Surface modification of hollow capsule by Dawson-type polyoxometalate as sulfur hosts for ultralong-life lithium-sulfur batteries
Mingliang Wang, Di Yin, Yundong Cao, Xinyang Dong, Guanggang Gao, Xun Hu,Cheng Jin, Linlin Fan, Jian Yu, Hong Liu
School of Materials Science and Engineering, Collaborative Innovation Center of Metal Nanoclusters & Photo/Electro-Catalysis and Sensing, University of Jinan, Ji’nan 250022, China
ABSTRACT Reasonable construction of sulfur host with high conductivity, large sulfur storage gap, strong chemical adsorption, and fast oxidation–reduction kinetics of polysulfide is very significant for its practical use in lithium-sulfur batteries (LSBs).In this paper, the surface modification of MIL-88A(Fe) is carried out by Dawson-type polyoxometalate (POM), and a hollow capsule shell material with P2W18, Fe3O4, and C components is synthesized by the subsequent carbonization process.When applied as the sulfur host,the hollow capsule shell material can efficiently improve the conductivity of sulfur electrode and restrain the volumetric change of active sulfur while charging and discharging.On this foundation, electrochemical analysis and density functional theory (DFT) calculation show that the P2W18 on the outer layer of the capsule shell have effective electrocatalytic activity and potent chemical bond on the lithium polysulfides (LiPSs), which is helpful to block the shuttle effect.Therefore, the as-assembled LSBs display the outstanding specific capacity and prominent cycle stability.Specifically, it delivers an excellent reversible capacity of 1063 mAh/g after 100 cycles of charge–discharge at a rate of 0.5 C, accounting for a preservation by 96% in comparison to that of the initial cycle.Moreover, even after 2000 cycles at 1 C,the reversible specific capacity of 585 mAh/g can still be maintained with an average decay rate of only 0.021%.
Keywords:Polyoxometalates Hollow capsule shell Catalytic activity Chemical bonding Lithium-sulfur batteries
The demands of large-scale energy storage systems and portable electronic devices present the growing trend at present,which motivates the search for energy conversion systems with high energy density and power density [1].Notably, lithium-sulfur batteries (LSBs) are considered to be one of the most potential next generation high-energy battery systems because of its excellent energy density and theoretical capacity [2,3].Nevertheless, the LSBs are still prevented by some thorny problems in practical commercial application: (1) the inferior conductivity of sulfur and its lithiation product (Li2S) causes the slow redox kinetics and low sulfur utilization, and thus limits the rate capacity; (2) the shuttle behavior of discharge intermediates (lithium polysulfides, LiPSs)dissolved in the electrolyte leads to poor cycle durability and low Coulomb efficiency (shuttle effect); (3) the serious volume expansion (about 80%) of sulfur in the lithiation can result in the structure collapse during the charge/discharge cycle [4–6].
To circumvent the above challenges, mammoth effort has been geared to promote the electrochemical properties of LSBs [7,8], including the construction of superior sulfur hosts [9,10], the development of functional membranes or modified interlayer, and the optimization of electrolyte additives [11].Among the reported strategies, the design of carbon-based matrix materials as the sulfur hosts is feasible, which can raise the electric conductivity and restrain the volume expansion of sulfur electrode to some extent[12–14].However, these carbon-based sulfur host materials cannot offer strong chemical affinity with polysulfide species, which seriously affect the cycle life of the LSBs [15,16].Furthermore, according to previous reports, polar inorganic metal-based compounds could supply rich polar active sites for the capture of LiPSs (such as metal nitrides, phosphides, carbides, sulfides, oxides) [17–24].For example, Zhanget al.prepared InN nanowires to modify the separators in LSBs.Both the electron-abundant nitrogen and indium cation of InN act as the polysulfide trapsviarobust chemical interaction.At the same time, in the process of electrochemical reaction, the fast electron transfer on polar InN surface expedites the transformation of polysulfides.Hence the InN can efficaciously subdue the shuttle effect [25].Sun’s group demonstrated nitrogendoped carbon nanosheets/molybdenum phosphide nanocrystal hollow nanospheres, which can be used as an electrochemical catalyst to intensify the redox reaction kinetics of LiPSs and reduce Li2S nucleation/dissolution interfacial energy barrier [26].
