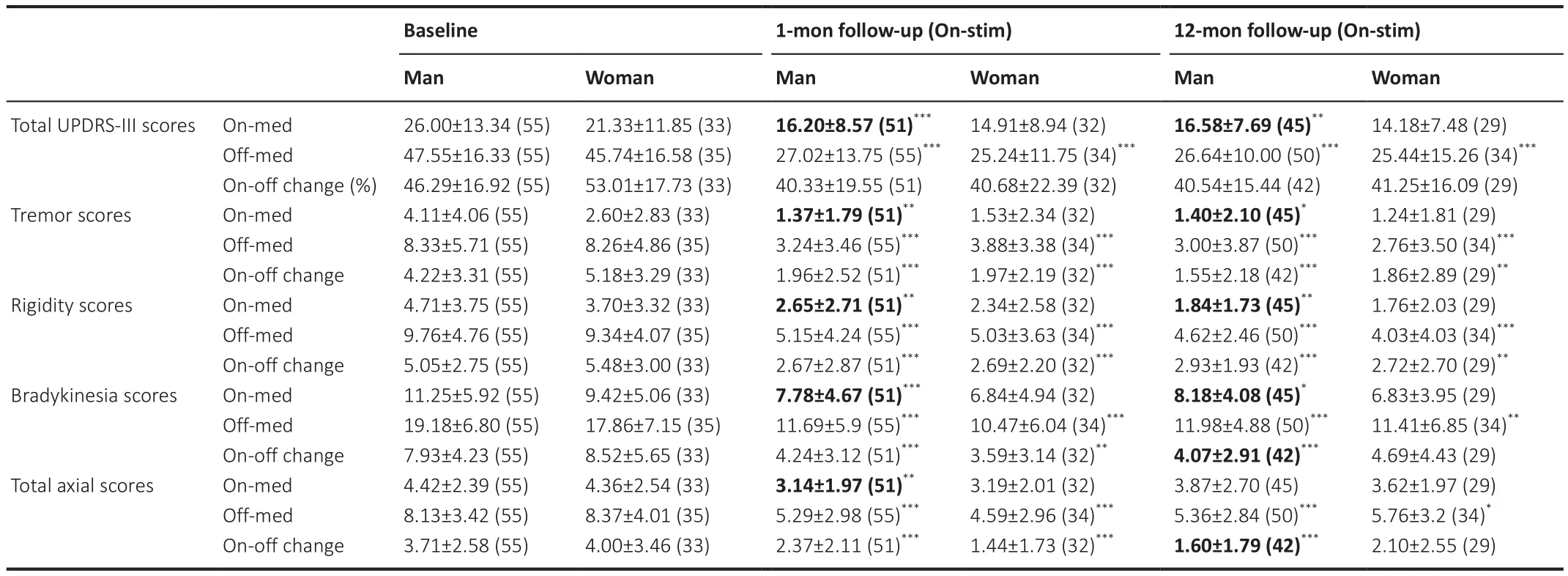
Table 2 |Changes in motor performance in the included patients from baseline to postoperative follow-up
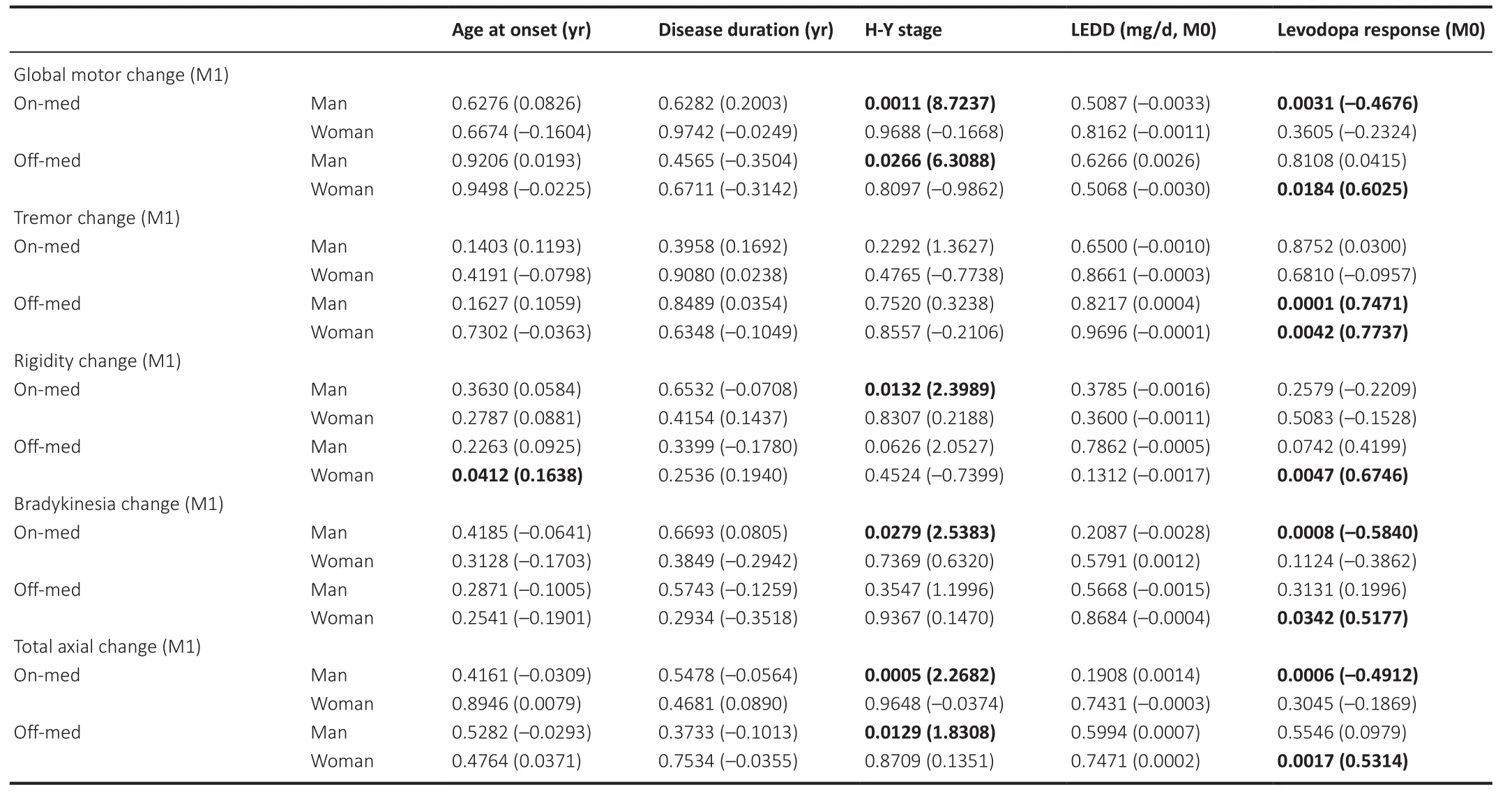
Table 3|Results of generalized linear model analyses for factors related to initial efficacy of subthalamic nucleus deep brain stimulation on motor symptoms
The GLMs showed that the H-Y stage was significantly and positively correlated with the initial (1 month post-operation) and chronic (12 months post-operation) effects of STN-DBS (the stimulation effects are summarized in Additional Table 3) on motor function in men, including changes in global motor performance, rigidity, bradykinesia, and axial symptoms (P
<0.05, see Table 3 and Table 4 for details).However, only improvements in axial symptoms in the On-med condition were significantly and positively correlated with the H-Y stage among women (P
= 0.0177).In contrast,although the LEDD was negatively correlated with the alleviation of motor dysfunction significantly, as well as with motor subscores among women in the Off-med condition at the 12-month follow-up (P
< 0.05 for every item),there were no such correlations among the men (Table 4).Age at onset was positively correlated with the initial (1 month post-operation) effect of STNDBS on rigidity in women (P
= 0.0412) but not men.The response to levodopa at the baseline was correlated with the efficacy of STN-DBS in both groups.Effects of sex on non-motor symptoms
