Pathophysiological mechanisms of chronic compressive spinal cord injury due to vascular events
Zhen-Xiao Ren, Jing-Hui Xu, Xing Cheng, Gui-Xing Xu, Hou-Qing Long,
Abstract Cervical spondylotic myelopathy is the main cause of non-traumatic spinal cord injury, with chronic static and/or dynamic compressive spinal cord injury as the unique pathogenesis.In the progression of this condition, the microvascular network is compressed and destroyed, resulting in ischemia and hypoxia.The main pathological changes are inflammation, damage to the blood spinal cord barriers,and cell apoptosis at the site of compression.Studies have confirmed that vascular regeneration and remodeling contribute to neural repair by promoting blood flow and the reconstruction of effective circulation to meet the nutrient and oxygen requirements for nerve repair.Surgical decompression is the most effective clinical treatment for this condition; however, in some patients,residual neurological dysfunction remains after decompression.Facilitating revascularization during compression and after decompression is therefore complementary to surgical treatment.In this review, we summarize the progress in research on chronic compressive spinal cord injury, covering both physiological and pathological changes after compression and decompression, and the regulatory mechanisms of vascular injury and repair.
Key Words: angiogenesis; cervical spondylotic myelopathy; hypoxia; inflammation; ischemia; spinal cord injury; surgical decompression
Introduction
Cervical spondylotic myelopathy (CSM) is the primary cause of non-traumatic spinal cord injury (Xu et al., 2017), accounting for 54-59% of all cases.In addition, static compression, such as disc herniation, ossification of the posterior longitudinal ligament (OPLL), intraspinal tumors, and dynamic compression, such as stretching and shearing of the spinal cord during cervical flexion and extension, are basic factors contributing to pathogenesis of CSM (Long et al., 2014).Chronic compressive spinal cord injury (CCSCI)is a pathogenic and regulatory mechanism unique to CSM.CCSCI is usually associated with disruption of the spinal cord vascular network with decreased blood vessel density (Cheng et al., 2015), particularly in the microvasculature,resulting in decreased blood supply to the spinal cord.This causes ischemia,hypoxia, and neurological dysfunction.These changes in the vascular system are sufficient to trigger neuropathology, suggesting that changes in the vasculature may exacerbate pathology in diseases where neurons are already affected by other factors (Hernandez-Gerez et al., 2019).Moreover,mechanical compression causes demyelination and apoptosis of neurons and oligodendrocytes and compresses and damages vascular endothelial cells,destroying the spinal blood-brain barrier and producing an inflammatory response.As a result, the blood supply to the spinal cord is reduced, and ischemia and hypoxia are aggravated, leading to different degrees of spinal cord damage (Karadimas et al., 2015).Owing to the morphology of the spinal cord and the specificity of the brain’s connection to the automatic and peripheral nervous systems, the severity and location of CCSCI determine the clinical symptoms of patients.
Surgical decompression is an effective method to treat CCSCI (Singleton and Hefner, 2022).Nevertheless, because most patients with CSM have obvious spinal cord dysfunction at the time of treatment, it is difficult to completely reverse pathological changes using surgical decompression alone: it has been reported that 11-38% of patients still have varying degrees of spinal cord dysfunction after surgical decompression (Karadimas et al., 2015; Dolan et al., 2016).However, acupuncture therapy, hyperbaric oxygen therapy, and other adjuvant therapies are mostly ineffective.Current understanding is that recovery of nerve function after spinal cord decompression is related to the improvement of vascular density and circulation (Halder et al., 2018); our previous studies also support this view (Long et al., 2015; Cheng et al., 2021).Although numerous studies have proposed various mechanisms for angiogenesis (Yang et al., 2021), those related to spinal angiogenesis have rarely been specified.Therefore, the mechanisms of vascular changes after compression and approaches to enhance spinal cord angiogenesis by promoting nerve function repair after decompression remains a key research focus (Figure 1).
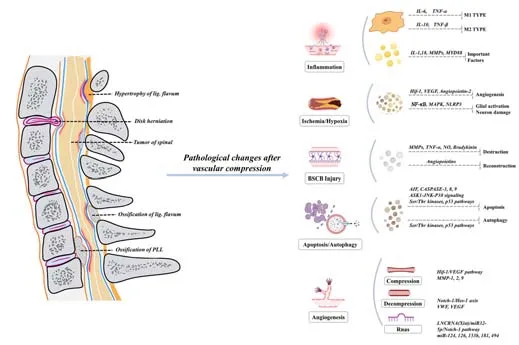
Figure 1| Flow chart showing the etiology of and pathological changes involved in chronic compressive spinal cord injury (CCSCI).
In this review, we summarize current progress in CCSCI research, focusing on the physiological and pathological changes after compression and decompression, vascular changes, and the mechanism of vascular regeneration.
Search Strategy
In this narrative review, we screened relevant literature published between January 2000 and January 2022 and searched the PubMed database using the search strings: ‘(chronic spinal cord compression) AND (vascular)’;‘(chronic spinal cord compression) AND (blood vessels)’; ‘(cervical spondylotic myelopathy) AND (vascular)’; ‘(cervical spondylotic myelopathy) AND (blood vessels)’.The title and abstract of the retrieved articles were screened.Firstly,we reviewed whether the title and abstract are relevant to our target content.If so, then we read the whole paper to ensure if there are descriptions of vascular events.Articles that meet the above criteria will be selected.More than half of the articles cited were published in the past 5 years.
