Flower-like NiCo2S4/NiFeP/NF composite material as an effective electrocatalyst with high overall water splitting performance
Jing Jiang, Fengyan Li, Hui Su, Yangqin Gao, Ning Li, Lei Ge,∗
a State Key Laboratory of Heavy Oil Processing, College of New Energy and Materials, China University of Petroleum Beijing, Beijing 102249, China
b Department of Materials Science and Engineering, College of New Energy and Materials, China University of Petroleum Beijing, Beijing 102249, China
ABSTRACT Rational design and building of high efficiency, secure and inexpensive electrocatalyst is a pressing demand and performance to promote sustainable improvement of hydrogen energy.The bifunctional electrocatalysts for oxygen evolution reaction (OER) and hydrogen evolution response (HER) with high catalytic performance and steadiness in the equal electrolyte are extra treasured and meaningful.Herein,a unique three-dimensional (3D) structure electrocatalyst for NiCo2S4 growing on the flower-like NiFeP was designed and synthesized in this study.The results show that the flower-like NiCo2S4/NiFeP/NF composite electrocatalyst has large specific surface area, appropriate electrical conductivity, and greater lively websites uncovered in the three-dimensional structure, and affords extraordinary electrocatalytic overall performance for the ordinary water splitting.In alkaline solution, the OER and HER overpotentials of NiCo2S4/NiFeP/NF only need 293 mV and 205 mV overpotential to provide the current densities of 100 mA/cm2 and 50 mA/cm2, respectively.This high electrocatalytic activity exceeds the catalytic activity of most nickel-iron based electrocatalysts for OER and HER process.Accordingly, the optimized NiCo2S4/NiFeP/NF sample has higher stability (24 h) at 1.560 and 10 mA/cm2, which extensively speeds up the overall water splitting process.In view of the above performance, this work offers a fine approach for the further improvement of low fee and excessive effectivity electrocatalyst.
Keywords:NiCo2S4 Electrocatalyst NiFeP Hydrogen evolution reaction Water splitting
In recent years, with the contradiction between global energy demand and resource, and environment constraints becoming increasingly prominent [1], we urgently need to find a green and efficient alternative energy.Compared with other green energy, hydrogen energy is a new type of energy with the most development potential [2], due to the fact of its wealthy resources, high energy density, free from geographical and environmental conditions.Among them, compared with chlor-alkali industrial [3], chemical raw materials [4–6] (methanol cracking, ethanol pyrolysis, liquid ammonia cracking,etc.), petrochemical resources [7,8] (oil cracking,water gas preparation method,etc.) and new preparation method to produce hydrogen [9–11] (biomass, photochemical,etc.), electrochemical water splitting will become the main technology of hydrogen production [12,13] due to the high purity, clean green with the development of new type catalysts.
At present, precious metal-based materials with high catalytic activity have limited large-scale applications due to high prices and relatively small reserves in nature [14].A large number of studies have showed that electrocatalysts containing nickel, iron, and cobalt are relatively inexpensive and have higher electrocatalytic activity [15–18].Recently, transition metallic phosphides [19,20]and transition metallic sulfides [21] have additionally been significantly explored as candidate substances for non-noble metal-based electrocatalysts for electrolysis of water.According to reports, transition metal phosphides will increase the charge transfer rate and change the electronic structure to optimize the sorption energy,however its inherent low conductivity and insufficient active centers severely limit the improvement of their catalytic performance for water splitting [19,22].Similarly, the ternary nickel-cobalt sulfide exhibits a synergistic effect between different components and has high oxygen evolution electrocatalytic activity, however the stability is still unsatisfactory [21,23–25].In addition, compared with single-component materials, the interaction between the two components of the composite structure shows excellent electrocatalytic performance and has become a research hotspot [26,27].Therefore, it is very necessary to accurately design and finely synthesize high-efficiency, durable, and low-cost dual-functional electrocatalysts.
Herein, stimulated by using the above ideas, we have assembled a three-dimensional flower-shaped NiCo2S4/NiFeP/NF electrocatalyst through an easy one-step hydrothermal method, phosphating, and electrodeposition technological to gain a surprisingly environmentally friendly and steady dual-function electrolysis for water splitting.NiCo2S4/NF, NiFe-LDH/NF and NiFeP/NF were synthesized as comparison samples, and the effect of threedimensional flowered NiCo2S4/NiFeP/NF composites on the catalytic activity was investigated and confirmed.As expected, the organized three-dimensional flower-like NiCo2S4/NiFeP/NF composite confirmed properly catalytic overall performance for both HER and OER in 1 mol/L KOH.The overpotential of HER at 50 mA/cm2was 205 mV, and that of OER at 100 mA/cm2was 293 mV.In addition, the three-dimensional flower-shaped NiCo2S4/NiFeP/NF showed 10 mA/cm2in a two-electrode system at 1.560 V to achieve high-efficiency overall water splitting activity, and exhibited remarkable durability for 24 h, which was a promising highperformance dual-function electrocatalyst.