At the baseline, we found no difference in restless legs syndrome (RLS) scores between men and women.However, women had more severe RLS scores than men at the 12-month follow-up (P
= 0.0385).Furthermore, compared with women, men showed significantly greater improvement in RLS score following STN-DBS (P
= 0.0373).Although women exhibited more severe anxious (P
= 0.0067) and depressive (P
= 0.0012) symptoms than men before surgery, these sex differences were absent at the 12-month follow-up (Figure 2), and both sexes demonstrated significant reductions in depression and anxiety compared with the baseline.Men had higher baseline MMSE scores than women, suggesting more severe cognitive impairments in women before surgery (P
= 0.0234).More details are shown in Table 5.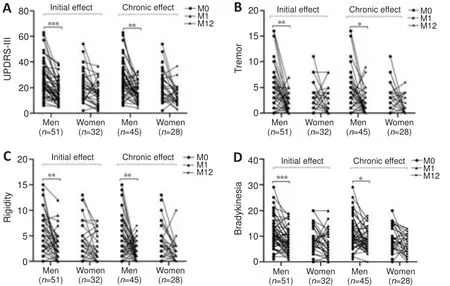
Figure 1| The initial and chronic efficacy of subthalamic nucleus deep brain stimulation for treating motor function.
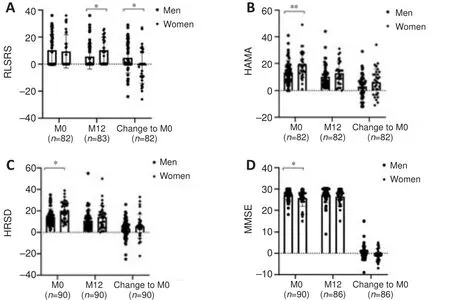
Figure 2 | Chronic effects of subthalamic nucleus deep brain stimulation on nonmotor symptoms.
Alleviation of RLS was significantly correlated with LEDD in men (P
=0.0368) but not women at the 12-month follow-up.The severity of RLS was significantly and positively correlated with anxiety in both sexes (P
< 0.05), but was associated with depressive symptoms in men only (P
< 0.05).Moreover,depression in men but not women was correlated with age at onset (P
