Nilotinib: from animal-based studies to clinical investigation in Alzheimer’s disease patients
Annalisa Nobili, Marcello D’Amelio, Maria Teresa Viscomi
Since their first description in the brains of patients suffering from Alzheimer’s disease (AD), more than 100 years ago, extracellular amyloid-β (Aβ)plaques and intracellular neurofibrillary tangles have been the principal focus of AD research.However, this focus has led to the failure of several long and promising clinical trials, and the efficacy of new Aβ-targeting drugs to slow down the disease progression is still controversial despite being successful in reducing the Aβ load.
Thus, despite the discouraging results, the lessons that have been learned from the Aβ debacle have prompted studies focusing on new molecular targets that regulate different cellular pathways and have formulated new hypotheses for investigating the involvement of different brain regions beyond the temporal lobe, including the hippocampal region.
There is evidence that several tyrosine kinases and their receptors are upregulated in the postmortem brains of patients with AD.Among them, the members of the Abelson (Abl) tyrosine kinase and discoidin domain receptor families have attracted a huge amount of attention in the last years.Indeed, it has been established that the activation of both Abl and discoidin domain receptors signaling causes synaptic plasticity disturbance, increases oxidative stress,alters homeostatic molecular mechanisms, and ultimately, causes neuronal death.Several studies on both patients and animal models have also revealed the upregulation of Abl in the key brain regions involved in AD progression, such as the hippocampus, cortex, and ventral tegmental area(VTA).
The VTA is a deep midbrain nucleus that is rich in dopaminergic neurons and that has recently been demonstrated to play a crucial role in the pathophysiology of AD.The VTA is the origin of the dopaminergic mesocorticolimbic system, and its implication in several cognitive and non-cognitive functions has been investigated in depth.Thus,the failure of dopamine release could explain the manifestation of deficits in both the cognitive and behavioral tasks that characterize AD.Of note,behavioral alterations, which are referred to as neuropsychiatric symptoms, are known to be evident at the prodromal phase of the disease and have a strong impact on a patient’s global disability, causing high levels of distress for the patients themselves and their caregivers.
Several independent clinical studies on patients suffering from mild cognitive impairment (MCI)and AD have described various functional,structural, molecular, and metabolic changes in the VTA and its target areas, underlining the involvement of the mesocorticolimbic pathway at the prodromal phase of the disease.Indeed,a structural magnetic resonance imaging study demonstrated a strict correlation between the VTA volume and both hippocampal size and memory performance (De Marco and Venneri,2018).Moreover, research investigating the functional connectivity of the VTA and its target areas in a cohort of patients with mild MCI due to AD and patients with AD identified a progressive functional disconnection at the onset of MCI that is likely related to cognitive and behavioral symptoms (Serra et al., 2018).Finally, a SPECT imaging study demonstrated a similar degree of reduction in dopamine transporter density in the VTA-projecting areas in both MCI and AD patients with dementia, underling the alterations in the dopaminergic system in the early phases of the disease (Sala et al., 2021).
The involvement of the VTA in early AD was also recently investigated in-depth in Tg2576 mice(Nobili et al., 2017), a validated experimental model of AD harboring the human amyloid precursor protein mutant allele that is linked to familial AD.It was proven that the degeneration of the dopaminergic neurons in the VTA precedes both the deposition of Aβ-plaques and neuronal loss in the hippocampus, and it is responsible for the insurgence of synaptic impairments and behavioral deficits.Moreover, VTA alterations,reductions in the dopamine transporter in the hippocampus, and the loss of tyrosine hydroxylasepositive fibers were also observed in other mouse models of AD.
Thus, taking all of these findings into account as well as the involvement of both tyrosine kinases and VTA in AD, a relevant hypothesis in favor of the role of c-Abl in neuronal loss in the VTA emerges.In this regard, it has recently been confirmed that the dopaminergic neurons in the VTA show high levels of phosphorylated c-Abl,which could cause or contribute to dopamine neuronal loss (La Barbera et al., 2021).Of note,these data are in strict compliance with previous observations that were obtained in experimental models of Parkinson’s disease (PD) focusing on the adjacent dopaminergic neurons from the substantia nigra pars compacta (Hebron et al.,2013), which share several common features with those in the VTA.In particular, mesocorticolimbic and nigrostriatal pathways originate from the dopaminergic midbrain that is affected in both AD and PD.Of note, PD is diagnosed as a movement disorder in which the nigrostriatal pathway is mainly affected, at least in the early phase of the disease.By contrast, the progressive degeneration of the mesocortcolimbic pathway may account for the cognitive and non-cognitive symptoms that characterize AD (Krashia et al., 2019).