Spinal Cord Vascular System
Intrinsic Arterial System
The intrinsic arterial system can be usefully categorized as central or peripheral arteries, with the central artery providing two-thirds of the blood to the spinal cord (Martirosyan et al., 2011).No pre-capillaries connect the two systems, and the relationship between these two systems is noncompensatory.Nevertheless, part of the spinal cord receives blood from both systems, due to overlap between distal branches.The overlap is seen in the inner quarter to the first third of the white matter and the outer margin of the gray matter.However, the back half of the posterior horn is completely supplied by the peripheral system (Martirosyan et al., 2011).
The various areas of the spinal cord are differently vascularized.Because of the extensive vascularity supplying the cervical and lumbosacral regions,infarction rarely occurs here.The thoracic and lumbar blood supply is mainly from segmental arteries that branch from the aorta, while the major points where the thoracic blood network feeds into the spinal cord are spaced more widely.The sacral region is supplied primarily by the lateral sacral artery(Martirosyan et al., 2011).The spinal cord has a rich supply of blood to the cervical and lumbosacral regions, which is not in the thorax.Due to poor collateralization of the vascular area in the thorax, disruption of blood flow can present a serious risk of ischemia.
Extrinsic Arterial System
Anterior spinal artery
The anterior spinal artery (ASA), which is the trunk artery of the central arterial system, provides most of the internal spinal blood vessels.Occlusion of the artery and anterior radicular artery (ARA) causes ischemia in the anterior two-thirds of the spinal cord.The diameter of the ASA gradually reduces from the beginning to the thoracic segment and the diameter of the ASA is relatively constant from the thoracic segment down.At the sacrococcygeal region, the branches of the ASA surround the spinal cone and join the posterior spinal artery of each limb (Martirosyan et al., 2011).ASA obstruction may lead to anterior spinal artery syndrome, and the clinical manifestations include a decrease or loss of temperature and pain sensation,while positional and vibrational sensations are relatively well preserved below the plane of injury (Gadepalli et al., 2010).
Posterior spinal artery
The posterior inferior cerebellar artery or the vertebral artery is where the posterior spinal artery (PSA) originates.The PSA has small branches proximal to the posterior and nerve roots at the lower end of the spinal marrow (Martirosyan et al., 2011).The blood supply to the posterior third of the spinal cord is provided by the PSA and vasocorona (anastomosis between the ASA, PSA, and anterior and posterior radicular arteries formed the pial plexus vasocorona;Martirosyan et al., 2011).Infarction of the PSA is rarer than the ASA, either because the PSA is paired or has greater collateral circulatory capacity, or because clinical diagnosis is underestimated.Zalewski et al.(2018) reported 15 cases of posterior spinal artery infarction; all patients presented with sudden sensory disturbances, often accompanied by pain and sensory ataxia.
Pial arterial plexus
Surface blood vessels branching off from the ASA and PSA form the pial plexus network that encircles the spinal cord.All penetrating branches are perpendicular to the surface of the spinal cord directly inward.These branches supply blood to the outer part of the spinal cord (Martirosyan et al., 2011).The CCSCI may result in flattening, extension, and absence of penetrating branches in the lateral pial plexus, thus affecting local perfusion(Badhiwala et al., 2020).
Radicular arteries
There are 31 pairs of radicular arteries, which supply blood to nerve roots,ASA, PSA, spinal ganglion, and dura mater.Different segments of the ASA receive different numbers of anterior radicular arteries (0-6 in the neck, 1-4 in the chest, and 1-2 in the lumber).The posterior radicular arteries appear more frequently in the caudal part of the spinal cord.Their number seems to be independent of that of anterior radicular arteries.When they appear in the same segment and side, they can form a joint stem external to the dura mater.The ASA and radicular arteries are markedly altered after chronic compression of the cervix, and this alteration may influence vascular density and significantly alter nerve function (Cheng et al., 2015).
Central arteries
The central artery is an important component of the spinal cord blood supply system.It arises from the ASA and reaches its maximum density in the neck(8-13 arteries per centimeter, compared to only 2-3 arteries per centimeter in the thoracolumbar spine) (Sinescu et al., 2010).Along with the ASA and the vasocorona, it provides the blood supply to the first two-thirds of the spinal cord (Martirosyan et al., 2011).
Adamkiewicz artery
The Adamkiewcz artery (the great radicular artery) is the largest vessel providing blood to the spinal cord.In approximately 50% of people, the vessel supplies one quarter of total blood volume to the spinal cord.It usually follows roots T9-T12 (75%) and is positioned mostly (approximately 80%) on the left side of the spinal cord.It is divided into two branches joining the ASA: a larger, descending branch and a smaller, ascending branch(Martirosyan et al., 2011).If the Adamkiewcz artery is occluded for a longtime, anterior spinal artery syndromes, such as paraplegia, result (Pennington et al., 2020; Figure 2).

Figure 2 | Vascular distribution in the transverse (A) and sagittal (B) view of the spinal cord.
Chronic Compressive Spinal Cord Injury Model
There have been few clinical studies on long-term spinal cord compression,and most such studies have been conducted in animal models (Krupa et al.,2020; Price et al., 2021).Reliable animal models of CCSCI are essential to the study of this condition.Dogs and rats are commonly used as models; however,due to the spontaneous recovery characteristics of rats, the experimental results may have a certain impact.Dogs may be a better choice, because the mechanism of spinal cord injury in dogs is similar to that of humans.In experimental animal studies, an understanding of diversity of the spinal cord blood supply among different animals is required in order to translate the findings into clinical studies in humans (Table 1).
