Regenerative peripheral nerve interface prevents neuroma formation after peripheral nerve transection
Zheng Wang, Xin-Zeyu Yi, Ai-Xi Yu
Abstract Neuroma formation after peripheral nerve transection often leads to severe neuropathic pain.Regenerative peripheral nerve interface has been shown to reduce painful neuroma in the clinic.However, no reports have investigated the underlying mechanisms, and no comparative animal studies on regenerative peripheral nerve interface and other means of neuroma prevention have been conducted to date.In this study, we established a rat model of left sciatic nerve transfection, and subsequently interfered with the model using the regenerative peripheral nerve interface or proximal nerve stump implantation inside a fully innervated muscle.Results showed that, compared with rats subjected to nerve stump implantation inside the muscle, rats subjected to regenerative peripheral nerve interface intervention showed greater inhibition of the proliferation of collagenous fibers and irregular regenerated axons, lower expressions of the fibrosis marker α-smooth muscle actin and the inflammatory marker sigma-1 receptor in the proximal nerve stump, lower autophagy behaviors,lower expressions of c-fos and substance P, higher expression of glial cell line-derived neurotrophic factor in the ipsilateral dorsal root ganglia.These findings suggested that regenerative peripheral nerve interface inhibits peripheral nerve injury-induced neuroma formation and neuropathic pain possibly via the upregulation of the expression of glial cell line-derived neurotrophic factor in the dorsal root ganglia and reducing neuroinflammation in the nerve stump.
Key Words: autotomy; dorsal root ganglia; glial cell line-derived neurotrophic factor; nerve injury; neuropathic pain; peripheral nerve; regeneration;regenerative peripheral nerve interface; retrograde axonal transport; traumatic neuroma
Introduction
A traumatic neuroma may develop from abnormal axonal regeneration and organization following peripheral nerve injury or amputation.The exact incidence of neuroma formation following injury remains unclear, although postoperative, symptomatic neuroma rates range from 1% to 30% (Neumeister and Winters, 2020).A symptomatic neuroma mainly causes abnormal hyperpathia or intractable pain, which severely decreases the quality of life of patients, especially amputees, because the neuroma can be stimulated by prostheses, resulting in patients abandoning their use.To date, various surgical treatments have been used to prevent the formation of traumatic neuromas, such as the implantation of a proximal nerve stump (PNS) into the muscle or vein (Balcin et al., 2009; Prasetyono et al., 2014) and covering the PNS with an acellular nerve allograft (Pan et al., 2021) or tissue-engineered cap (Onode et al., 2019; Zhou et al., 2019; He et al., 2020; Pi et al., 2022).Currently, however, no consensus on the optimal technique for providing long-term benefits is available.
The regenerative peripheral nerve interface (RPNI) was recently reported as a reproducible and practical surgical procedure to reduce painful neuroma formation in the clinic (Kubiak et al., 2018, 2019; Hooper et al., 2020).An RPNI is constructed by implanting a PNS into a free skeletal muscle graft and was originally designed to transduce and amplify neural signals for controlling a neuroprosthetic limb (Kung et al., 2014; Sando et al., 2016; Frost et al.,2018; Vu et al., 2020).The use of free muscle grafts during RPNI surgery offers a vast supply of denervated muscle targets for regenerating axons sprouting from the PNS (Santosa et al., 2020; Hu et al., 2021).This distinguishing characteristic differentiates the RPNI from the conventional technique of implanting a PNS into the adjacent muscle because the regenerating axons of a PNS cannot create functional connections with an already innervated local muscle belly (Vu et al., 2020).In contrast, RPNIs are capable of generating compound muscle action potentials of substantive amplitude as early as 1 month after implantation, which indicates that new neuromuscular junctions(synaptogenesis) form concurrently within the muscle graft (Kung et al., 2014;Ursu et al., 