In this scenario, the results obtained from a different research field, such as cancer research,have made several interesting pharmacological approaches against tyrosine kinases available,and these approaches appear to be a promising strategy in neurodegenerative research as well.Thus, several pharmacological cancer treatments have been investigated in AD and Nilotinib seems to be particularly interesting in neurodegenerative diseases.Nilotinib was approved by the US FDA in 2010 and was initially authorized for the treatment of adults with chronic myeloid leukemia positive for the Philadelphia chromosome.The pharmacokinetic evidence proved that Nilotinib crosses the blood-brain barrier and targets c-Abl and discoidin domain receptors efficiently.This, together with the evidence that c-Abl and discoidin domain receptors are upregulated in several neurodegenerative disorders, such as in AD and PD, was the rationale for c-Abl inhibition inin vitro
andin vivo
experimental models of AD.Biochemical, histological, and behavioral assays proved that Nilotinib prevents the formation of Aβ-plaques and neurofibrillary tangles as well as improves cognitive performance in AD animal models (Lonskaya et al., 2013).Moreover, c-Abl is responsible for alterations in the autophagy mechanism, a key homeostatic pathway that regulates the removal of protein aggregates and damaged organelles, guaranteeing neuronal survival.Interestingly, it has recently been demonstrated that Nilotinib increases autophagy flux in Tg2576 mice, promoting both the reduction of Aβ levels and the survival of dopaminergic VTA neurons.Thus, Nilotinib also leads to dopamine level rescue in VTA targets, such as the hippocampus, and improvements in cognitive performance (Figure 1; La Barbera et al., 2021).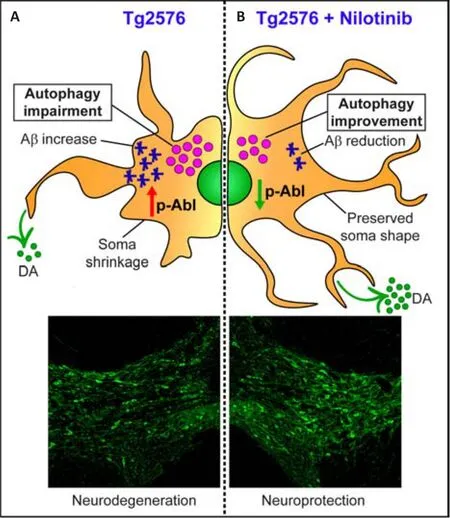
Figure 1| Nilotinib protects ventral tegmental area (VTA) neurons from degeneration by the improvement of autophagic flux.
Indeed, besides preclinical investigations focusing on the molecular and histological effects of Nilotinib, its potential therapeutic role is also currently being tested by clinical trials in patients suffering from AD.The encouraging results that have been obtained in the completed phase II(ClinicalTrials.gov Identifier: NCT02947893) have prompted the scientific community to have high expectations for the phase III trial, which have only just begun (ClinicalTrials.gov Identifier:NCT05143528).In patients, as in experimental models, Aβ burden was significantly reduced in the frontal lobe, and the cerebrospinal fluid levels of the Aβand Aβpeptides were reduced at 6 and 12 months, respectively.Of note, the hippocampal volume, which was investigated by volumetric magnetic resonance imaging, was also higher in subjects receiving Nilotinib versus the placebo group (Turner et al., 2020).
Neuroinflammation is another early event that contributes to AD pathology, exacerbating disease progression (Tondo et al., 2021).Inflammatory cytokines appear before the clinical onset of dementia, and there is a strong link between neuroinflammation and Aβ and tau accumulation.There are several pieces of evidence indicating that among the cytokines upregulated in the human AD brain, tumor necrosis factor-α has been shown to induce caspase cleavage of c-Abl at the C terminus, leading to nuclear accumulation and contributing to apoptosis.Moreover, different works have demonstrated that the overexpression or activation of glial c-Abl leads to inflammation that contributes to neurodegeneration.Of note,tyrosine kinase inhibitors, including Nilotinib,are able to modulate microglia morphology and astrocyte proliferation, thus reducing inflammation in several animal models of AD and PD (Lonskaya et al., 2015).Furthermore, the recent demonstration of Nilotinib’s effectiveness in rescuing the astrocytes’ mitochondrial bioenergetics and biogenesis through the NF-κB signaling pathway in a mouse model of AD(Adlimoghaddam et al., 2021), highlights the crosstalk between mitochondrial dynamics and inflammation also indicates a new Nilotinibtargeted therapeutic strategy for AD.
Collectively, the results that have been obtained in recent years have allowed us to further expand our knowledge of the early events occurring in AD.However, clinical research needs to consider several aspects that have not been considered until now and that could be essential to improving the efficacy of therapeutic interventions.To test the efficacy of Nilotinib in VTA protection,it will necessary to include this area in the next imaging studies, as recently done (De Marco and Venneri, 2018; Serra et al., 2018).Indeed, the evidence that Nilotinib prevents the degeneration of dopamine neurons in VTA in experimental models of AD (La Barbera et al., 2021) suggests that pharmacological interventions targeting the mesolimbic system should be considered as a possible candidate for successful treatments in clinical settings.Moreover, no evaluation has been carried out on the inflammatory profiles of the patients enrolled in the recently concluded clinical trial.