Scheme 1 shows the synthesis process of NiCo2S4/NiFeP/NF on a nickel foam substrate.Under the action of using urea as a chelating agent and ammonium fluoride as a structure directing agent,the OH−released in urea reacts with the added Fe3+and Ni2+ions to form NiFe-LDH through a simple hydrothermal reaction.Specifically, ammonium fluoride (NH4F) assists the morphology evolution of NiFe-LDH nanomaterials [28].Using the as-synthesized NiFe-LDH as the precursor, NiCo2S4/NiFeP/NF electrocatalyst was prepared by 350 °C high temperature phosphating and electrodeposition process.
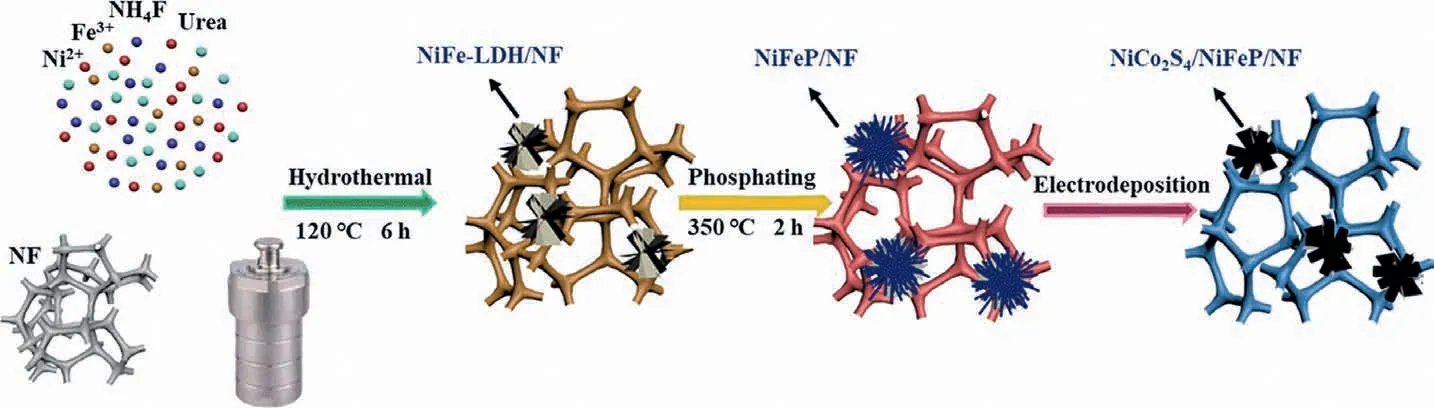
Scheme 1.Schematic diagram of NiCo2S4/NiFeP/NF synthesis process on nickel foam.
The X-ray diffraction pattern of NiCo2S4/NiFeP/NF (Ni:Co = 1:1)is illustrated in Fig.1.Since the diffraction peak intensity of the foamed nickel substrate is too powerful, it has masked the diffraction peaks of the electrocatalyst, so the electrocatalyst sample has been scraped off the foamed nickel substrate for XRD measurement (Fig.S1 in Supporting information).Among them, the 19.8°diffraction peak may be caused by the low purity of the sample scraped from the foamed nickel.As shown in Fig.1, all diffraction peaks in NiCo2S4/NiFeP/NF composite correspond to NiCo2S4(JCPDS No.200782), Ni2P (JCPDS No.741385) and FeP (JCPDS No.781443).NiCo2S4/NiFeP/NF sample has three sharp diffraction peaks at 31.58°, 50.46°, 55.33°, corresponding to the (311), (511),(400) crystal planes of NiCo2S4, respectively.The peaks at 47.36°and 54.19° are attributed to the (210) and (300) crystal planes of Ni2P, and the peaks at 46.29° and 48.31° are attributed to the (112)and (211) crystal planes of FeP.Therefore, it can be speculated that NiCo2S4/NiFeP/NF have successfully prepared.
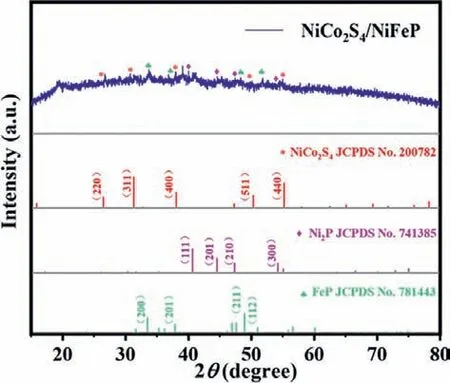
Fig.1.XRD patterns of NiCo2S4/NiFeP powder peeled off on nickel foam.