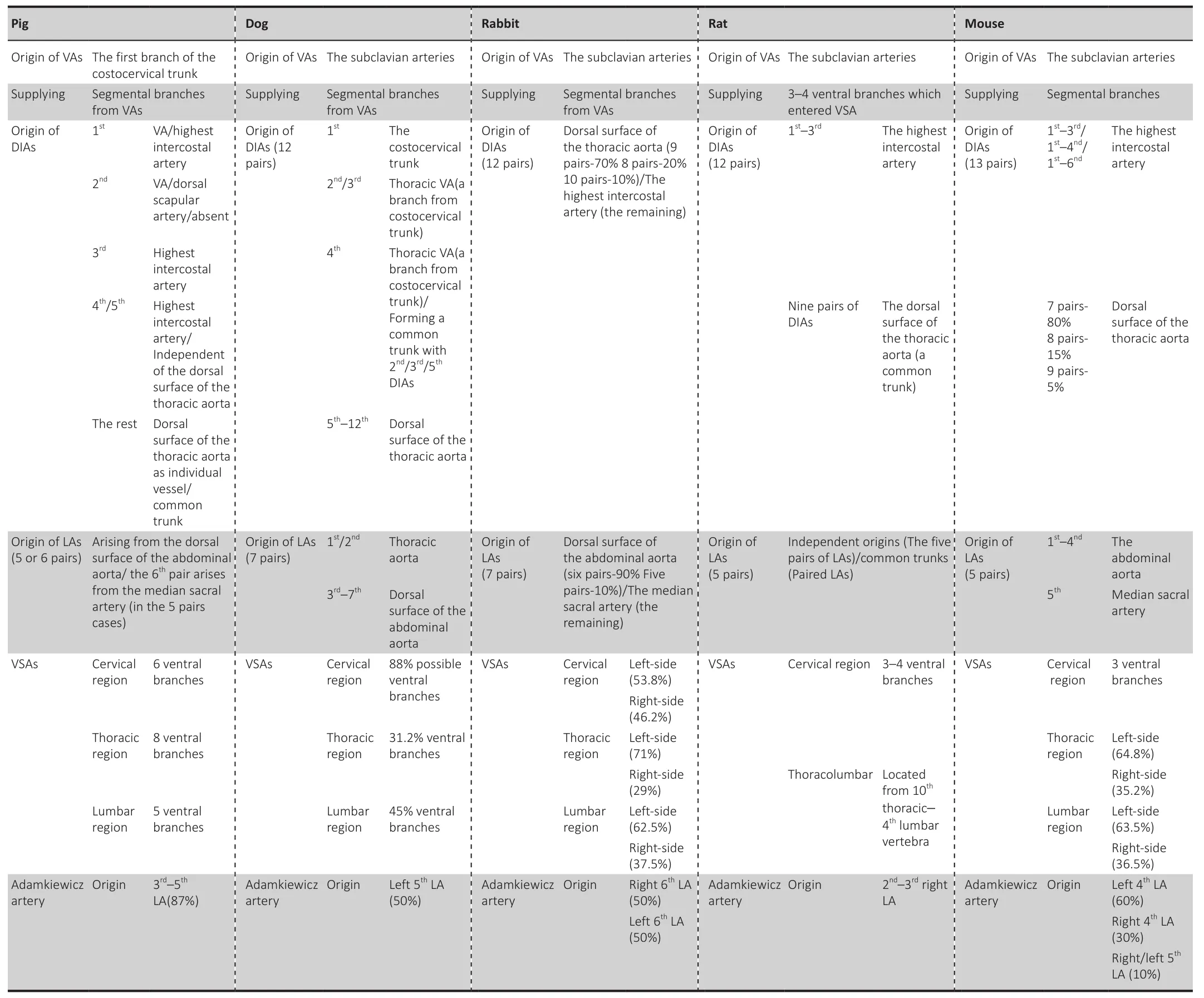
Table 1 |Characteristics of blood supply in different animal models
Existing methods of preparing CCSCI models include: titanium screw drilling(Lee et al., 2012b), balloon catheters (Krupa et al., 2020), epidural tumor cell seeding (Price et al., 2021), polyether-wife phosphate anions to guide calcium phosphate precipitation and form osteoids (Kanbara et al., 2020), and the TWY/TWY rat model method (Yu et al., 2021a).However, these methods have certain limitations.
A rat model that uses a slow-release membrane to wrap absorbent material((14)-3,6-anhydro-α-L-galactopyranosyl-(13)-β-D-galactopyranan) to compress the spinal cord, which reaches a maximum volume within 24 hours (Hu et al., 2011), may be a better choice.The membrane can control the swelling rate in CCSCI.This model can be used to investigate the effect of differentcompression rates on the spinal cord and pathological progression of CCSCI(Kubota et al., 2011).In addition, cutting the membrane into a blade implant can significantly reduce the rate of SCI (Kanbara et al., 2020).Ijima et al.(2017)indicated that polymer sheets with the capacity for 200% volume expansion are ideal for constructing models of cervical compression myelopathy.Owing to high compatibility and controllability of the membrane, this method is commonly used to prepare CCSCI models.
Vascular Events
Vascular alteration
Spinal cord compression can be categorized as acute compressive spinal cord injury (ACSCI) and CCSCI.The disease resulting from ACSCI can be usefully divided into phases: acute, subacute, and chronic (Liu et al., 2019).Vascular changes are different in these different phases.The earliest event following spinal cord compression is a severe injury to the local vascular system (capillaries and venules), manifested as hemorrhage, thrombosis,and vasospasm, which results in the interruption of spinal microcirculation(Chamankhah et al., 2013).After vascular injury, reactive oxygen species and other toxic substances produced during the breakdown of hemoglobin or fibrinogen in the blood cause further damage to the spinal cord.In the acute phase, the blood vessels are in a state of disorder and distortion, but the basic structure of the entire injury area has not changed.Bleeding due to the disorganized and distorted vascular morphology may be the main reason for decreased blood flow during this phase.In the subacute phase, the density of microvasculature increases significantly, and the blood vessels dilate.In the chronic phase, there is a decrease in vessel volume and the number of vessels, but an increase in the separation between vessels (Liu et al., 2019).Vascular changes in the chronic phase may be similar to those observed in CCSCI.