=0.0094), disease duration (P
= 0.0156), and H-Y stage (P
= 0.0007) at baseline.MMSE scores were positively correlated with education in both sexes.More details are shown in Additional Table 1.
Table 4 |Results of generalized linear model analyses for factors related to chronic efficacy of subthalamic nucleus deep brain stimulation on motor symptoms
Effects of sex on quality of life
Table 6 summarizes the PDQ-39 results for men and women at baseline and following STN-DBS.At the baseline, women had lower emotional wellbeing than men (P
= 0.0274), while no difference was found between the sexes at the 12-month follow-up.Both men and women showed significant improvements in ADL but only men reported improvements in mobility (P
=0.0027), stigma (P
= 0.042), and PDQ-39 SI (P
= 0.0032) after STN-DBS.The results of the GLM (summarized in Additional Table 2) revealed no significant sex-specific factors influencing quality of life.Effects of sex on dopaminergic therapy and stimulation intensity
Although neither stimulation intensity (TEED) nor dopaminergic dose (LEDD)differed significantly between the sexes at the 12-month follow-up, we observed a sustained reduction in LEDD in both sexes (P
< 0.001; Table 1).We established GLMs of stimulation intensity and dopaminergic therapy at the 12-month follow-up for each sex (Table 7).In men, greater stimulation intensity was correlated with more advanced H-Y stage (P