The surface morphologies of NF, NiFe-LDH/NF, NiCo2S4/NF,NiFeP/NF, NiCo2S4/NiFeP/NF were characterized by the scanning electron microscope (SEM).In Fig.S2a (Supporting information), it can be seen that the surface of NF is smooth and clean.According to the SEM image of NiCo2S4/NF (Figs.S2b and c in Supporting information), it can be seen that a good deal of NiCo2S4nanosheets with a thickness of several nanometers are deposited on the surface of NF, and they are connected with each other, which is conducive to promoting the diffusion of electrolyte ions.Figs.S2d and e (Supporting information) show the NiFe-LDH/NF nanosheets with a thickness of a few nanometers and a diameter of 200–500 nm grown under the guidance of the ammonium fluoride structure.The flower-like nanostructures assembled in sheet form and the ultra-thin nanosheets provide the possibility for the electrolyte to fully contact the active center.Compared with NiFe-LDH/NF, the size of NiFeP/NF catalyst does not change under high temperature phosphating at 350 °C, and the nano flakes are slightly damaged,however the nano-curd structure is basically maintained, as shown in Figs.S2f and g (Supporting information).In Fig.2a and Fig.S2h (Supporting information) NiCo2S4/NiFeP/NF, the thickness of the nanosheets increases, which may be caused by the sedimentation of smaller NiCo2S4nanosheets surfacewise of the large NiFeP nanosheets.The multi-level structure is conducive to the formation of more abundant space, further increasing the exposure of active sites, thereby obtaining more excellent electrocatalytic performance [29].The element mapping of the NiCo2S4/NiFeP/NF sample is presented in Figs.2b–i.It can be seen that the Ni, Fe, Co, P and S elements are even-distributed on the surface of the sample, so the EDS results are in accordance with the XRD results.NiFe-LDH/NF is assembled from a large number of nanosheets, and the lattice fringe of 0.26 nm matches the (012) crystal plane of NiFe-LDH, as shown in Fig.3b.Transmission electron microscopy (TEM) results show that NiFeP/NF sample presents a porous nano-sheet structure, which is conducive to electrolyte penetration and contact.
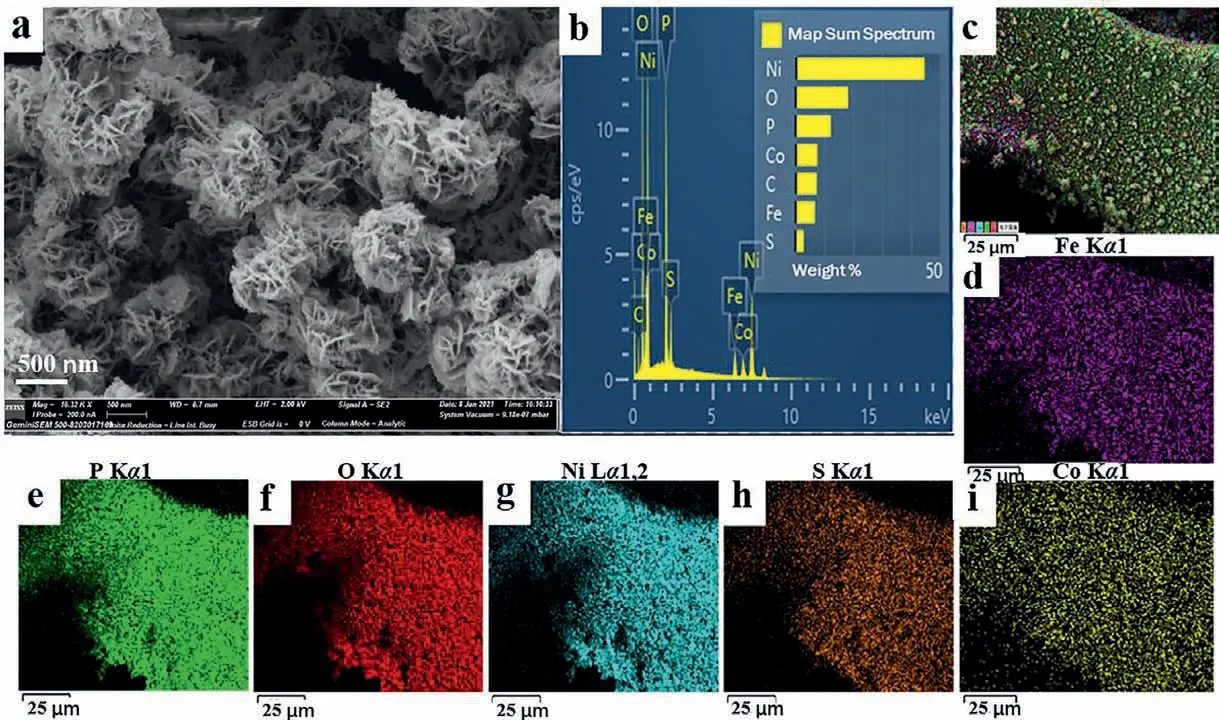
Fig.2.(a, b) SEM images and EDS (c–i) images of NiCo2S4/NiFeP/NF sample.
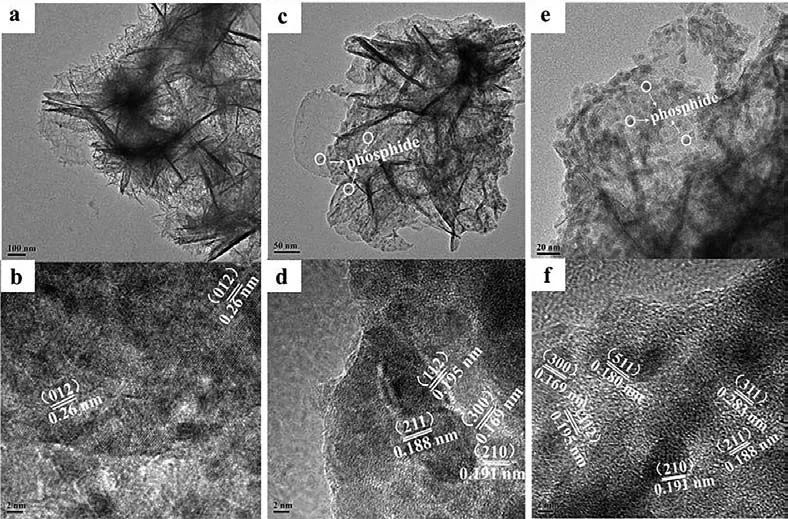
Fig.3.(a, b) The TEM and HRTEM images of NiFe-LDH/NF (c, d) NiFeP/NF (e, f) NiCo2S4/NiFeP/NF.