There is no certain staging of CCSCI.In the early stages of chronic compression, reduced blood flow may result from local compression, which leads to obstruction of the vessels as the tissue swells and pressure rises(Kurokawa et al., 2011).As the compression process continues, blood vessels in the compressed area may become non-functional or even necrotic due to a chronic lack of blood supply; the number of vessels also decreases, leading to a further reduction in the effective blood supply to the injury.Small vessels are formed during this process, but cannot function properly due to the continuous pressure.Ischemia, hypoxia, inflammation, and apoptosis occur in spinal cord cells.These factors may together lead to the loss of neurons,accompanied by motor dysfunction (Akter et al., 2020).In addition, as compression persists, myelin destruction and loss of axons in the spinal cord cells, and loss of gray matter neurons and white matter oligodendrocytes occur in the compressed area (Akter and Kotter, 2018), resulting in neurological symptoms.Understanding vascular events during compression thus contributes significantly to improving treatment of and recovery from CCSCI.
As compression progresses, hemodynamics in the spinal cord are affected.Ischemia mainly results from compression or injury of ASA and ARA in the cervical spine, as they provide 60-75% of the blood supply to this area,and are more susceptible to compression (Lee et al., 2012a).However, the posterior spinal and posterior radicular arteries have little significance in the etiology of ischemia due to their location and blood supply characteristics(Cheng et al., 2015).Therefore, changes in the ASA and ARA after compression and healing is more relevant for a better understanding of the etiology of CCSCI.Our previous studies indicated that the diameter of ARA reaches a minimum at 28 days and increased significantly at 42 days after compression.For ASA, the diameter was smallest at 42 days, and significantly increased at 70 days after compression.The overall change process may affect the density of blood vessels (Cheng et al., 2015).In addition, the angle between ARA and ASA in normal rats was approximately 90°, but by day 70 following compression, this angle had become acute (Cheng et al., 2015).Thechanges in compressed blood vessels at different time points may be related to the compensatory mechanism.Similarly, the diameters and distribution densities of vessels and the existence of compensatory mechanisms may together affect hemodynamics at the compressive site.
To describe this pathological process in more detail, several ultrastructural studies are currently in progress.The destruction of the compressed vessels may be associated with changes in endothelial cells, pericytes, astrocytes,and other cell types (Xu et al., 2017).The blood-spinal cord barrier (BSCB) is an extension of the blood-brain barrier.However, they are different in form and function.The BSCB prevents white blood cells and red blood cells from entering the spinal cord and provides a stable microenvironment for the spinal cord.Altered permeability of the BSCB is an important stage in this process, and may be mediated by extracellular pathways, independent of the loss of tight junctions (TJs) between cells (Nahirney et al., 2016).Conversely,Bartanusz and colleagues believe that permeability of the BSCB may depend on paracellular transport through the TJs (Bartanusz et al., 2011).Our previous research indicated that BSCB permeability increased with increasing numbers of vacuoles in the endothelium at 28 days after compression in adult rats (Xu et al., 2017).In addition, we found that there was a reduction of TJ proteins in ischemic tissues, confirmed by our observation of TJ fluctuation.Fluid-filled spatial clustering at the TJ appears to support the sustained movement of vesicles/vacuoles across the endothelium (Xu et al., 2017).These studies together suggest that compressive spinal cord permeability in rats is based on a transcellular mechanism.Osmotic gradients may also be disrupted by increased protein and ion transport into pericytes, endothelial cells, and glial cells, leading to perivascular swelling and water influx.
The neurovascular unit (NVU) is the functional unit comprising the basic structure of the central nervous system.It has a transport function in the BSCB (Wei et al., 2022).Structural and functional changes in the NVU have been reported to be associated with nerve injury, neurodegeneration, and other central nervous system diseases (Jullienne and Badaut, 2013; Janigro et al., 2020).The NUV is composed of vascular endothelial cells, extracellular matrix, pericytes, astrocytes, neurons, and sustentacular cells.Loss and dysfunction of these cells lead to BSCB dysfunction, the most important manifestation of which is change in BSCB permeability.Pericytes are major contributors to altered BSCB permeability, regulating this process by secreting matrix metalloproteinases (MMPs) to degrade the basement membrane(Yang et al., 2021).In addition, pericyte loss due to mutations may cause increased vascular permeability and microaneurysms (Armulik et al., 2010).The increase in swelling and coverage of pericytes after compression may be a compensatory regulatory mechanism in BSCB permeability.Apart from this, glycogen accumulation, swelling, and mitochondrial disintegration of astrocytes have been also observed in CCSCI (Xu et al., 2017).Microscopic studies such as these provide important guidance for vascular reconstruction.
Remodeling
After chronic compression of the epidural space, a prominent increase in vascular density is observed in the anterior horn of the spinal cord.Vascular endothelial growth factor (VEGF) and von Willebrand factor labeling also demonstrated that angiogenesis increased only in the anterior horn of the spinal cord (Yu et al., 2021b).This may be because the blood supply to the compressed site is affected, and a denser blood vessel network in the noncompressed site is required to compensate.In addition, vascular regeneration after injury is associated with glial scar formation (Hesp et al., 2018).NG2-expressing pericytes are involved in glial scar formation, and promote vascular formation after injury (Cheng et al., 2018).However, the formation and effect of the glial scar on spinal angiogenesis in CCSCI models are currently unclear.