= 0.0032) and lower on-off change (P
= 0.0036), while no factors related to TEED were identified in women.In both sexes, patients using higher antiparkinsonian medication doses exhibited significant greater motor symptom changes between the Onmed and Off-med conditions (P
< 0.05).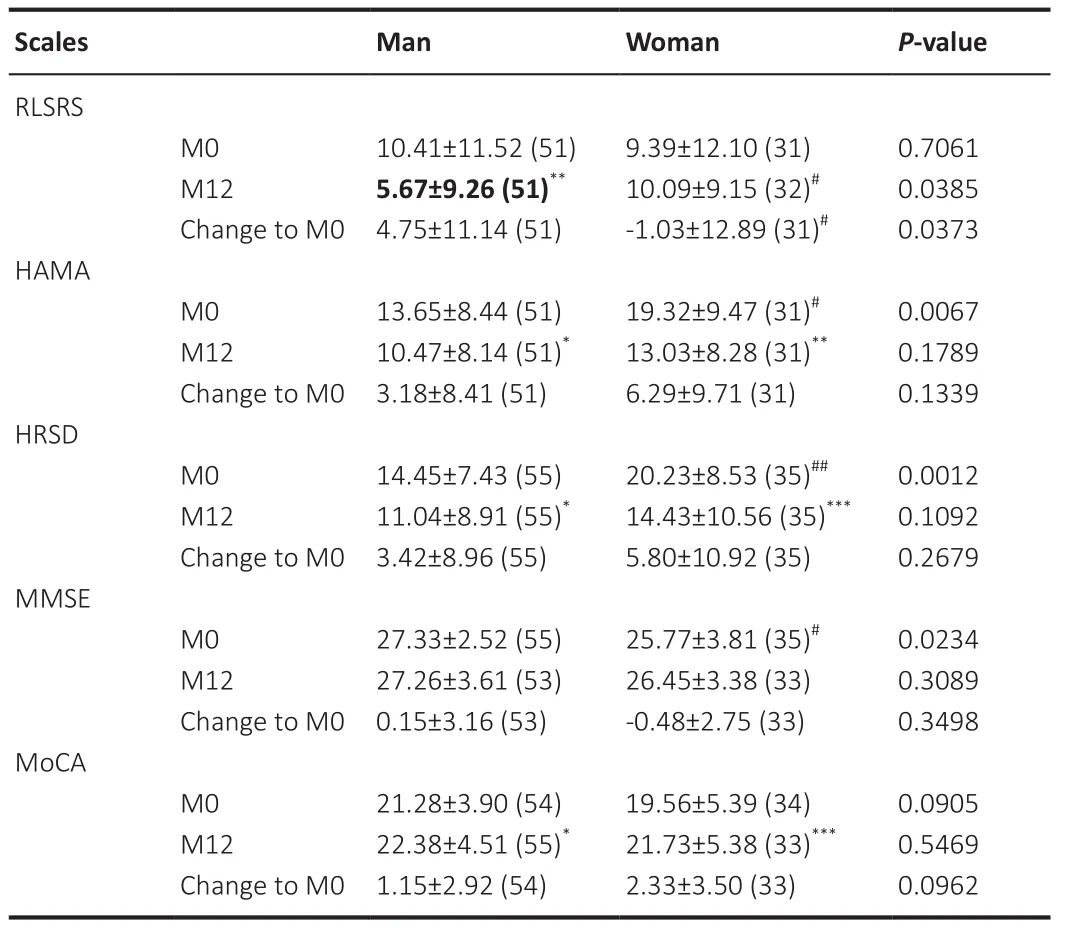
Table 5 |Efficacy of STN-DBS on non-motor performances
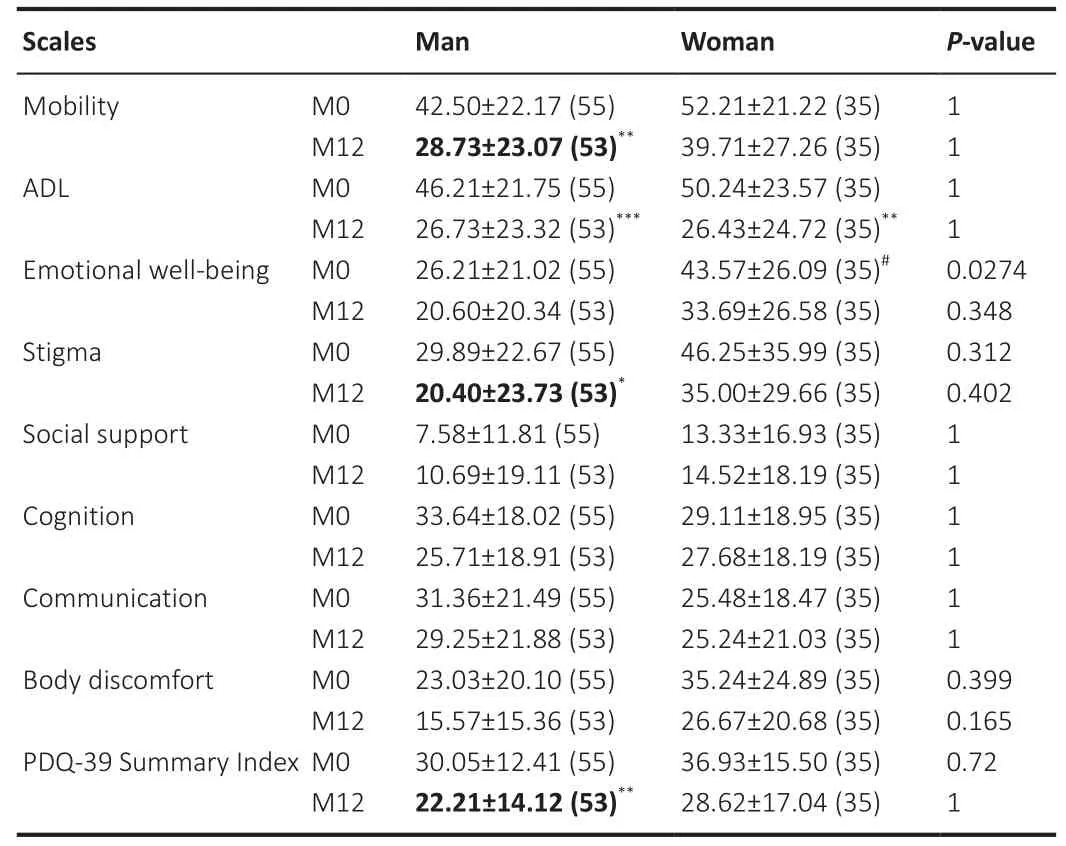
Table 6 |Efficacy of STN-DBS on quality of life assessed using PDQ-39

Table 7 |Results of generalized linear model analyses for factors related to stimulation intensity and dopaminergic therapy
Discussion
Our data demonstrate that sex influences the therapeutic effects of STNDBS on various PD motor and non-motor symptoms, drug responses, and even quality of life.These differences emerged despite similar baseline conditions, including age at onset, disease duration, and disease severity (H-Y stage), as well as significant improvements in both sexes compared with the preoperative state.These sex differences should be considered in patient selection and comprehensive treatment planning.
A PD diagnosis is based primarily on the presence of motor symptoms, and motor deficits are the predominant symptoms in most patients throughout the initial disease course (Guan et al., 2022).Thus, differential effects of STN-DBS on motor symptoms could have a large influence on sex-associated outcome disparities.Accolla et al.(2007) found that bradykinesia in women was less responsive to both pharmacological and neurosurgical treatment one year after electrode implantation.Similarly, Romito et al.(2010) reported higher motor responsiveness in men compared with women at a 1-year follow-up.While previous studies have focused on different motor symptoms,collectively, these findings suggest that motor symptom improvement following STN-DBS is greater in men than in women.In the current study,men but not women showed significant alleviation of global motor function and sub-items during combined STN-DBS and dopaminergic therapy at 1-month and 12-month follow-ups, consistent with previous studies.The onmedication condition represented the optimal state in the patients’ daily life,although the period of improved motor function (on-time) was decreased in advanced patients.Our data demonstrate that only men achieved motor improvement during the on-time after STN-DBS, which might result from sex differences in the response to dopaminergic therapy (Kompoliti et al.,2002).Previous studies have reported that women have a greater risk of motor fluctuations that take less time to develop than in men, which also indicates the sex differences in the effects of levodopa treatment (Warren Olanow et al., 2013).In conclusion, these findings suggest that men receive more benefits than women from STN-DBS, particularly in the on-medication condition.