The microstructures of NiFe-LDH/NF, NiFeP/NF and NiCo2S4/NiFeP/NF samples were further explored by transmission electron microscopy (TEM).The sample was separated from the NF by an ultrasonic apparatus.Figs.3a, c and e further confirms the ultrathin nano-spherical structure of the electrocatalyst.In Fig.3b, the lattice fringes at 0.26 nm can be attributed to the (012) crystal plane of NiFe-LDH.As shown in Fig.3c, after high-temperature phosphating, phosphide clusters appear on the ultra-thin nanospheres.In Fig.3d, the lattice fringes at 0.191 nm and 0.169 nm can be attributed to the (210) and (300) crystal planes of Ni2P, and the lattice fringes at 0.195 nm and 0.188 nm act in response to the (112) and (211) crystal planes of FeP, respectively.Typically, as shown in HRTEM, NiCo2S4/NiFeP/NF not only shows the lattice fringes of Ni2P and FeP, but also confirms the existence of NiCo2S4phase.As shown in Fig.3f, the 0.283 nm and 0.180 nm lattice fringes match the (311) and (511) crystal planes of NiCo2S4.TEM and HRTEM results indicate the successful preparation of NiCo2S4/NiFeP/NF composite sample.
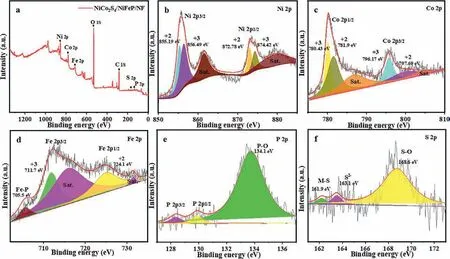
Fig.4.(a) XPS measurement spectrum and high-resolution XPS spectrum of NiCo2S4/NiFeP/NF in (b) Ni 2p, (c) Co 2p, (d) Fe 2p, (e) P 2p, (f) S 2p region.
The surface chemical composition and state of NiCo2S4/NiFeP/NF were characterized by XPS technique.Fig.4a confirms the presence of Ni, Co, Fe, P and S elements, which is consistent with EDS results.For the high-resolution X-ray photoelectron spectroscopy of Ni 2p (Fig.4b), the two characteristic peaks with binding energies of 855.9 eV and 873.5 eV are attributed to Ni 2p3/2and Ni 2p1/2orbitals, respectively.The two peaks at 861.7 eV and 879.9 eV are attributed to the satellite peaks of Ni 2p3/2and Ni 2p1/2, respectively.It is well worth noting that the peaks at 855.19 eV and 872.78 eV are attributed to Ni2+species, and the peaks at 856.49 eV and 874.42 eV are attributed to Ni3+, which confirms that the valence states of Ni in NiCo2S4/NiFeP/NF are both +2 and +3 [30].As shown in Fig.4c, Co2+(781.9 eV, 797.69 eV) and Co3+(780.43 eV, 796.17 eV) species can also be identified [31].Among them, it has been confirmed that the Ni and Co peaks shift relative to a single sample when NiCo2S4and NiFeP form the composite material, indicating that a strong chemical bond is formed between NiCo2S4and NiFeP [32].The high-resolution Fe 2p-XPS spectrum (Fig.4d) presents that the Fe element exhibits the coexistence of +2 valence (724.1 eV) and +3 valence (711.7 eV).Among them, the peaks with binding energies of 716.6 eV and 731.0 eV are satellite peaks, and the peaks at 705.5 eV are related to Fe-P species, which is consistent with previous work [32–34].Fig.4e shows that the XPS peaks with binding energies of 128.6 eV and 129.5 eV belong to P 2p3/2and P 2p1/2orbitals.The peak corresponding to P-O bond (134.1 eV)is formed due to the oxidation of NiCo2S4/NiFeP/NF in air [35].Correspondingly, S 2p can be divided into three peaks of 161.9 eV(metal-sulfur bond), 163.1 eV (low coordination sulfite ion), and 168.6 eV (S-O bond) [36,37], which is consistent with previous reports (Fig.4f).XPS analysis results indicate that NiCo2S4/NiFeP/NF has the +2, +3 valence of Ni, Fe, Co and the mixed valence of S2−.
