Development of environment-insensitive and highly emissive BODIPYs via installation of N,N’-dialkylsubstituted amide at meso position
Junping Bi, Junling Zhou, Xin Ji, Nnnn Wng, Xiochun Dong,∗, Wei Wu,∗,Weili Zho,,∗
a School of Pharmacy, Institutes of Integrative Medicine, Fudan University, Shanghai 201203, China
b Key Laboratory for Special Functional Materials of Ministry of Education, School of Materials Science and Engineering, Henan University, Kaifeng 475004,China
ABSTRACT Fluorescent dyes play a crucial role in fluorescence imaging and sensing technology.However, there is a dilemma that they are usually intrinsically hydrophobic which lacks of emission in water and modification with ionic groups to access water solubility may result in poor membrane permeability.Fluorescent dyes with strong fluorescence emission in both nonpolar and polar solvents are highly desirable.In this manuscript, we reported a strategy to develop fluorescent BODIPY dyes via installation of amide moiety at meso position of 1,3,5,7-tetramethyl-BODIPY and discovered that N,N’-dialkylsubstituted BODIPY amides possessed highly fluorescent emission with favorable environment-insensitive properties.
Keywords:Fluorescent dyes BODIPY Environment-insensitive Bioimaging
Fluorescent imaging and sensing with high sensitivity and spatio-temporal resolution is a powerful tool for understanding of complex physiological and pathological dynamic processes [1].Fluorescent dyes are gradually playing a crucial role in imaging and sensing technology, such as super-resolution microscopy techniques [2,3], biomarkers [4,5], molecular probes [6,7], and tumor targeting imaging applications [8–10].However, these fluorescent molecules usually are highly fluorescent in organic media,but frequently lack sufficient water solubility and encountered aggregation-caused quenching (ACQ) in aqueous enviroment.Recently, we have developed ACQ-based bioimaging strategy for studingyin vivofate of nanocarriers [11].However, water soluble fluoreophores are essential for the majority of biological applications [12].Classic way to improve water solubility of fluorophores(such as CF, Dy light, ATTO, and Alexa dyes) was accessed through installation of sulfonate or phosphonate group(s).However, these structural modifications often produced cell-impermeant polar dyes.On the other hand, staining cells with hydrophobic dyes may not correctedly indicating the actual distribution of biological species detected because of ACQ.Along with the advances of analytical equipment and broaden applications of fluorescence imaging, fluorescent probes have been developed for various scientific applications.However, these utilizations still heavily rely on the traditional fluorophores.Fluorophores which are highly fluorescent in both organic medium and aqueous enviroment are still lacking.Herein, we developed a series of environment-insensitive brillant dyes on 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) family and illustrated imaging applications.
BODIPY dyes are well-known fluorophores in fluorescent probes and bioimaging applications [13,14].1,3,5,7-Tetramethyl-BODIPYs are most frequently used BODIPY dyes for various applications.However, these BODIPYs often suffer from ACQ in polar solvents,causing insufficient brightness and invalid excitation in aqueous solution.Existing strategies to enhance water solubility usually modified on the positions of 2,6-, 3,5- or 8–mesoposition(s) using ionic hydrophilic groups (quaternary ammonium salts [15,16],sulfonates [17–19] carboxylates [20], zwitterions moieties [21]), or oligo(ethylene glycol) moieties [22,23].
We are particularly interested in modifications atmesoposition due to the electronic effect of meso–substituent wherein photo-induced electron transfer (PET) plays a vital role in the regulation of fluorescence performance of BODIPYs [22,24].Notable examples lie in themesocarboxy modifications.Whilemeso–methoxycarbonyl-1,3,5,7-tetramethyl-BODIPY 1 (Scheme 1,λabs=511 nm,λem=526 nm,ΦF=0.019, in PBS using rhodamine 6 G as standard) exhibited poor fluorescence emission in aqueous solutions,meso–carboxyl-1,3,5,7-tetramethyl-BODIPY 2 (Scheme 1,λabs=496 nm,λem=511 nm,ΦF=0.58, in PBS with fluorescein as reference dye) revealed dramatic increment of fluorescence [25,26].Such a distinct change was useful to design various stimuliresponsive fluorescent probes [22,25,27-29].