Approximately 10-50% of PD patients experience RLS (Klepitskaya et al.,2018), and Martinez-Martin et al.(2012) found that RLS was more common and severe in women than in men.A previous report described a significant decrease in RLS symptoms after STN-DBS (Klepitskaya et al., 2018), but sex disparities were not explored.In the present study, we found no sex differences in RLS at the baseline, but men showed greater improvement in RLS than women at the 12-month follow-up.As neither LEDD nor TEED differed between sexes at this time, the greater RLS response to STN-DBS in men may arise from genetic, environmental, or hormonal influences(Manconi et al., 2012).Depression and anxiety are among the most common and consequential non-motor symptoms of PD.In accordance with several previous studies, we found that women had higher Hamilton Anxiety Rating Scale and Hamilton Rating Scale for Depression scores than men before surgery (Fernandez et al., 2000; Song et al., 2014).Regression analyses revealed that the effects of DBS on depression and anxiety were associated with other clinical factors, notably RLS, which was positively correlated with the improvement in the severity and degree of depression and anxiety in men as well as anxiety in women.Thus, RLS could be a potential indicator to guide treatment, and PD patients with severe RLS should be assessed to determine whether they have comorbid mood disorders.Finally, men scored significantly higher on the MMSE than women, in line with other studies (Song et al.,2014).This might relate to the higher level of education in men than women in the sample.
Accolla et al.(2007) reported that the ADL after STN-DBS improved to a greater extent in women than in men.Similarly, Hariz et al.(2013) found that health-related quality of life improved to a greater extent in women according to the PDQ-39.In the present study, women reported lower emotional wellbeing than men before surgery, and exhibited significant improvements in the ADL after STN-DBS.In contrast, men reported a positive effect of STNDBS on mobility, ADL, stigma, and PDQ-39 SI at the 12-month follow-up.Taken together, these findings show that women with PD experience more difficulties, especially in terms of emotional health, and benefit less from STN-DBS than men in daily life.A previous study involving 7209 participants with PD reported that women received fewer caregiving resources and had a poorer health-related QOL than men according to the PDQ-39 (Dahodwala et al., 2018).Women with PD may face greater difficulties because they shoulder a larger share of the caregiving burden and are more likely to be living alone because of their longer life expectancy (Wanneveich et al., 2018).These burdens that associated with increased stress could influence how women self-report their symptoms and how they act in a clinical assessment, which might result in higher rates of anxiety and depression, and ultimately a poorer quality of life.
One of the factors most strongly recognized as related to STN-DBS efficacy is levodopa responsiveness.Welter et al.(2002) proposed that, among advanced cases of PD, the ideal candidate for STN-DBS is highly responsive to levodopa without levodopa-induced side effects.To our knowledge, few studies have examined sex-specific factors influencing STN-DBS efficacy on motor function.The GLMs indicated that the H-Y stage, which is widely used to describe PD progression (Suo et al., 2017), was a sex-specific factor for men.Men with a more advanced H-Y stage achieved greater amelioration of motor symptoms at both the 1-month and 12-month follow-ups.Thus, STNDBS might improve motor function even in men with advanced PD, while women with severe PD might be more likely to experience dyskinesia and other complications (Zappia et al., 2005).Similarly, among women, LEDD was negatively correlated with STN-DBS efficacy for motor performance in the Off-med condition at the 12-month follow-up only, suggesting that women requiring higher doses of levodopa will show less long-term motor improvement following STN-DBS.Finally, H-Y stage was also associated with stimulation intensity in men.Both men and women receiving higher LEDD exhibited a greater on-off change, but this change was negatively correlated with stimulation intensity in men only.Thus, higher stimulation intensity might help reduce drug-related motor fluctuations in men.Collectively, these sex-specific factors may contribute to the observed disparities in disease progression, rates of complication emergence, and treatment responsiveness between men and women.