Using a traditional three-electrode system, the HER performance of NiCo2S4/NiFeP/NF sample was investigated in 1 mol/L KOH electrolyte.First of all, the HER performance of the electrocatalyst is affected by synthesis temperature.In this experiment, NiFe-LDH/NF was phosphated at 300, 350 and 400 °C heating rate of 2 °C/min, and the optimal phosphating temperature of the precursor NiFeP/NF (Fig.S3 in Supporting information) was determined to be 350 °C.Too low phosphating temperature is not conducive to the formation of Ni2P and FeP, and too high phosphating temperature will destroy the original structure of the catalyst [38].In addition, the Ni/Co ratio of the electrodeposited NiCo2S4and the electrodeposited ring layer also affect the activity of the NiCo2S4/NiFeP/NF electrocatalyst.As shown in Fig.S4a(Supporting information), according to the linear sweep voltammetry (LSV) curves of hydrogen evolution with different Ni/Co ratios, the ratios of Ni:Co are 0:5, 1:4, 2:3, 3:2, 4:1, 5:0.When Ni:Co= 1:1, NiCo2S4/NiFeP/NF shows the best hydrogen evolution activity.Fig.S4b (Supporting information) presents that the HER activity of NiCo2S4electrodeposited 4 turns on NiFeP/NF is the best.For comparison, the HER performance of NiCo2S4/NF, NiFe-LDH/NF,and NiFeP/NF were also tested.As expected, the NiCo2S4/NiFeP/NF electrocatalyst performed the best HER activity (Fig.5a), which can be attributed to the synergy and interaction between NiCo2S4and NiFeP species.NiCo2S4/NiFeP/NF reaches current density of 50 mA/cm2with an overpotential of 205 mV, which is decrease than pure NiCo2S4/NF (295 mV) and NiFeP/NF (225 mV) electrocatalysts.
To further investigate the catalytic mechanism, the linear part of the polarization curve was fitted, and the calculated Tafel slope was used to estimate the catalytic kinetics.According to Tafel equation:η=blogj+a, whereηis the overpotential,jis the current density, andbis the Tafel slope.The Tafel slope of NiCo2S4/NiFeP/NF is 99 mV/dec, which is much lower than NiCo2S4/NF (156 mV/dec), NiFe-LDH/NF (123 mV/dec), NiFeP/NF(114 mV/dec), indicating good electron transport and reaction kinetics, As shown in Fig.5b.In addition, the best HER activity of NiCo2S4/NiFeP/NF is related to its high electrochemically active specific surface area (Fig.5c, Fig.S6 in Supporting information), which is mainly due to the increase in the quantity of active sites in the composite structure [39].For HER,the double-layer capacitance (Cdl) value of NiCo2S4/NiFeP/NF is determined to be 31.27 mF/cm2, which is significantly greater than NiCo2S4/NF (2.84 mF/cm2), NiFe-LDH/NF (6.4 mF/cm2) and NiFeP/NF (14.08 mF/cm2).In order to explore the charge transfer capability, electrochemical impedance spectroscopy (EIS) is evaluated in a 1 mol/L KOH aqueous solution.As shown in Fig.5d, the charge transfer resistance ranges from NiCo2S4/NF(Rct= 153.03Ω), NiFeP/NF (Rct= 40.27Ω) to NiCo2S4/NiFeP/NF(Rct= 9.23Ω), indicating that the composite structure can minimize ohmic loss and effectively promote the integration of electronics and Hads[26,40].To evaluate the stability of NiCo2S4/NiFeP/NF, the electrocatalytic activity is measured in 1.0 mol/L KOH with current density of 10 mA/cm2for at least 24 h by chronopotentiometry, no significant catalytic performance degradation is observed, indicating high catalytic stability (Fig.5e).In addition, in Fig.5f, compared to NF, NiCo2S4/NF, NiFe-LDH/NF and NiFeP/NF, NiCo2S4/NiFeP/NF shows excellent hydrogen evolution activity.High electrocatalytic activity and long-term stability establish NiCo2S4/NiFeP/NF as a promising HER electrocatalyst with potential applications.