The hypoxia-inducible factor 1/vascular endothelial growth factor (HIF-1/VEGF) pathway is thought to be critical for protection and vascular remodeling in the compressed spinal cord.Hypoxia promotes angiogenesis mainly by inducing HIF-1/VEGF.Autophagy at the injured site also promotes angiogenesis via this pathway (Li et al., 2019).Studies have proven that VEGF can protect the vascular endothelium, improve local blood flow, promote angiogenesis, and increase vascular density, and also has anti-apoptotic functions, protects damaged neurons and nourishes nerves, and reduces secondary injury (Xu et al., 2020; Micheli et al., 2021).Paradoxically, VEGF may aggravate secondary injury, especially in the acute and subacute phases.This may cause increased BSCB permeability, which can lead to reactions such as excitotoxicity, leukocyte infiltration, and tissue edema (Long et al., 2012).
MMPs not only play a pivotal role in angiogenesis but also affect other processes such as proliferation, differentiation, and cell migration (Cui et al.,2017).During revascularization following hypoxia, the level of MMP-1,2,9 expression increases, and MMP-1 can help endothelial cells undergo matrix degradation and chemotaxis (Sinescu et al., 2010).MMP-9 is regulated by oxygen levels and HIF-1α; it is mainly expressed in the proliferative margins of blood vessels and also in activated astrocytes (Edsberg et al., 2015).MMP-2 induces VEGF expression by binding to αVβ3 receptors and activating the P13K/AKT pathway (Sapieha, 2012).In addition, VEGF and angiogenic factors are associated with the expression of MMP-9 and can promote the expression of MMP-9 (Sinescu et al., 2010).The regulation of HIF-1α, VEGF, and MMP-9 may have a useful therapeutic effect on ischemic hypoxia following CCSCI.In our previous studies, we found that von Willebrand factor and VEGF expression increased after decompression, while the expression of MMP-2 and MMP-9 decreased.In addition, expression of the Notch-1/Hes-1 axis increased after decompression, and promoted angiogenesis (Cheng et al., 2020, 2021).These changes are important for blood vessel formation after decompression.
Mechanisms in Vascular Events
During the development of CCSCI, pathological changes such as ischemia,hypoxia, inflammation, BSCB injury, and apoptosis occur at the site of compression.However, the pathological mechanisms for this have not been fully elucidated.RNAs are involved in the regulation of these processes,and mainly regulate angiogenesis.The relationship between autophagy and angiogenesis has become a hot research topic in recent years.This section covers inflammation, ischemia and hypoxia, BSCB injury, apoptosis, and autophagy.
Ischemia and hypoxia
Lack of blood supply to the compressed area usually results in chronic hypoxia.Halder et al.(2018) found that chronic mild hypoxia can strongly accelerate vascular remodeling in the spinal cord.It was found that hypoxia promotes angiogenesis mainly via induction of Hif-1, VEGF, and angiopoietin-2(Pichiule and LaManna, 2002; Chen et al., 2018).The expression of angiogenic proteins, fibronectin, and TJ proteins in endothelial cells were also found to increase during chronic mild hypoxia (Halder et al., 2018).This suggests that chronic mild hypoxia-mediated molecular changes are a major mechanism of vascular remodeling during compression.
Oligodendrocytes and oligodendrocyte progenitors may become damaged during chronic hypoxia, leading to axonal demyelination.Ischemia also leads to decreased adenosine triphosphate (ATP) levels, abnormal sodium/potassium (Na/K) ATPase function, and changes in mitochondrial membrane permeability (Chamankhah et al., 2013).Hypoxia induces the production of a Hif-1 heterodimer.Hif-1 consists of Hif-1α and Hif-1β; Hif-1α induces the activation of nuclear factor-kappa B (NF-κB) and its inhibitors.Genes encoding VEGF, erythropoietin, glycolysis, and glucose transporters, which are associated with oxygen transport and cell metabolism during hypoxia, are also activated by HIF-1α (Long et al., 2015).Hif-2α is produced and remains active for longer during chronic hypoxia, and may thus have a more substantial role in promoting vascular development and increasing erythropoiesis (Hernandez-Gerez et al., 2019).Another complication of ischemia is ischemia-reperfusion injury, which leads to necrotic damage to astrocytes, oligodendrocytes,neurons, and endothelial cells.Hypoxia, cytokines, activated complement,reactive oxygen species, and apoptosis-related signal cascades are involved in this process (Chamankhah et al., 2013).Several experiments have shown that NF-κB links hypoxia to the innate immune response (Rius et al., 2008) and has a significant role in various neurodegenerative diseases.It induces anti-apoptotic proteins and superoxide dismutases by regulating gene expression in glial cells, thus promoting neuronal survival (Karadimas et al., 2013).Dysfunction in astrocyte mitochondria and microglia leads to the production of reactive oxygen molecules, which then activate NF-κB, mitogen-activated protein kinase (MAPK), and pyrin domain-containing 3 inflammasome, leading to glial activation and neuron damage (Zhou et al., 2020).