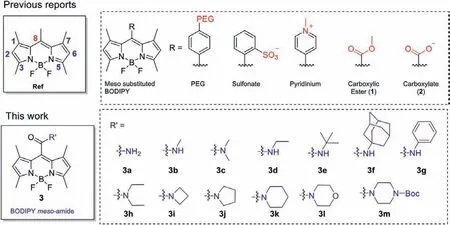
Scheme 1.Previous modifications on BODIPY scaffold to improve water solubility and our strategy for developing novel BODIPY dyes.
Our design concept is displayed in Scheme 1.We speculated that once a polar non-ionic amide functionality was installed in themesoposition of 1,3,5,7-tetramethyl-BODIPY, the steric repulsion of the 1,7-dimethyl groups might drive the amide functionality to twist out of the BODIPY plane to reduce or prevent hydrophobicπ-πpacking of BODIPY, and the twisted polar amide might be easily hydrated by water, therefore may ensure reasonable solubility both in organic media and in aqueous environment.Literature search indicated that the only known publication of BODIPYmesoamides were recently reported by Kim’s group based on 3,5-dimethyl-BODIPY scaffold with highly emissive nature [30].
The amides designed were prepared straight forward from the BODIPY carboxylate (2) which was obtained from hydrolysis of BODIPY carboxylic ester (1) shown in Scheme S1 (Supporting information).All designed compounds were synthesized in reasonable yields (Supporting information).The initial tests to varify our hypothesis were carried out using compounds 3a, 3b, and 3c in comparison with BODIPY carboxylic derivatives (1 and 2) with classsic hydrophobic dye of 1,3,5,7,8-pentamethyl BODIPY (Ref) as a reference.The spectroscopic properties of these dyes in various solvents were collected in Fig.1, Table 1, Table S1 and Figs.S1-S6 (Supporting information).
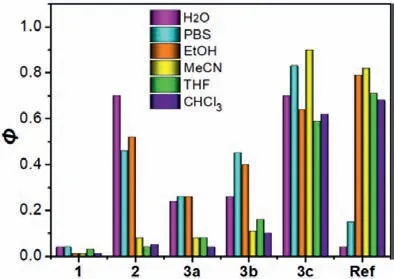
Fig.1.Fluorescence quantum yields of 1, 2, 3a–3c and Ref in various solvents.
All BODIPYmesocarboxylic derivatives showed longer absorption maxima than the Ref dye (Table 1).The absorption maxima of BODIPY amides are nearly independent of the substitution pattern in chloroform with absorption maxima around 512 nm which is longer thanmesocarboxylate 2 (502 nm) but slightly shorter thanmesoester 1 (515 nm).In aqueous environment, while BODIPY carboxylate 2 and amides (3a, 3b, 3c) showed small hypsochromic shift compared to organic medium, the BODIPY ester 1 formed J-aggregate with broadening of absorbance band and red-shifted absorption.However, Ref dye encountered H-aggregation in water with blue-shifted absorption maxima.