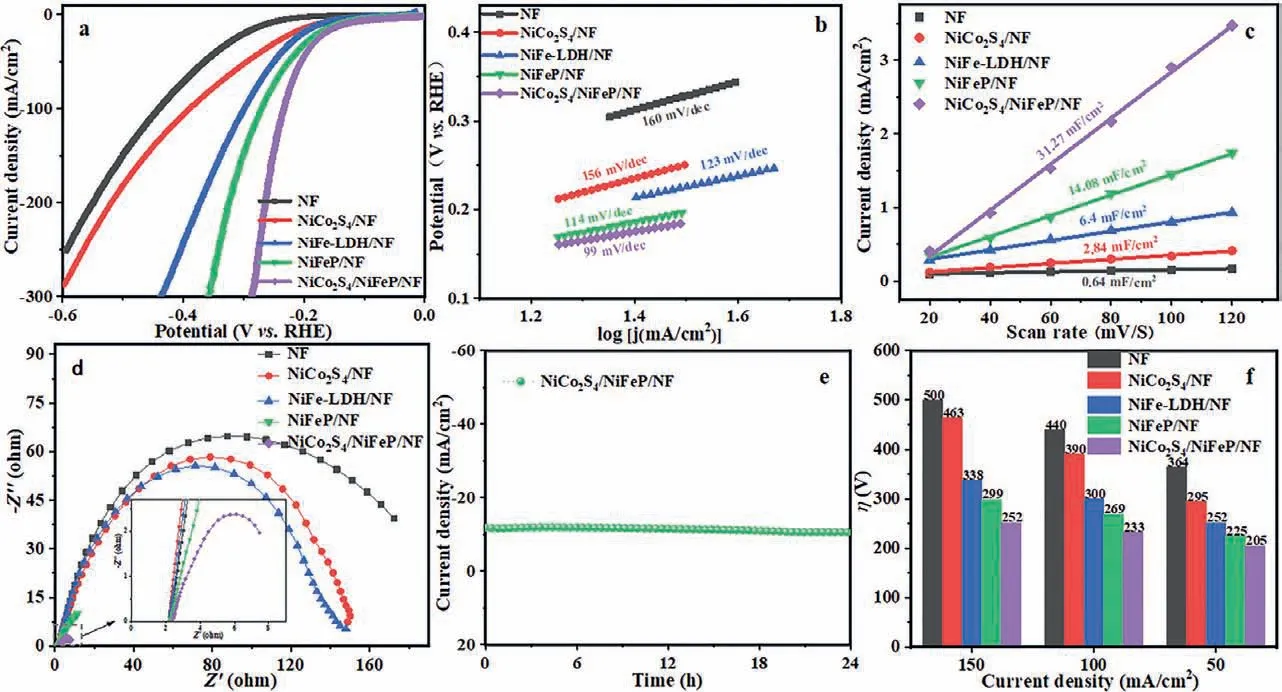
Fig.5.Electrocatalytic HER performance of NiCo2S4/NF, NiFe-LDH/NF, NiFeP/NF, NiCo2S4/NiFeP/NF composite system under 1 mol/L KOH: (a) LSV, (b) Tafel, (c) Cdl, (d) EIS curves and (e) long-term stability test of NiCo2S4/NiFeP/NF, (f) η required at different current densities.
Under the same test conditions as HER, the OER performance of NiCo2S4/NiFeP/NF was further evaluated.Similarly, the optimal NiCo ratio of NiCo2S4/NiFeP/NF and the electrodeposited ring layer was investigated (Fig.S5 in Supporting information).NiCo2S4/NiFeP/NF has the best oxygen evolution activity when Ni:Co = 1:1 and the electrodeposited ring layer is 4 circles.For OER, NiCo2S4/NiFeP/NF requires 293 mV overpotential at current density of 100 mA/cm2, (Fig.6a), which is significantly lower than that of pure NiCo2S4/NF (470 mV) and NiFeP/NF (340 mV).The high OER activity of NiCo2S4/NiFeP/NF is mainly attributed to the synergistic effect between NiCo2S4and NiFeP, as well as the rapid electron transfer and abundant active sites of NiCo2S4/NiFeP/NF three-dimensional flower-like composite structure.As shown in Fig.6b, NiCo2S4/NiFeP/NF has the lowest Tafel slope (110 mV/dec)compared with the NiCo2S4/NF sample (193 mV/dec) and NiFeP/NF sample (123 mV/dec), which means that it has faster oxygen evolution kinetics.It is conducive to the electro-absorption of OH−and the generation of OH∗[41].At the same time, the doublelayer capacitance (Cdl) value of the electrode was also measured by cyclic voltammetry (CV) at a scan rate of 20–120 mV/s on the sample, and calculated the electrochemical surface area is shown in Fig.6c.For OER, the double-layer capacitance (Cdl) value of NiCo2S4/NiFeP/NF is 31.1 mF/cm2, which is significantly greater than NiCo2S4/NF (3.34 mF/cm2), NiFe-LDH/NF (9.2 mF/cm2) and NiFeP/NF (12.1 mF/cm2), which is mainly due to the combination of NiCo2S4and NiFeP/NF increasing the number of active sites.In addition, compared with NiCo2S4/NF (Rct= 17.13Ω) and NiFeP/NF(Rct= 3.52Ω), NiCo2S4/NiFeP/NF (Rct= 3.36Ω) has fast charge transfer capability, as shown in Fig.6d.Previous work has confirmed that the strong electron interaction between two separate components in the composite electrocatalyst can effectively change the electronic structure, reduce the free energy of the intermediate adsorption orption, and thus improve the catalytic activity [42–45].In the stability test of 1.0 mol/L KOH, NiCo2S4/NiFeP/NF could keep stable for at least 24 h at 10 mA/cm2without significant degradation of performance (Fig.6e).In addition, in Fig.6f, compared to NF, NiCo2S4/NF, NiFe-LDH/NF and NiFeP/NF, NiCo2S4/NiFeP/NF shows excellent oxygen evolution activity.