2016; Vu et al., 2020).Thus, fewer regenerating axons will be available to form a stump neuroma.However, to the best of our knowledge,there were only clinical studies comparing RPNI with other conventional methods for the treatment of painful neuromas (Kubiak et al., 2019; Hoyt et al., 2021).Moreover, these studies assessed pain relief without histological data on the outcomes of neuroma formation.Thus, detailed mechanisms of the RPNI in inhibiting neuroma pain remain unclear.Several mechanisms may be involved in this type of neuropathic pain, such as peripheral and central sensitization, structural changes, and abnormally high expressions of α-smooth muscle actin (α-SMA) and sigma-1 receptor (S1R) in the neuroma(Shen et al., 2017; Lu et al., 2018).α-SMA is involved in painful neuromas as an indirect marker of local mechanical stimuli, whereas S1R is a molecular biomarker of nerve injury and neuroinflammation, and both play active roles in neuroma-associated pain (Yan et al., 2012; Onode et al., 2019).Changes in the expression of neurotrophins (NTs) following nerve transection have also received increasing attention.Following nerve injury, retrograde axonal signaling via molecular kinases decreases the expression of regenerationassociated glial cell line-derived neurotrophic factor (GDNF), proximally within the sensory neurons of the dorsal root ganglia (DRG) and injury sites (Nagano et al., 2003; Shi et al., 2011).Previous studies have shown that GDNF is mainlyproduced by skeletal muscle in the peripheral nervous system (Suzuki et al.,1998; Wehrwein et al., 2002; Zhang and Song, 2012) and can alleviate or even eliminate neuropathic pain following peripheral nerve injury (Ossipov,2011; Zhong et al., 2020).As mentioned above, neuromuscular junctions can form between the muscle graft and the PNS after RPNI surgery.Thus,we speculated that the reinnervated muscle, which serves as a target organ,could secrete target-derived GDNF, which may be retrogradely transported to the cell bodies of neurons in the DRG and alleviate neuropathic pain following peripheral nerve transection.
In this study, we established a rat model of sciatic nerve stump neuroma to compare the RPNI with PNS implantation inside a fully innervated muscle, a common PNS management approach for preventing painful neuroma in the clinic, by histologically evaluating which technique generates less neuroma.In addition, we investigated the effect of RPNI surgery on neuropathic pain relief and whether this effect is associated with changes in GDNF expression within the ipsilateral DRG.
Methods
Ethics statement
All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, Eighth Edition (2011) and approved by the Experimental Animal Welfare Ethics Committee of Zhongnan Hospital of Wuhan University under animal protocol number ZN2021094 on July 22, 2021.All experiments were designed and reported according to the Animal Research: Reporting ofIn Vivo
Experiments(ARRIVE) guidelines (Percie du Sert et al., 2020).Animal grouping
Ninety male specific-pathogen-free Sprague-Dawley rats aged 6-8 weeks and weighing 200-250 g were purchased from the Experimental Animal Center of Wuhan University, Wuhan, China (animal use license No.SYXK [E] 2020-0025).Animals were kept in groups of three in plastic cages with floor covered with soft bedding at 25°C with 50-60% relative humidity and maintained on a light/dark cycle of 12-hour day/night.Food and water were made available ad libitum.The rats were randomly divided into three groups: no treatment(control,n
= 30), nerve stump implantation inside a fully innervated muscle(NSM,n
= 30), or nerve stump implantation inside a free muscle graft (RPNI,n
= 30).All rats in each group were randomly divided into two subgroups and sacrificed at 4 (n
= 10) or 8 weeks (n
= 20) after surgery.Twenty rats in each group were used for behavioral analyses once per week after surgery.At 4 weeks post-surgery, five rats in each group were sacrificed for histological and immunohistochemical analyses, and the remaining five rats in each group were sacrificed for western blot analysis.Similarly, after completing the behavioral analyses at 8 weeks post-surgery, 10 of the 20 rats in each group used for behavioral analyses were randomly selected for histological,immunohistochemical, and western blot analyses (Additional Figure 1).Surgical procedures