As expected, the Ref dye displayed highly fluorescent nature in organic solvents (ΦF0.68–0.82), however, due to hydrophobic packing in aqueous media, fluorescence was largely quenched(ΦFof 0.15 in PBS; 0.04 in water).The ester 1 was weakly fluorescent in organic solvents (ΦF0.01–0.03) owing to PET effect[25].In aqueous media, aggregation-induced emission (AIE) was found in accordance with literature report [26].In stark contrast,though carboxylate 2 had strong emission in polar environments, it only exhibited weak fluorescence in less polar or non-polar organic media (ΦF<0.10).Amide 3a possessed quite similar emission behavior to 2 though with lower fluorescence quantum yields in polar media.Monomethylation of amide (3b) slightly enhanced fluorescence quantum yields in all solvents investigated.Interestingly, bismethylation (3c) led to striking increment of fluorescence quantum yields (ΦF0.59–0.90) and provided balanced emission both in organic solvents and aqueous media.To get a hint behind this unique behavior, we carried out fluorescence lifetime analysis.The results were shown in Fig.S7 (Supporting information) and data were collected in Table S2 (Supporting information).The depressed quantum yields of BODIPYs often reflected large rate constants for nonradiative deactivation.Particularly fast nonradiative deactivation (knr>109s–1), which was believed to be associated with deviations from planarity of the boradiazaindacene ring system in the ground state [26], was found for ester 1 in all kinds of solvents investigated.For carboxylate 2,knrvalue in CHCl3was about 5-fold of it in water, indicating weaker emission in CHCl3.For primary amide 3a and secondary amide 3b, the trends of solvent-dependence ofknrare similar to 2, however, the difference between non-polar solvent and polar solvent is smaller.Striking difference was found for tertiary amide 3c whereinknrvalues were smaller than those of other dyes nearly in all circumstances with comparable rates to radiative deactivation (kr) in all solvent systems, leading to solvent-independent highly emissive characteristics.To the best of our knowledge, such a solvent-independent brilliant fluorophore has not been reported and such a prominent feature may be highly attractive for biological applications, thus detailed evaluations were carried out afterwards focusing on compounds 3a, 3b, and 3c (Fig.2).
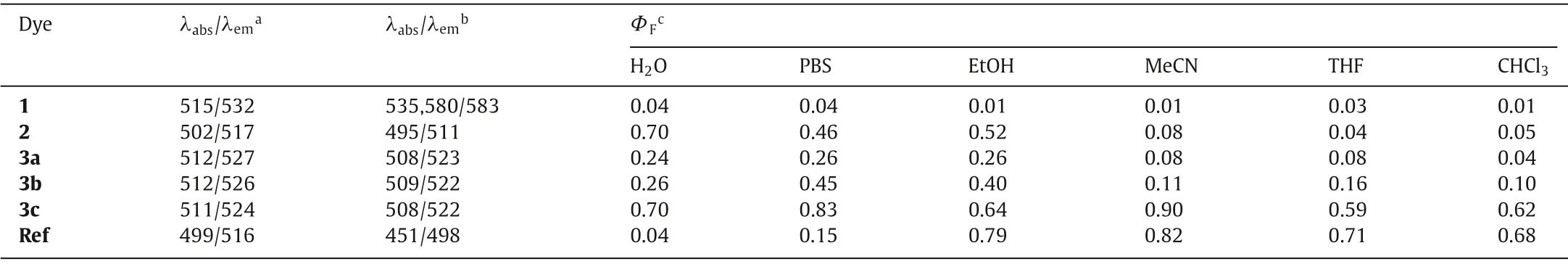
Table 1 Spectroscopic properties of BODIPY dyes.
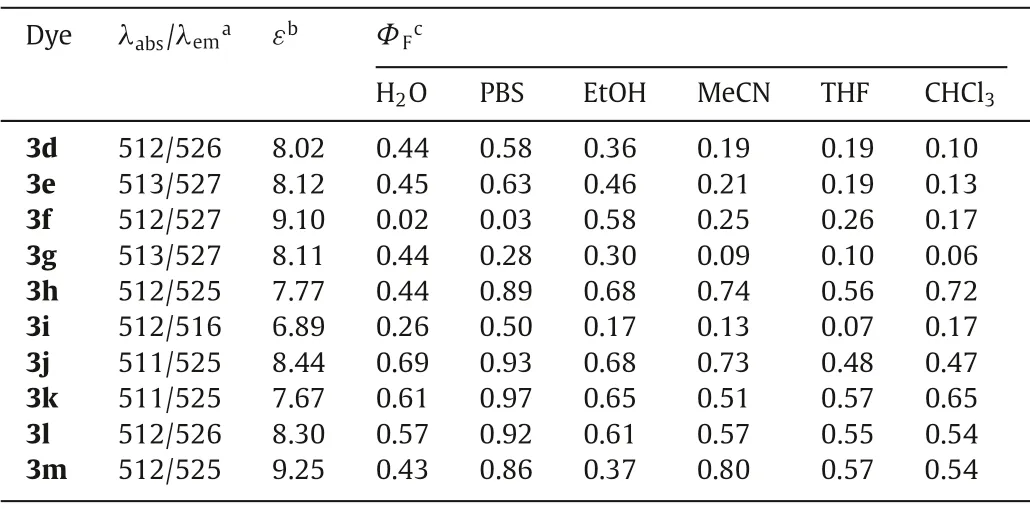
Table 2 Spectroscopic properties target compounds.