For further check out the origin of OER activity, the NiCo2S4/NiFeP/NF sample after 24 h stability test was characterized.It is observed that the NiCo2S4/NiFeP/NF still maintains the three-dimensional nano-spherical structure, however the spherical thickness increased, as shown in Fig.7a.Among them, the peak at 853.0 eV is attributed to Niδ+ (δis close to 0), which may be the exposed Ni matrix after the sulfate falls off [46].XPS tests illustrate that the metal elements all move towards high binding energy after oxygen evolution (Figs.7b–d), indicating a tendency to form Ni3+, Fe3+, and Co3+species, promoting M-OOH production, similar phenomenon has also been observed in many previous studies[47–49].As shown in Fig.S8 (Supporting information), after the OER reaction in the Raman spectrum, NiOOH peaks were observed at both 473 cm−1and 553 cm−1[50], the peak of NiCo2S4at 682.5 cm−1becomes weaker [51], and the peak of CoOOH is shown at 824.6 cm−1[52].Correspondingly, the shift of the binding energy of P element and S element is mainly attributed to the electronic interaction between M (Ni/Fe/Co) [32,33,37,53].Moreover,NiCo2S4/NiFeP/NF produces phosphate species [54,55] (Fig.7e) and sulfate [56,57] (Fig.7f) after the oxygen evolution reaction.Nevertheless, the relevant literature has also shown that the real active species for oxygen evolution is the corresponding metal hydroxide[54–57].Therefore, high electrocatalytic activity and long-term stability have established NiCo2S4/NiFeP/NF as a promising OER electrocatalyst with potential applications.
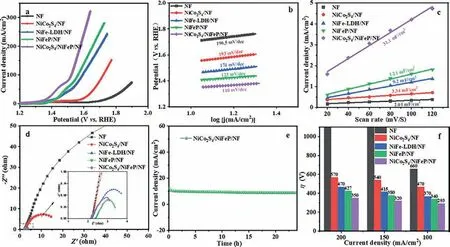
Fig.6.Electrocatalytic OER performance of NiCo2S4/NF, NiFe-LDH/NF, NiFeP/NF, NiCo2S4/NiFeP/NF composite system under 1 mol/L KOH: (a) LSV, (b) Tafel, (c) Cdl, (d) EIS curves and (e) long-term stability test of NiCo2S4/NiFeP/NF, (f) η required at different current densities.
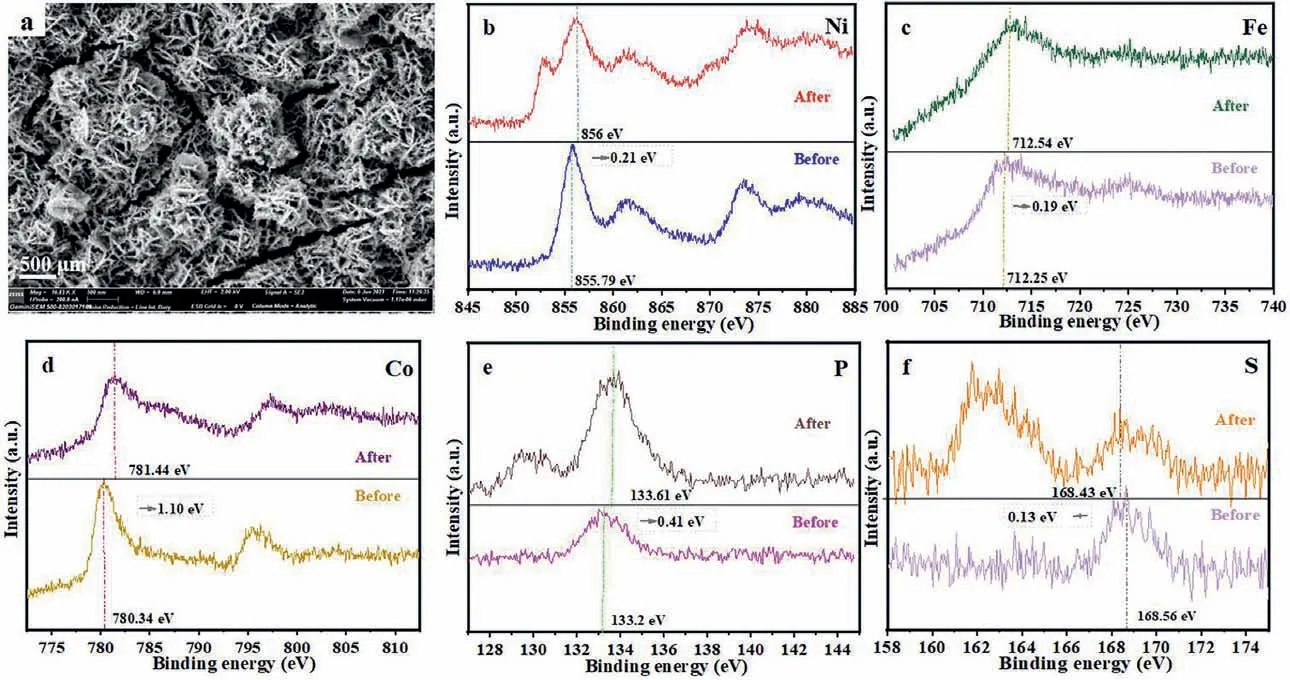
Fig.7.(a) SEM image of NiCo2S4/NiFeP/NF after OER stability test.XPS spectra of (b) Ni, (c) Fe, (d) Co, (e) P, (f) S before and after the reaction.