The rats were anesthetized using intraperitoneal pentobarbital sodium (1%, 5 mL/kg; Sigma-Aldrich, St.Louis, MO, USA).The left sciatic nerve was exposed using the posterior thigh approach and sharply transected 1 cm from the original branch of the posterior gluteal nerve.Then, approximately 1-1.5 cm of the distal nerve stump was removed to create a gap between the ends to prevent spontaneous reinnervation.In the RPNI group, a free muscle graft measuring approximately 1.0 × 0.5 × 0.3 cmwas harvested from the nearby adductor magnus muscle along the direction of the muscle fibers.The PNS was placed in the middle of the muscle graft, parallel to the muscle fibers,and secured distally with 10-0 nylon sutures in an epimysium-to-epineurium fashion.The muscle graft edges were then wrapped around the PNS and secured with 7-0 nylon sutures to complete the construction of the RPNI(Figure 1A).In the NSM group, the PNS was implanted into a muscle sac of the nearby adductor magnus muscle through a small incision along the direction of the muscle fibers.The epineurium of the sciatic nerve was fixed with the epimysium by two 10-0 nylon sutures (Figure 1B; Prasetyono et al., 2014).In the control group, the PNS was leftin situ
with no treatment (Figure 1C).The left leg incision was closed in layers using 4-0 sutures in all groups.When the animals recovered from anesthesia, they were injected subcutaneously with a meloxicam analgesic (0.2 mg/mL/kg; Boehringer-Ingelheim, Mainz-Bingen,Germany).Each rat recuperated for 4 weeks to facilitate wound healing and reinnervation of the free muscle graft.Histological and immunohistochemical analyses
At 4 and 8 weeks after surgery, five rats in each group were anesthetized by intraperitoneal pentobarbital sodium and sacrificed by cervical dislocation.The sciatic PNSs (n
= 5) were collected and processed for histological analysis.The specimens were fixed in 4% paraformaldehyde at 4°C and cut into 5-μm transverse sections.We then randomly selected 20 sections from each group for subsequent staining analysis.To label the collagenous fibers, the sections were deparaffinized and stained using Masson’s Trichrome Stain Kit (Baiqiandu,Wuhan, China) following the manufacturer instructions.Briefly, the samples were placed in Bouin’s solution for 40 minutes, Weigert’s hematoxylin solution for 10 minutes, Biebrich scarlet solution for 10 minutes, phosphotungstic acid-phospholimbic acid solution for 15 minutes, and aniline blue solution for 2 minutes.The samples were washed with running water before each change in dye solution.Finally, samples were rinsed in a 1% acetic acid bath,treated with alcohol for rapid color separation, permeabilized with xylene,and mounted.To label the regenerative axons, the sections were stained with 1% toluidine blue (Baiqiandu) for 20 minutes and subsequently treated with alcohol for rapid color separation, permeabilized with xylene, and mounted.For the immunohistochemistry assay, an anti-α-SMA antibody (fibrosis marker;mouse, 1:200, Boster, Wuhan, China, Cat# BM0002, RRID: AB_2811044) was used to evaluate myofibroblast activity, and an anti-S1R antibody (rabbit;1:500, Proteintech, Beijing, China, Cat# 15168-1-AP, RRID: AB_2301712) was used to examine neuroinflammation in the PNS.Briefly, the sections were deparaffinized and rehydrated.After antigen retrieval, the sections were blocked with 3% bovine serum albumin for 30 minutes, incubated with an anti-α-SMA antibody or anti-S1R antibody overnight at 4°C, and incubated with an anti-mouse immunoglobulin G (IgG) secondary antibody (1:200, KPL,Gaithersburg, MD, USA, Cat# 5220-0341, RRID: AB_2891080) or an anti-rabbit IgG secondary antibody (1:200, KPL, Cat# 5220-0336, RRID: AB_2857917) for 1 hour at 25°C.
The positive areas were morphometrically analyzed using the ImageJ software 1.8 (National Institutes of Health, Bethesda, MD, USA) (Schneider et al.,2012).The percentage of the collagen fiber area, transverse nerve fibers, and α-SMA- and S1R-positive areas were calculated as the positive staining area/the total image area × 100%.