Time-dependent fluorescence responses in water were investigated during 24 h.Unlike AIE fluorescence of 1 which gradually declined upon standing (data not shown), all intensities of 3a–3c remained relatively stable even though their fluorescence intensities are different (Fig.2a and Fig.S8 in Supporting information).Therefore, modificationviaintroduction of amide could effectively prevent ACQ.
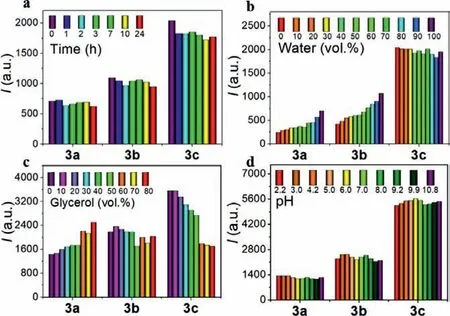
Fig.2.(a) Time-dependent fluorescence intensities of 3a–3c (5 μmol/L) in water monitored at emission maxima.(b) The polarity-dependent emission behaviors of BODIPY meso amides 3a–3c (5 μmol/L) were in depth studied in mixtures of acetonitrile/water.(c) Fluorescence intensities of BODIPY dyes (10 μmol/L) in waterglycerol mixtures.(d) Fluorescence intensities of 3a–3c monitored at emission maximum in phosphate buffers.Excitation of 460 nm was used.
The polarity-dependent emission behaviors of BODIPYmesoamides 3a–3c were in depth studied in mixed solvent of acetonitrile/water in various ratios (Fig.2b, Figs.S9 and S10 in Supporting information).While absorption spectra were only marginal affected by water content, the fluorescence emission behaviors were strikingly different.3a and 3b became steadily more brilliant along with the increment of water content whereas 3c remained stable emission.Therefore, 3c turned out to be a polarity-independent and highly emissive fluorophore.
We were also interested in viscosity influence.As shown in Fig.2c and Fig.S11 (Supporting information), when the content of glycerol in water became greater, 3a gradually turned brighter while 3c darkened but maintained fluorescent nature at the similar level of 3a and 3b under very sticky environment.Interestingly,3b seemed to be marginally affected by the viscosity.Overall, all the BODIPY amides were not very sensitive to viscosity.
We then carried out studies of pH effect.From Fig.2d and Fig.S12 (Supporting information), it can be concluded that the luminescence outputs for 3a–3c were not affected by the pH change in the range of 2.2–10.8.This characteristic implies the advantage of BODIPY fluorophores.
Photostability is a very important parameter of a fluorophore.BODIPY dyes have been well-known to be fairly photo stable.To differentiate the bleaching rate of various BODIPY fluorophores, we used very powerful Xe white light to irradiate the solution of our compounds.The photo-stabilities of our dyes were shown in Fig.S13 (Supporting information).Unsubstituted amide (3a) possessedthe best photostability.Mono- and disubsitituted amides (3b and 3c) showed identical photostability.All amide-derived fluorophores(3a–3c) were found to be more stable than carboxylate 2.
The evaluations carried out so far suggested that the disubstituted amide 3c possessed favorable environment-insensitive property with brilliant emission.We further studied the concentration effect of 3c and the results were shown in Fig.S14 (Supporting information).While the absorbance followed aquasi-linear relationship to concentrations investigated (1–50 μmol/L), the fluorescence intensity deviated slightly from linear response especially in high concentration circumstance.Therefore, 3c remained highly fluorescent even at high concentration without obvious ACQ effect.