BSCB
A major function of the BSCB is to protect the spinal cord from neurotoxic injury (Jin et al., 2021).After ACSIC, altered BSCB permeability is one of the earliest events, which impairs tissue recovery (Cohen et al., 2009).BSCB permeability reaches a maximum 24 hours after the initial damage (Figley et al., 2014).The permeability of BSCB decreases gradually after injury, but even 56 days after injury, the BSCB remains damaged.Pathological changes may extend along the longitudinal axis of the spinal cord (Cohen et al.,2009).In ACSCI, caudal BSCB permeability reaches a peak in the acute phase and recovers to a relatively stable level in the subacute and chronic phases.The permeability of BSCB in the injury epicenter decreases gradually with time, and reaches its lowest point in the chronic period.The reduction in permeability in caudal and epicenter of injury site are marked.On the rostral side, BSCB permeability during the subacute phase is higher than during the other two phases.Research has indicated that the delayed reconstruction of rostral BSCB may hinder the endogenous repair process of this area after SCI(Cohen et al., 2009).Moreover, the mechanism of BSCB injury in CCSCI is not quite the same as that of ACSCI.Increased BSCB permeability is a doubleedged sword; as long as BSCB injury continues, its protective effect on the spinal cord is reduced, but there is also a unique opportunity for therapeutic drugs and molecules to enter the central nervous system (Figley et al., 2014).Therefore, the different permeability of BSCB at different times of disease progression may guide the treatment strategy.Improved therapeutic effects may be obtained by using drugs or surgery at specific times; however, there is no clear indication of the most appropriate time for different intervention methods, which requires further study.
The BSCB is destroyed when the spinal cord is chronically compressed,resulting in increased permeability.MMPs, tumor necrosis factor α, nitric oxide,bradykinin, and endothelin are involved in the destruction of the BSCB (Kumar et al., 2017).In addition, the expression of immunoglobulins IgG and IgA, and intrathecal albumin Q also increases during this process.The expression of MMPs is crucial for vascular remodeling and BSCB permeability for several reasons.Firstly, MMPs directly degrade the TJs between the endothelial cells and extracellular matrix; thus the integrity and structural stability of the NVU is undermined, and the filtration of immune cells is increased (Long et al., 2015).Secondly, during chronic compression, MMPs can induce the activation and migration of glial cells, reducing the number of local glial cells.The aggregation and expression of astrocytes can lead to glial scarring, which may prevent axon regeneration via physical barriers or by chemical means, via the release of inhibitory molecules (Silver and Miller, 2004).MMP-9 promotes glial scar formation and enhances inflammatory cell infiltration and cytokine production.
In turn, MMP-2 can inhibit this process by degrading chondroitin sulfate proteoglycan, thus promoting growth of neurites (Pan et al., 2020).Thirdly,in ischemic and hypoxic sites resulting from long-term compression, MMPs activate microvascular hyperplasia, thus initiating a cascade reaction leading to excitotoxicity and oxidative stress (Long et al., 2012).Finally, MMPs can affect the structure and function of the BSCB.Tissue inhibitors of MMPs, secreted by pericytes in the NVU, antagonize the decomposition of extracellular matrix by MMPs.When the expression of MMPs and their inhibitors is unbalanced,the structure and function of BSCB becomes abnormal (Anik et al., 2011).Therefore, prevention of the damage of BSCB and promotion of BSCB injury healing are key steps in the treatment of CCSCI.
Inflammation
Chronic inflammation is an important pathological factor in CCSCI, the process may be either detrimental or protective and the details would be described as follows (Badhiwala et al., 2020).
The early inflammatory response results from neutrophil signal transduction.However, the number of white blood cells in the injury site is greatest in the first week, and they disappear by the third week.Activated microglia,monocytes, and macrophages are the main cells involved in chronic inflammation; they can remain for weeks to months (Sinescu et al., 2010).Microglia activate the inflammatory response, and are thus important for the inflammatory process (Yu et al., 2011).Activated microglia have M1 and M2 phenotypes; the proportion of these two phenotypes determines the recovery of SCI.When the spinal cord is continuously compressed, T helper 1 (Th1) cytokines (tumor necrosis factor [TNF]-α, interleukin [IL]-6) continue to be expressed, and microglia increase in number and convert to M1 type; this aggravates the inflammatory response and accelerates neuronal death, leading to nervous system damage.M2 type microglia produce anti-inflammatory mediators (IL-10, transforming growth factor [TGF]-β) and express high levels of Arg-1, which can inactivate pro-inflammatory cells.A greater M2:M1 ratio is thus beneficial to the recovery of spinal cord function(Hirai et al., 2013).Experimental regulation of M2 polarization has not yet been reported; however, Yu et al.(2021a) reported that the polarization of microglia toward M1 could be prevented by blocking the MK2 signaling pathway, thus reducing the inflammatory response and promoting recovery from SCI.In this process, TNF-α may be the most important Th1 cytokine involved in glial differentiation into M1 type cells (Hirai et al., 2013).However,it was shown that the increased expression of neurotropic factors does not reverse increased expression of pro-inflammatory cytokines or neuronal and glial apoptosis (Matsunaga et al., 2008).Therefore, surgical decompression may be the only effective way to treat the causes CCSCI.
The Fas/Fasl system inhibits inflammation by inhibiting microglial activation;it can also promote the recovery of neurological function after injury by increasing MMP-2 levels (Yu and Fehlings, 2011).In addition, lack of phase,anti-competitive inhibition of phase, or neutralization of Fasl with anti-Fasl antibodies can contribute to the recovery of normal neurobehavior in SCI animal models (Yu et al., 2011).Growing evidence has shown that the Fas/Fasl system is a potential therapeutic target for SCI as a complement to surgical decompression (Zhou et al., 2019; Liu et al., 2021).