Behavioral analysis
The autotomy behaviors of the rats in each group (n
= 20) were monitored once per week for 8 weeks to assess neuropathic pain levels.The scoring scale devised by Wall et al.(1979) was used to document points based on the severity of autotomy.Briefly, 1 point was given for the removal of two or more toenails; 1 point was added for the attack of each distal half digit; 1 extra point was assigned when a proximal half of each digit was lesioned.Thus, if all digits were attacked, a score of 11 was given.The percentages of the rats with autotomy in each group were also calculated.Western blot analysis
At 4 and 8 weeks after surgery, five rats in each group were sacrificed for western blot analysis to evaluate the expression of substance-P, c-fos, and GDNF from the pooled L4-L5 DRG (n
= 5).Equal amounts of proteins from the DRG were loaded onto 5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes(Millipore, Billerica, MA, USA).The membranes were blocked in 5% nonfat dry milk (Baiqiandu) in Tris-buffered saline Tween (Baiqiandu) buffer for 1 hour at 25°C and incubated at 4°C overnight with monoclonal antibodies to detect substance-P (rabbit; 1:2000, Affinity, Cincinnati, OH, USA, Cat#DF7522, RRID: AB_2841021), c-fos (mouse; 1:1000, Santa, Dallas, TX, USA,Cat# sc-166940, RRID: AB_10609634), and GDNF (rabbit; 1:1000, ABclonal,Boston, MA, USA, Cat# A14639, RRID: AB_2761514).In addition, β-actin(rabbit; 1:1000, Abcam, Cambridge, UK, Cat# ab227387, RRID: AB_2909447)was used as an internal control.After washing in Tris-buffered saline Tween three times, the membranes were incubated with a secondary antibody,horseradish peroxidase-conjugated goat anti-rabbit IgG (1:5000, KPL, Cat#5220-0336, RRID: AB_2857917) or horseradish peroxidase-conjugated goat anti-mouse IgG (1:5000, KPL, Cat# 5220-0341, RRID: AB_2891080), at 25°C for 30 minutes.Membranes were washed three times in Tris-buffered saline Tween, and the labeled protein bands were visualized using an Enhanced Chemiluminescence Detection Kit (Baiqiandu) and analyzed using the ImageJ software.The relative levels of substance-P, c-fos, and GDNF were normalized to β-actin.Statistical analysis
The sample size was largely determined by similar previous publications(Onode et al., 2019; Zhou et al., 2019; He et al., 2020; Pi et al., 2022).No animals or data points were excluded from the analysis.Histological analyses were conducted in a blinded manner.All relevant data are presented as means± standard deviations (SDs), and GraphPad Prism 7.0 (GraphPad Software,San Diego, CA, USA, www.graphpad.com) was used for data analysis.For comparisons among the three groups, we used a one-way analysis of variance followed by Tukey’spost hoc
test.Statistical significance was set atP
< 0.05.Results
RPNI inhibits neuroma formation following sciatic nerve transection as assessed by macroscopic appearance and histopathology
To observe the growth of neuromas in each group, the PNS was assessed by macroscopic and histological examination.Four weeks after the surgery,considerable swelling of the PNS was observed in both the control and NSM groups, whereas the PNS in the RPNI group remained similar to a normal nerve.Typical bulbous neuromas were formed 8 weeks after neurectomy in the control and NSM groups.In contrast, none of the rats in the RPNI group developed bulbous neuromas after neurotomy (Figure 1D-I).
Masson’s trichrome staining showed blue-stained collagenous fibers in the neuromas.At 4 weeks post-surgery, there was a large number of irregular and haphazard blue-stained collagenous fibers in the control and NSM groups.Muscle tissues also grew into the PNS in the NSM group.Furthermore, the collagenous fibers gradually proliferated, gathered together, and wrapped around the nerve fibers over time in both the control and NSM groups.In the NSM group, more muscle tissues invaded the nerve stump at 8 weeks postsurgery than at 4 weeks post-surgery.In contrast, no obvious collagenous fibers or muscle tissues were observed in the RPNI group at 4 or 8 weeks post-surgery (Figure 2).

Figure 1| Effect of the RPNI on neuroma prevention as evaluated by macroscopic appearance.