We thus intrigued to know whether the trend observed was general and further modifications on amide moiety were allowed.In this regard, we installed various side chains (3d–3m in Scheme 1) on amide portion and the results were collected in Table 2, Table S3 (Supporting information), and were shown in Figs.S15-S20 (Supporting information).It could be seen that in organic solvents, the substituents on amide moiety did not alter the absorption maxima.In aqueous media, similar conclusion was obtained except for 3f which contained bulky hydrophobic adamantane side chain and formed aggregation.Such aggregation was also reflected from the fluorescence quantum yield of 3f wherein the emission was largely quenched in water or PBS.When small alkyl presented (3d, 3e), the solvent effects were similar to those for 3b with only slightly higher fluorescence quantum yields and favorable luminescence in polar environments (water, PBS, and EtOH).When phenyl was installed onto amide (3g), the dye usually possessed declined brightness.In the situation wherein diethyl presented (3h), delightful solvent-independent highly emissive pattern was seen.To our pleasant, most cyclic amides (3j–3m) showed brilliant solvent insensitive emission except for azetidine-derived BODIPY (3i) with lower fluorescence quantum yields presumably due to the strain of azetidine moiety [32].
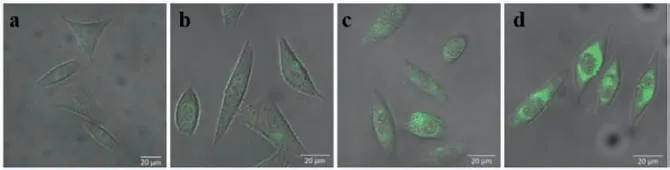
Fig.3.Confocal fluorescence images (λex=488 nm) of BODIPYs (10 μmol/L) in HeLa cells: (a) 2; (b) 3a; (c) 3b; (d) 3c.Scale bar: 20 μm.
To demonstrate the feasibility of dyes for biological applications, the cellular toxicity was evaluated using 3c as a representative dye was performed.No obvious toxicity on the HUVEC cell was observed for 3c (≤20 μmol/L) as shown in Fig.S21 (Supporting information).We subsequently examined the imaging ability in living HeLa cells for various compounds by confocal fluorescence microscopy.From the images shown in Fig.3, the green emission was hardly visualized in HeLa cells incubated with carboxylate 2, confirming the poor permeability of BODIPY carryingmesocarboxylate moiety.By contrast, green images were seen in the order of brightness as 3c>3b>3a, suggesting that improved permeability amide-derived BODIPYs.Overall, disubstituted amide 3c possessed the most favorable properties such as highly emissive, solvent-independent, polarity-independent, pH-independent,viscosity-insensitive, least quenching, and cell-permeable etc.Additionally, 3c was co-stained with Lyso-tracker Red/Mito- Mito-Tracker Deep Red in HUVEC cells (Fig.S22 in Supporting information) and was found that there is no organelle-targeting at all.
In conclusion, we have discovered thatN,N’-dialkylsubstituted BODIPY amides possessed highly fluorescent emission with favorable environment-insensitive brilliant emission.We also preliminary discovered that BODIPYmesoamide can be easily modified to fulfill various detection and imaging applications.Our preliminary studies indicated that our amide BODIPY strategy can be easily transformed to reaction-based fluorescent probe, as well as subcellular targeted probe.The detection of ROS and lysosome imaging applications with amide BODIPY derivatives are current undergoing and will be reported shortly.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (No.82030107).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.02.017.
 Chinese Chemical Letters2022年9期
Chinese Chemical Letters2022年9期
- Chinese Chemical Letters的其它文章
- A review on recent advances in hydrogen peroxide electrochemical sensors for applications in cell detection
- Rational design of nanocarriers for mitochondria-targeted drug delivery
- Emerging landscapes of nanosystems based on pre-metastatic microenvironment for cancer theranostics
- Radiotherapy assisted with biomaterials to trigger antitumor immunity
- Programmed polymersomes with spatio-temporal delivery of antigen and dual-adjuvants for efficient dendritic cells-based cancer immunotherapy
- The effect of organic ligand modification on protein corona formation of nanoscale metal organic frameworks