There are documented structural differences between male and female brains that could, in principle, contribute to sex-specific STN-DBS responses.For instance, a multimodal neuroimaging study reported distinct patterns of regional brain atrophy and altered connectivity between male and female PD patients (Tremblay et al., 2020), and an electrophysiological survey reported sex differences in the human basal ganglia response to levodopa (Marceglia et al., 2006).Sex chromosome genes may also contribute to disparities in PD.Czech et al.(2012) reported that the sex-determining region on the Y chromosome (SRY) was expressed in brain regions with enriched dopamine,such as the substantia nigra and ventral tegmental area.This suggests that SRY genes might directly influence the nigrostriatal dopaminergic system (Vegeto et al., 2020).Moreover, like many other aspects of behavior, cognition, and neuropharmacology, sex hormones could theoretically contribute to sex differences in PD expression and therapeutic response, although there is little experimental support for any specific mechanism.Estrogens have been reported to protect dopaminergic neurons in PD models (Thadathil et al.,2021), but other studies found no such effect (Miller and Cronin-Golomb,2010).Hormonal cycle changes in women may also influence PD symptoms,and young women with PD were reported to have symptoms that fluctuated with their menstrual cycles (Kompoliti et al., 2000).In sum, these findings support the idea that the development of PD may be influenced by different factors in men and women, resulting in unique symptom expression profiles.
This exploration of sex differences in STN-DBS efficacy could help with patient selection, prognosis, comprehensive patient management, and the development of stimulus programming regimens to optimize therapeutic benefits for male and female patients.However, our analysis has several limitations.First, the follow-up period in this study encompassed the first year after the stimulator implant.However, longer follow-up periods are needed to verify the long-term effects of sex differences.Second, mechanistic studies with animal models and minimally invasive studies on human patients (e.g.,using functional MRI, near-infrared spectroscopy, electroencephalography,blood biochemistry) are needed to help identify the potential mechanism underlying the observed sex differences in DBS response.
Conclusion
This study identified numerous sex-specific differences in outcomes after STNDBS for PD treatment.Initial and chronic improvements in motor symptoms were detected in men but not women in the on-medication condition, and women exhibited poorer amelioration of RLS than men at a 12-month followup assessment.Furthermore, PD had a greater impact on the quality of life of women both before and after STN-DBS.Finally, H-Y stage was correlated with motor symptom improvement in men, while LEDD was associated with motor response in women only.This information may be valuable for prognoses and the promotion of reasonable expectations for the outcome of STN-DBS.
Author contributions:
Tian-Shuo Yuan, Ying-Chuan Chen: Conceptualization,Methodology, Writing-Original Draft.De-Feng Liu, Ting-Ting Du, Ruo-Yu Ma,and Xin Zhang: Software, Investigation.Guan-Yu Zhu, and Jian-Guo Zhang:Conceptualization, Funding Acquisition, Resources, Writing-Review & Editing.All authors approved the final version of the paper.
Conflicts of interest:
The authors declare no conflicts of interest.
Availability of data and materials:
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Workflow of patient identification.
Ethical Approval Documentation (Chinese).
Results of generalized linear model analyses for factors related to STN-DBS efficacy on non-motor symptoms.
Results of generalized linear model analyses for factors related to STN-DBS efficacy on quality of life.
Efficacy of STN-DBS on motor performance at postoperative follow-up.
- 中国神经再生研究(英文版)的其它文章
- Neural and Müller glial adaptation of the retina to photoreceptor degeneration
- Agomelatine: a potential novel approach for the treatment of memory disorder in neurodegenerative disease
- MicroRNAs: protective regulators for neuron growth and development
- In vivo astrocyte-to-neuron reprogramming for central nervous system regeneration: a narrative review
- Intranasal nerve growth factor for prevention and recovery of the outcomes of traumatic brain injury
- Altered O-GlcNAcylation and mitochondrial dysfunction,a molecular link between brain glucose dysregulation and sporadic Alzheimer’s disease