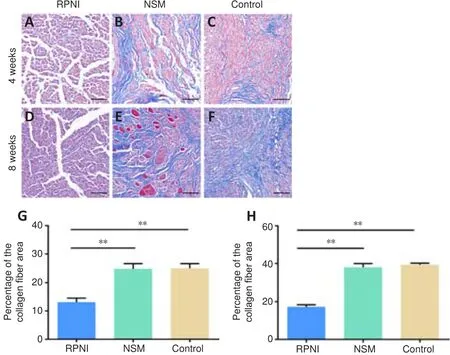
Figure 2 | Effect of the RPNI on the inhibition of the proliferation of collagenous fibers in the PNS.
The fibrosis marker α-SMA in the PNS was evaluated using immunohistochemistry staining.At 4 weeks post-surgery, there were no significant differences in the percentage of the α-SMA-positive area in the PNS among the three groups (allP
> 0.05).However, the percentage of the α-SMA-positive area in the control and NSM groups increased significantly at 8 weeks post-surgery, and was significantly higher than that in the RPNI group(bothP
< 0.01; Figure 3).Toluidine blue staining results showed that the regenerated nerve fibers(stained in blue) were densely and chaotically distributed in the NSM and control groups at 4 weeks post-surgery, whereas those in the RPNI group maintained clear and regular morphology.At 8 weeks after axotomy, further regeneration of axons occurred, including many transverse and longitudinal nerve fibers, many of which were clustered together in the NSM and control groups.In contrast, most nerve fibers in the RPNI group were normally sized,regularly distributed, and arranged in an insulated pattern.Furthermore,only a small number of transverse regenerated axons were observed in the RPNI group (Figure 4).Altogether, these findings suggested that RPNI surgery inhibits the accumulation of collagenous fibers and the disordered sprouting of axons at the injury site following neurectomy.

Figure 3 | Effect of the RPNI on the inhibition of myofibroblast activity in the PNS.
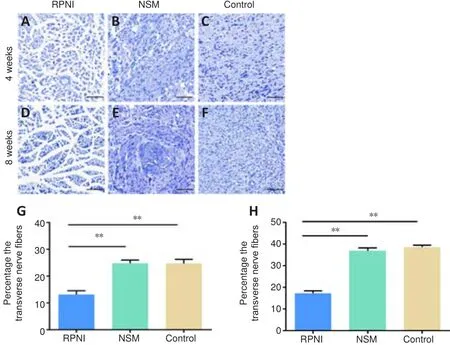
Figure 4 | Effect of the RPNI on the inhibition of the proliferation of irregularly regenerated axons in the PNS.
RPNI reduces S1R immunopositivity in the PNS after sciatic nerve transection
To evaluate the neuroinflammation of the PNS in each group, we examined S1R expression using immunohistochemistry staining.Histomorphological analysis showed that the percentage of the S1R-positive area in the RPNI group was significantly lower than that in the NSM and control groups at 4 and 8 weeks post-surgery (allP
< 0.01).In contrast, the percentage of the S1R-positive area in the NSM group was significantly lower than that in the control group at 8 weeks only (P
< 0.01; Figure 5).Collectively, this suggested that RPNI surgery prevents neuroinflammation following nerve transection and offers a better anti-inflammatory effect than NSM surgery.RPNI relieves autotomy behaviors following sciatic nerve transection
To evaluate neuropathic pain in each group, autotomy behavior was observed once per week for 8 weeks.The representative gross appearances of the hind paw after neurectomy without intervention, assessed by the autotomy score,are shown in Figure 6A-D.At the end of the experiment, the development rate of autotomy was 90% in the control group and 70% in the NSM group,whereas only 25% of the rats in the RPNI group showed autotomy (Figure 6E).The time-dependent changes in autotomy score in each group are shown in Figure 6F.The average autotomy score in the RPNI group was significantly lower than that in the control and NSM groups at all time points (P
< 0.01 andP
< 0.05, respectively) except for the first 2 weeks, when autotomy behaviors were similar among the three groups (allP
> 0.05).Taken together, these results indicated that RPNI surgery relieves axotomy-induced neuropathic pain.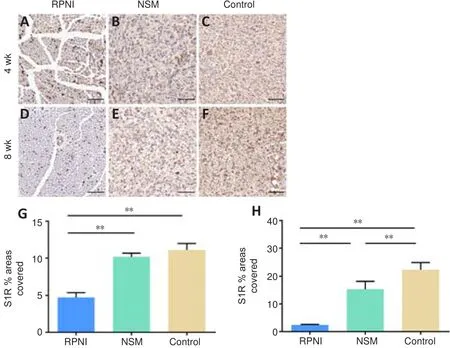
Figure 5 | Effect of the RPNI on the inhibition of neuroinflammation in the PNS.