According to the above analysis, it is found that NiCo2S4/NiFeP/NF has superb hydrogen evolution and oxygen evolution things to do at the same time.NiCo2S4/NiFeP/NF are used as each cathode and anode to consider the overall water splitting endeavor in 1 mol/L KOH with a two-electrode system.Under the constant voltage current density without IR compensation and the scanning speed of 5 mV/s, the catalytic performance of electrocatalyst in practical application can be more accurately reflected [10].As shown in Fig.8a, when NiCo2S4/NiFeP/NF gives a current density of 10 mA/cm2, it solely needs a battery voltage of 1.560 V to attain the overall water splitting in 1 mol/L KOH.The general water splitting overall performance of the dual-function electrocatalyst is comparable to the reported composite structure electrocatalyst.For example, CoMoS4/Ni3S2(1.568 V) [58],NiFe2O4/CoNi–S (1.570 V) [59] and CoNi2S4/Ni3S2(1.65 V) [60].Furthermore, the stability of NiCo2S4/NiFeP/NF was tested, and the current density of 20 mA/cm2can be stable operation at 80.2% after 24 h (Fig.8b).As proven in Fig.S9 (Supporting information), both the cathode and the anode of the dual-electrode system have bubbles emerging, indicating that the overall water splitting performance of NiCo2S4/NiFeP/NF is excellent.Finally,using commercially available solar cells (2 V, Tianjin Shixun,54 mm × 54 mm) for NiCo2S4/NiFeP/NF||NiCo2S4/NiFeP/NF cell power supply, and the continuous evolution of H2and O2bubbles in the two electrodes, confirmed NiCo2S4/NiFeP/NF composite electric catalyst application value, as shown in (Fig.8c).
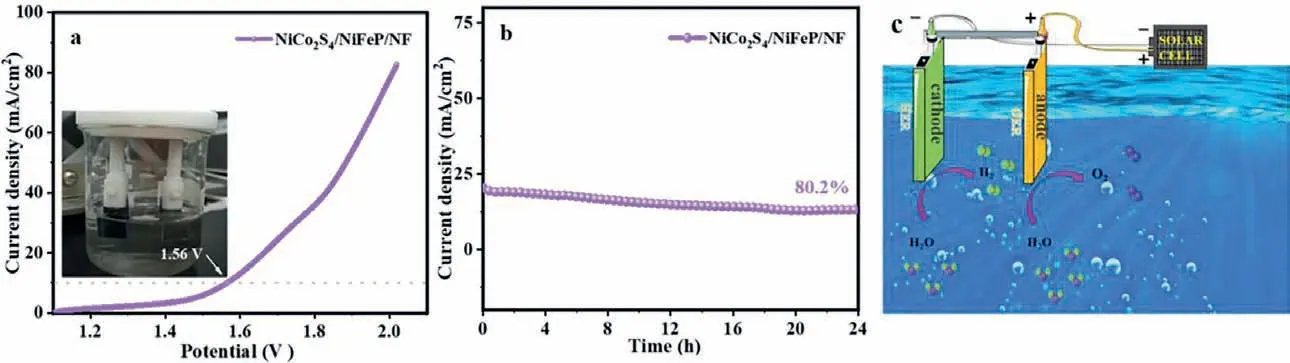
Fig.8.(a) LSV and (b) stability test curves of NiCo2S4/NiFeP/NF under 1 mol/L KOH.(c) Illustration of electrocatalytic water splitting over NiCo2S4/NiFeP.
In summary, a three-dimensional flower-like NiCo2S4/NiFeP/NF electrocatalyst was assembled through a simple one-step hydrothermal method, phosphating and electrodeposition techniques.Furthermore, the reaction mechanism of NiCo2S4/NiFeP/NF and the reasons for hydrogen evolution and oxygen evolution activity were systematically discussed.Notably, the superb electrocatalytic activity and stability of NiCo2S4/NiFeP/NF are attributed to the unique three-dimensional flower-like structure, which affords a high specific area and swarming active sites for the reaction.At the same time, the synergistic effect of NiCo2S4and NiFeP promotes the rapid transfer of charges, improves the binding energy of the reaction intermediates, and thereby increases the catalytic activity.In the two-electrode system, the high efficiency integral hydrolysis of 10 mA/m2is driven at 1.560 V and shows remarkable durability for 24 h.The consequences indicate that the composite is a promising excellent performance bifunctional electrocatalyst.This work gives a high-quality approach for the similar improvement of low-priced and high-efficiency electrocatalysts.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by National Key R&D Program of China (No.2019YFC1907602) and National Natural Science Foundation of China (Nos.51572295, 21273285 and 21003157).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.12.028.
 Chinese Chemical Letters2022年9期
Chinese Chemical Letters2022年9期
- Chinese Chemical Letters的其它文章
- A review on recent advances in hydrogen peroxide electrochemical sensors for applications in cell detection
- Rational design of nanocarriers for mitochondria-targeted drug delivery
- Emerging landscapes of nanosystems based on pre-metastatic microenvironment for cancer theranostics
- Radiotherapy assisted with biomaterials to trigger antitumor immunity
- Development of environment-insensitive and highly emissive BODIPYs via installation of N,N’-dialkylsubstituted amide at meso position
- Programmed polymersomes with spatio-temporal delivery of antigen and dual-adjuvants for efficient dendritic cells-based cancer immunotherapy