Another crucial aspect that needs to be considered is the age and the heterogeneity of the enrolled subjects.In fact, AD is a progressive and complex neurodegenerative disease in which genetic, epigenetic, and environmental factors are involved in disease onset, which can beginup to 20 years before symptoms appear.Current diagnostic procedures fail to intercept very early clinical manifestations of the disease, meaning that experimental drugs are delivered in advanced stages.Thus, it could be necessary to define new diagnostic criteria to individuate and include a subset of patients to enroll in new clinical trials at a very early stage.In this scenario, the fascinating results obtained from patients with MCI and AD regarding VTA functional connectivity and dopamine transporter density suggest that these aspects could be considered as potential clinical markers to discriminate MCI due to AD subjects in the early phases of the disease.
In conclusion, significant progress has been made to clarify key aspects of the underlying pathophysiology of AD in the last few years.Now,it is necessary to detect efficient pharmacological therapies that go beyond the amyloid-based ones and the specific cluster of patients.Thus, although Tau- and ApoE-directed clinical approaches remain at an early stage of development and even though both hold great potential going forward, other avenues must also be pursued.The increasing acknowledgment of the complexity of AD, the diversity of pathology, and the dynamic interactive network of players that make up the disease at the different stages suggest the need to pursue multiple lines to fight this complex progressive neurodegenerative disorder.The recognition of this complexity indicates that multiple targetbased combination therapies could be needed for successful AD treatment, as evidenced by the success obtained with other diseases, such as cancer and multiple sclerosis.Thus, Nilotinib,because of its broad-spectrum effects, would be an ideal candidate that is suitable for clinical testing in combination with different drugs.The clinical trial on mild to moderate AD patients demonstrated that this drug is safe and well-tolerated.In fact, no mood swings and behavioral changes are recorded in patients treated with 150 mg, while agitation and irritability appear with 300 mg dosage.Other reported side effects of Nilotinib treatment include gastrointestinal symptoms, transient elevations of pancreatic and liver enzymes, leukopenia, and thrombocytopenia, which resolve spontaneously(Turner et al., 2020).Interestingly, in the phase 3 trial lower doses of Nilotinib are under evaluation in subjects with MCI or AD.
Collectively, current and future studies should attempt to recognize the different pathways that characterize different disease trajectories.This step will be essential to identify specific drugs to realize a personalized treatment plan, which means dealing with the complexity of each AD patient’s biology.Ongoing intensive investigations in this area will be critical to making key discoveries that will ultimately unveil novel therapeutic approaches leading to truly disease-slowing medications.
This work was supported by Linea D.1.2021 Università Cattolica del S.Cuore (to MTV) and by the Italian Ministry of Health (IT) [Research Grant:RF-2018-12365527, to MDA and MTV].MDA was supported by the American Alzheimer’s Association[AARG-21-851219], and by Fondazione Roma(Rome, Italy).
Annalisa Nobili, Marcello D’Amelio,Maria Teresa Viscomi
Department of Medicine and Surgery, University Campus Bio-Medico; Department of Experimental Neuroscience, IRCCS Santa Lucia Foundation,Rome, Italy (Nobili A, D’Amelio M)Department of Life Science and Public Health Section of Histology and Embryology, Università Cattolica del Sacro Cuore; Fondazione Policlinico Universitario “A.Gemelli”, IRCCS, Rome, Italy(Viscomi MT)
*Correspondence to:
Marcello D’Amelio, PhD,m.damelio@unicampus.it; Maria Teresa Viscomi,PhD, mariateresa.viscomi@unicatt.it.https://orcid.org/0000-0001-6526-1832(Marcello D’Amelio)https://orcid.org/0000-0002-9096-4967(Maria Teresa Viscomi)Date of submission:
April 5, 2022Date of decision:
June 6, 2022Date of acceptance:
June 18, 2022Date of web publication:
September 16, 2022https://doi.org/10.4103/1673-5374.350700
How to cite this article:
Nobili A, D’Amelio M,Viscomi MT (2023) Nilotinib: from animal-based studies to clinical investigation in Alzheimer’s disease patients.Neural Regen Res 18(4):803-804.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:
Md Imamul Islam, University of Manitoba, Canada.
- 中国神经再生研究(英文版)的其它文章
- Neural and Müller glial adaptation of the retina to photoreceptor degeneration
- Agomelatine: a potential novel approach for the treatment of memory disorder in neurodegenerative disease
- MicroRNAs: protective regulators for neuron growth and development
- In vivo astrocyte-to-neuron reprogramming for central nervous system regeneration: a narrative review
- Intranasal nerve growth factor for prevention and recovery of the outcomes of traumatic brain injury
- Altered O-GlcNAcylation and mitochondrial dysfunction,a molecular link between brain glucose dysregulation and sporadic Alzheimer’s disease