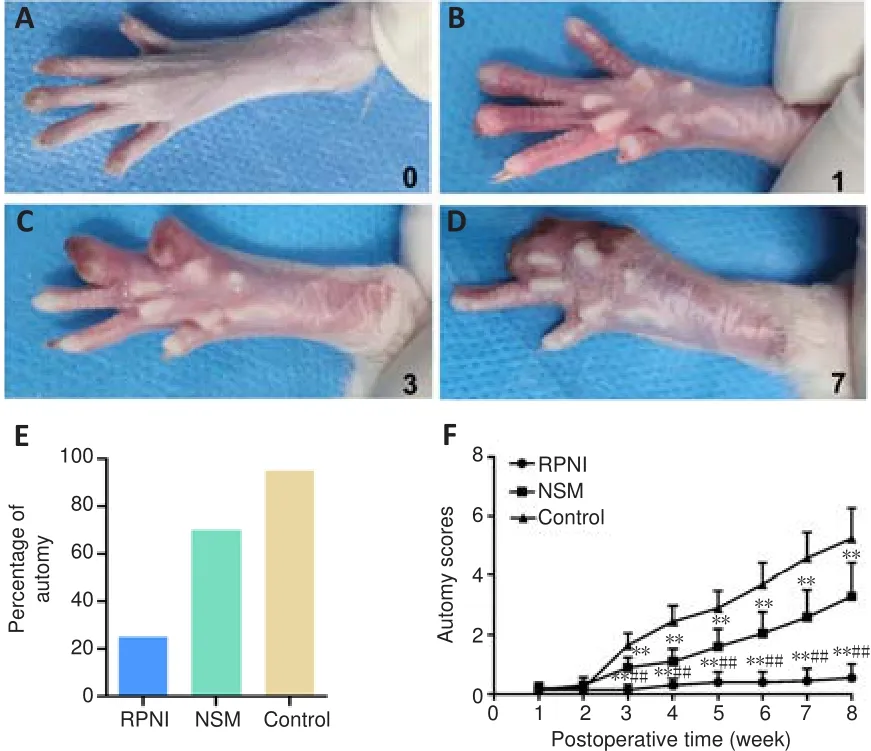
Figure 6 | Effect of the RPNI on relieving autotomy behaviors following sciatic nerve transection.
RPNI decreases the expression of pain-related markers and upregulates GDNF expression in the ipsilateral L4-L5 DRG following sciatic nerve transection
Western blot analysis was used to evaluate the expression of pain-related markers (i.e., substance P and c-fos) and GDNF in the ipsilateral DRG in each group.At 4 weeks post-surgery, significantly lower expressions of substance P and c-fos were observed in the RPNI group than in the control and NSM groups (P
< 0.01 andP
< 0.05, respectively).Similar results were obtained at 8 weeks post-surgery (bothP
< 0.01).At 4 weeks post-surgery, the expression of GDNF in the RPNI group was significantly higher than that in the control and NSM groups (bothP
< 0.01).The expression of GDNF in the RPNI group increased at 8 weeks post-surgery and remained higher than that in the control and NSM groups (P
< 0.01 andP
< 0.05, respectively).However, no significant difference in GDNF expression was found between the NSM and control groups throughout the experiment (P
> 0.05; Figure 7).Overall,these results demonstrated that RPNI surgery attenuates axotomy-induced neuropathic pain, and this effect was associated with the increased expression of GDNF in the ipsilateral DRG.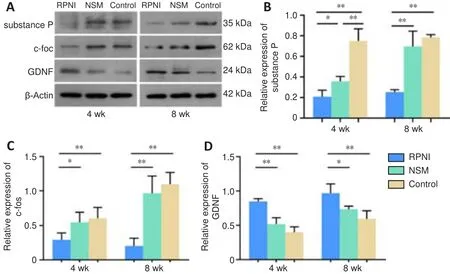
Figure 7 | Effect of the RPNI on the modulation of the expression of pain-related markers and GDNF in the ipsilateral DRG following sciatic nerve transection.