Interleukin-18 is a cytokine produced in the first phase of inflammation, which regulates both innate and adaptive immune responses by affecting T cells, B cells, natural killer (NK) cells, monocytes, and dendritic cells (Srivastava et al.,2010).It also promotes the production of interferons by T cells and NK cells.Studies have demonstrated that autoantibodies can be detected in patients with CCSCI.The delayed production and accumulation of antibodies initiates a classic complement-activated enzyme chain reaction via the complement C1q pathway.This allows microglia and macrophages to gather in the injury site to participate in the inflammatory response (Lucin et al., 2007).In addition,the B cell-mediated immune response is the main immune response in the chronic phase.Thus, greater attention should be paid to immune function in the treatment of CCSCI in future (Chamankhah et al., 2013).
Apoptosis and autophagy
After hypoxia and SCI, programmed cell death begins, and the expression of apoptosis-inducing factors, such as apoptosis-inducing factor and caspase-3,increases (Ibrahim et al., 2021).Apoptosis-inducing factor is a mitochondrial intermembrane protein that can promote apoptosis, and caspases are vital mediators of apoptosis.As part of an independent apoptotic pathway,apoptosis-inducing factor degrades DNA in the nucleus.Activation of caspase-3 promotes cell death.In addition, caspase-3, -8, and -9 are associated with Fas-mediated apoptosis of oligodendrocytes and neurons(Kurt et al., 2014).Moreover, this pathological process also promotes neuronal and oligodendrocyte apoptosis via the ASK1-JNK-P38 signaling cascade (Takenouchi et al., 2008).Evidence has shown that the anti-apoptotic effects of novel drugs that inhibit Fas-mediated apoptotic pathways, c-Jun N-terminal kinase, and calcium-activated calpain may be options for the treatment of CCSCI (Dolan et al., 2016).
Autophagy is involved in regulating the redox state of cells and it is known to be a key regulator of SCI repair (Kardideh et al., 2019).In recent years,studies have highlighted cross-regulation between autophagy and apoptosis,with mutual inhibition.They may be jointly regulated by the Ser/Thr kinases and p53 pathways, and biochemical crosstalk occurs between them (Song et al., 2014).Autophagy may play a protective role in inflammatory cellmediated apoptosis (Beljanski et al., 2019), and mitochondrial autophagy inhibits apoptosis in several diseases such as cancer (Prabhu et al., 2013).A moderate level of autophagy can protect nerve cells, regulate inflammation,and inhibit apoptosis following CCSCI.In addition, autophagy can help regulate the expression of VEGF by stabilizing the phenotype of Hif-1α in a hypoxic environment, promoting angiogenesis.However, the role of spinal angiogenesis in CCSCI has not been described (Li et al., 2019).The interaction between autophagy and apoptosis and the effect of autophagy on angiogenesis may shed new light on potential treatments and further studies relating to angiogenesis after CCSCI.
RNAs
Previous studies indicate that microRNAs (miRNAs) are pivotal in many cellular processes, such as cell proliferation, differentiation, and apoptosis.miR-124,miR-126, miR-133b, and miR-494 all have significant roles in spinal cord repair following SCI (Huang et al., 2020).Remodeling of nerve cells is also regulated by miRNAs, including miR-451 and miR-494 (Wang et al., 2018).miR-181 participates in astrocyte-related inflammation, and reduction in the level of this miRNA affects the expression of glial cell line-derived neurotrophic factor and VEGF.This may reduce the vulnerability to neurons and enhance neurogenesis (Hutchison et al., 2013).An increasing number of studies have shown that the regulation of specific miRNAs may provide a new therapeutic target in SCI.
In addition, long noncoding RNA (lncRNA) is of great significance for angiogenesis.We demonstrated that Notch-1 promotes endogenous nerve repair and angiogenesis after surgical decompression (Cheng et al., 2021); in subsequent studies, we found that the lncRNA Xist regulates the expression of Notch-1 through sponging miR-32-5p to promote angiogenesis (Cheng et al.,2020).In addition, the notch signaling pathway is also regulated by miR-134,miR-153-3p, Xist/miR-137/32-5p, and others (Zhao et al., 2020), though their role in CCSCI has not been established using animal models of this condition.Therefore, it is necessary to continue to explore mechanisms that participate in angiogenesis following CCSCI.
Treatment
For patients in the early stage of CCSCI, when nerve compression is not severe, a conservative treatment strategy is usually used, including physical therapy, medication and epidural steroid injection.For those with moderate to severe clinical symptoms, and those in whom conservative treatment has not succeeded, surgical treatment should be considered.The specific operative method should be determined according to the status of the individual patient’s disease (Table 2).Surgical treatment is the most effective method of treating CCSCI.However, since most people with CCSCI present with obvious neurological symptoms, surgical decompression alone cannot completely reverse the pathological changes.It is reported that between 11% and 38% of patients still have some degree of dysfunction after decompression (Karadimas et al., 2015), and delayed treatment can lead to worse outcomes or even lifelong disability.At present, hyperbaric oxygen, neurotrophy, acupuncture,and moxibustion are used in clinical targeted treatment strategies, but the effect is limited.Therefore, early detection of surgical indications and earlier surgical treatment will yield a better prognosis.