Discussion
Despite the availability of various surgical options, preventing the development of painful neuromas remains a challenge (Lu et al., 2018).We compared the prevention of painful neuroma development between NSM and RPNI surgery.We found that RPNI inhibited neuroma formation induced by nerve transection in both macroscopic appearance and histopathology and was superior to NSM surgery, a common PNS management approach to prevent painful neuromas in the clinic.Moreover, the neuroinflammation in the PNS following sciatic nerve transection was reduced after RPNI surgery.Notably, the expression of GDNF in the ipsilateral DRG was increased after RPNI surgery, which may be attributed to the modulation of pain behaviors following RPNI.
In several studies, NSM surgery reduced the incidence of pain, possibly because the recipient’s muscle belly protected the neuroma from external stimuli, such as pressure from the socket of a prosthetic device (Balcin et al., 2009; Prasetyono et al., 2014).In fact, the PNS undergoes a process of disordered axonal sprouting and elongation because the muscle belly is already fully innervated by its native motor nerve, and thus the regenerating axons of the PNS are not introduced to any targets to reinnervate (Vu et al., 2020).The eventual formation of a neuroma is inevitable following surgery.In contrast, an RPNI is constructed by implanting the PNS into a free skeletal muscle graft, which concomitantly serves as denervated targets for regenerating axons sprouting from the PNS (Kubiak et al., 2018).
In this study, we found that the distribution of the collagenous fibers was more structured and less dense in the RPNI group than in the NSM and control groups.We also found that considerable muscle tissue invaded the nerve stump in the NSM and control groups.This observation is consistent with previous studies that showed that regenerating axons wrap around a large number of surrounding tissues, including muscles, during proximal nerve axonal sprouting following nerve transection (Stokvis et al., 2010;He et al., 2020).In contrast, no obvious muscle tissues were observed in the RPNI group.Furthermore, the expression of α-SMA, which reflects myofibroblast activity (Pi et al., 2022), was lower in the RPNI group than in the other two groups.Moreover, the regenerating axons in the control group were irregular and disorganized, whereas the nerve fibers in the RPNI group maintained clear and regular morphology.These findings suggested that the physiological process of muscle reinnervation inhibits the accumulation of collagenous fibers and the growth of disordered axonal sprouting at the injury site following neurectomy.We found no significant differences in the collagenous fibers between the NSM and control groups, which indicated that implanting the PNS inside the muscle did not prevent neuroma formation.Previous studies have shown that neuroinflammation plays an active role in both central and peripheral pain modulation (Onode et al., 2019;Chen et al., 2022).S1R is a specific molecular biomarker of nerve injury/neuroinflammation, as shown in rat models of neuroma and neuropathic pain (Shen et al., 2017).S1R may be expressed during both acute and chronic inflammation phases following nerve injury and is associated with acute and“memorizing” pain (Onode et al., 2019; Bravo-Caparrós et al., 2020).Because our experiment was conducted over a long period, and neuroma formation isoften accompanied by chronic inflammation and “memorizing” pain (Kakinoki et al., 2008), we chose S1R as the neuroinflammation-related biomarker.We found that the expression of S1R in the RPNI group was significantly lower than that in the control and NSM groups at 4 and 8 weeks post-surgery.This indicated that once the muscle graft had regenerated and reinnervated 1 month after implantation, it could isolate the PNS from the inflammatory cascade for a long period.
Autotomy behaviors previously described in rats are considered a reliable equivalent of human neuropathic pain (Wall et al., 1979).In the current study, we found that the autotomy behavior was suppressed significantly more in the RPNI group than in the control and NSM groups from 2 weeks after surgery.However, because this behavior is an indirect indicator of pain,more specific pain-related markers are required for further study.To better reflect the pain status of rats following neurotomy, we selected c-fos and substance P as pain-related markers.Previous studies have demonstrated that the expression of c-fos and substance P in the DRG is significantly decreased when terminal neuroma formation or neuropathic pain is suppressed (He et al., 2020; Pan et al., 2021).Our results showed that c-fos and substance P within the DRG were significantly downregulated in the RPNI group at 4 and 8 weeks post-surgery.Taken together, these results indicated that RPNI surgery relieves neuroma-induced pain.
Some patients who undergo NSM surgery continue to experience discomfort or pain at the site where the neuroma was previously located (Kakinoki et al.,2008), which may be attributed to the modulation of NTs expression, such as GDNF, in the ipsilateral DRG (Nagano et al., 2003; Shi et al., 2011).Previous studies have shown that GDNF is produced primarily by skeletal muscles in the peripheral nervous system (Suzuki et al., 1998; Wehrwein et al., 2002)and can alleviate or even eliminate neuropathic pain following peripheral nerve injury (Ossipov, 2011; Zhong et al., 2020).To determine whether the inhibition of neuroma pain following RPNI surgery is associated with increased GDNF expression in the DRG, we examined the protein expression of GDNF in the ipsilateral DRG in all three groups.In the RPNI group, the ipsilateral DRG expressed more GDNF than it did in the control and NSM groups at 4 and 8 weeks post-surgery.We speculate that the reinnervated muscle serving as a target organ in the RPNI group was able to secrete target-derived GDNF,which was then retrogradely transported to the DRG.In contrast, there was no significant difference in GDNF expression between the NSM and control groups, which may be related to the lack of target organs of neurons in the NSM group, and thus no GDNF could be synthesized and retrogradely transported to the DRG.
Our study had several limitations.First, although we speculated that the increased GDNF in the ipsilateral DRG following RPNI surgery was produced from the reinnervated muscle and retrogradely transported, the exact mechanism underlying the GDNF elevation remains unclear.Moreover,Schwann cells may also proliferate during the formation of neuromuscular junctions after RPNI surgery.Thus, GDNF was likely derived from Schwann cells, which secrete numerous types of neurotrophic factors (Cintron-Colon et al., 2022).Future research confirming the mechanism and transport process of this molecular event is needed.Another limitation is that we only measured the expression of GDNF, and it is unclear whether the expression of other NTs, such as the nerve growth factor and brain-derived neurotrophic factor, is altered in the ipsilateral DRG following RPNI surgery.Therefore,changes in other NTs should be evaluated in future studies.Third, because estrogen can increase sensitivity to pain in female rats (Maurer et al., 2016),we only used male rats to establish an end-neuroma model to avoid the effect of sex differences on axotomy-induced pain.Further studies are necessary to assess the effect of RPNI on neuroma pain in female rats.
In summary, as a reproducible and practical surgical solution to prevent neuroma formation by leveraging a physiological biologic process, RPNI surgery could reduce the disordered accumulation of collagen fibers, irregular axonal regeneration, and neuroinflammation in the PNS.Furthermore, RPNI surgery could attenuate axotomy-induced pain, which we revealed was associated with the increased expression of GDNF in the ipsilateral DRG.This provides evidence support for the clinical prophylaxis of neuroma formation and neuropathic pain after peripheral nerve transection by RPNI surgery.
Author contributions:
Study design and conception: AXY; experiment implementation, data collection and analysis: ZW, XZY; data interpretation:ZW, XZY, AXY; manuscript draft: ZW, XZY.All authors approved the final version of this manuscript for publication.
Conflicts of interest:
The authors declare no competing interests concerning the materials or methods used in this study or the findings specified in this paper.
Availability of data and materials:
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:
Helmar C.Lehmann, University of Cologne, Germany.
Additional files:
The experiment processes.
Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Neural and Müller glial adaptation of the retina to photoreceptor degeneration
- Agomelatine: a potential novel approach for the treatment of memory disorder in neurodegenerative disease
- MicroRNAs: protective regulators for neuron growth and development
- In vivo astrocyte-to-neuron reprogramming for central nervous system regeneration: a narrative review
- Intranasal nerve growth factor for prevention and recovery of the outcomes of traumatic brain injury
- Altered O-GlcNAcylation and mitochondrial dysfunction,a molecular link between brain glucose dysregulation and sporadic Alzheimer’s disease