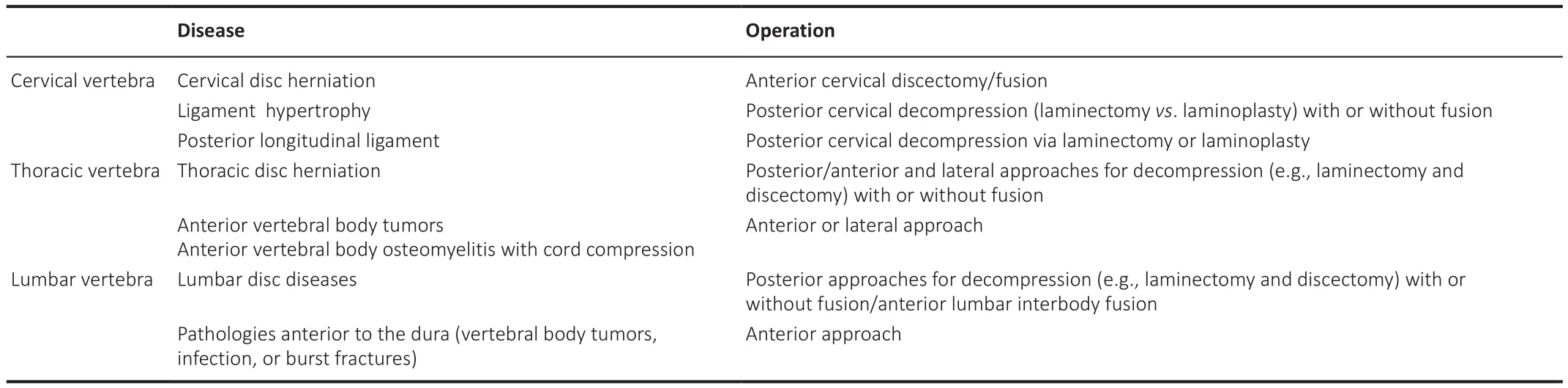
Table 2|Surgical procedures used for chronic compressive spinal cord injury
Moreover, early surgical treatment, defined as surgery within 24 to 72 hours after acute injury, also appears to be beneficial for ACSCI.Spinal cord compression is time-dependent for behavioral and electrophysiological recovery, and changes in blood supply (Furlan et al., 2011).The duration of compression also affects postoperative recovery.Together, these factors suggest that early surgical decompression may be feasible, and may improve outcomes.However, studies have shown that there is no significant difference in therapeutic effect between patients with early versus delayed surgical decompression (Furlan et al., 2011; Wilson et al., 2017).However, results may have been affected by sample quality, severity, level of injury, timing of follow-up, and other factors.Therefore, more evidence from further studies is needed to determine the need for early decompression and the precisetiming of surgical intervention (Furlan et al., 2011; Wilson et al., 2017).
In recent years, stem cell regenerative medicine has been applied in SCI to a certain extent, showing promising results (Papa et al., 2020).In addition,nanotherapy, in which nanocarriers deliver drugs to the site of injury, may also provide another avenue for treatment.With the use of nanocarriers,lower drug doses can achieve similar therapeutic effects at the cellular level as higher drug doses without the use of nanocarriers (Song et al., 2019).Some nanocarriers also enable the slow release of drugs, which can thus act on the injured site for a longer time and achieve a better therapeutic effect.Furthermore, it may be a good way to focus on inflammation, BSCB injury,and apoptosis to treat CCSCI.Elevated levels of angiogenesis may promote nerve repair by promoting the reestablishment of effective circulation,boosting blood flow, and meeting the nutritional and oxygen needs of oligodendrocytes and axon cells.As a result, angiogenic, neuroprotective, and neurotrophic factors may represent novel therapeutic tools.
Limitations
Our work has several limitations.First, our review was conducted using PubMed to find all relevant literatures written in English, so selection bias may exist in our article.Secondly, articles that have not been published yet,or were published in languages other than English, were not selected; thus,there may be reporting bias in our review.Thirdly, due to lack of available studies on some topics covered, we based our discussion on our own longterm work.Therefore, more research is needed to validate these points.
Conclusion and Outlook
Vascular disruption and reconstruction are important processes in the progression of CCSCI.However, the mechanisms of vascular injury and reconstruction at the compressed site require further clarification through more research.Vascular injury connects BSCB injury, inflammation of compressed parts, ischemia, and hypoxia, which together affect the function of the spinal cord.Therefore, research is currently focused on methods to reduce vascular injury after compression and promote repair after a vascular injury.Excitingly, with the development of molecular medicine in recent years, an increasing number of angiogenic mechanisms have been proposed.Although most of these have not yet been proven to be effective in the promotion of angiogenesis after compression, this may increase the number of therapeutic options in CCSCI.It is worth noting that there are few clinical reports on the mechanism of CCSCI, and consistency between the results of basic research and clinical practice remain unclear.In the near future, more vascular repair methods after CCSCI and therapeutic agents may be verified and used to treat CCSCI.
Author contributions:
Manuscript writing: ZXR.Manuscript revision: HQL,ZXR, JHX, XC.Figure preparation and revision: GXX, HQL, ZXR.All authors read and approved the final manuscript.
Conflicts of interest:
All authors report that they have no disclosures relevant to this manuscript.
Availability of data and materials:
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:
Chen Chen, Indiana University School of Medicine, USA.
Additional file:
Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Neural and Müller glial adaptation of the retina to photoreceptor degeneration
- Agomelatine: a potential novel approach for the treatment of memory disorder in neurodegenerative disease
- MicroRNAs: protective regulators for neuron growth and development
- In vivo astrocyte-to-neuron reprogramming for central nervous system regeneration: a narrative review
- Intranasal nerve growth factor for prevention and recovery of the outcomes of traumatic brain injury
- Altered O-GlcNAcylation and mitochondrial dysfunction,a molecular link between brain glucose dysregulation and sporadic Alzheimer’s disease