As we all know, polyoxometalates (POMs) have been widely used in the electrochemical field due to their good proton transfer ability and catalytic activity [27–33].However, there are few reports on the application of POMs in LSBs.Specifically, Yaoet al.synthetized bifunctional separators constituted of Keggin[PW12O40]3−/Super P composite retarding layer, the POMs can act as a Lewis acid site to further enhance the adsorption of the polysulfides [34].Ye and co-workers found that the LiPSs can be intensively adsorbed on the {AgIPW11O39} cluster by spectroscopic investigations, and the Ag(I) ion of POMs cluster can be used as a Lewis acid site to robustly assimilate S-moieties [35].More interestingly, Choiet al.designed a series of POMs, which were used in sulfur electrode.They found that POMs have multiplicate redox potentials containing the range of equilibrium potentials for sulfur redox processes, which was beneficial to accelerate charge and discharge reactions.Density functional theory (DFT) calculations revealed that the redox potentials of POMs are adjustable,which allowed the optional design of appropriate POMs for specific battery systems [36].Furthermore, metal–organic frameworks(MOFs) are a class of crystalline organic–inorganic hybrid materials with large specific surface area, high porosity, and excellent stability, which show the application potential in hydrogen storage, catalysis, electrochemistry, and sensors.According to previous reports, MOFs, such as ZIF-8, HKUST-1, and ZIF-67 [37–39] were fabricated and annealed into hierarchical porous carbon as host carriers of sulfur cathode.The resulting carbon materials with porous structure and high specific surface area were beneficial to the sulfur confinement.Therefore, we speculate that it should be possible to exploit MOF–POM hybrids for practical application of LSBs.
Motivated by the above considerations, we have designed a hollow capsule shell containing P2W18(K6P2W18O62·14H2O, a classic Dawson-type POM), Fe3O4, and C components derived from MIL-88A(Fe)/P2W18hybrids by chemical etching and carbonization.MIL-88A(Fe)-derived carbon shell surface has hierarchical porous structure and the partial sulfur confinement in ultra-small mesoporous carbon, combined with hollow capsule structure, guaranteeing the high sulfur loading and effectively suppressing volume expansion of the sulfur upon cycling.More significantly, P2W18on the surface of capsule shell, as a dual-function assistant, can not only have the strong chemical adsorption for polysulfide, but also show the effective catalytic activity for LiPSs.Benefiting from these favorable factors, in the present study, the hollow capsule shell utilized as a multi-functional sulfur host exhibits superior reversible capacity, extraordinary rate capability and long-life cycle stability.When the current density is set as 0.5 C (1 C = 1675 mAh/g), the specific capacity of 1063 mAh/g can be reached upon 100 cycles.More strikingly, it can be stabilized for 2000 cycles at 1 C, in which the average attenuation rate of per cycle is only 0.021%.
In this work, the synthesis procedure of hollow capsule shell is shown in Scheme 1.MIL-88A(Fe) is initially synthesized by the method reported in the literature [40] and used as the template material.Then P2W18is successfully loaded on the surface of MIL-88A(Fe) nanorods by hydrothermal reaction.Under acidic conditions, the combination between Fe3+and organic ligands is weakened due to the presence of P2W18, and thus MIL-88A(Fe) slowly releases Fe3+.The Fe3+can be adsorbed on the surface of MIL-88A(Fe)viaP2W18.Due to the Kirkendall effect [41], the diffusion speed of Fe3+in MIL-88A(Fe) is greater than that in solution,therefore, a cavity structure appears in the interior of MIL-88A(Fe),forming the MIL-88A(Fe)/P2W18composite with the hollow capsule structure.Subsequently, MIL-88A(Fe)/P2W18is carbonized to obtain composite containing P2W18, Fe3O4, and C, and the original hollow capsule shell structure can be well retained.Finally, the sulfur monomer is diffused into the capsule shell material by melt diffusion method.

Scheme 1.The synthetic procedure of hollow capsule sulfur host.
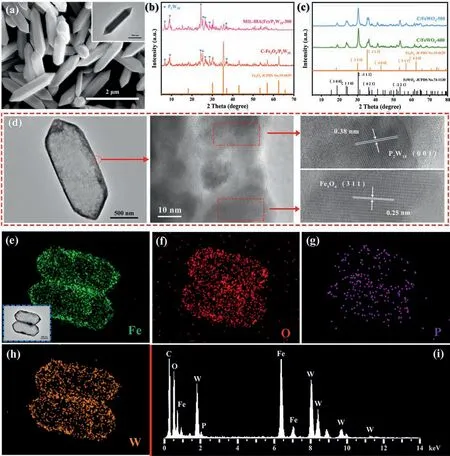
Fig.1.(a) SEM image of MIL-88A(Fe) (the insert is TEM image).(b) XRD patterns of MIL-88A(Fe)/P2W18-300 and C-Fe3O4/P2W18.(c) XRD patterns of C/FeWO4-500 and C/FeWO4-600.(d) TEM and HRTEM images of C-Fe3O4/P2W18.The elemental mapping of (e) Fe, (f) O, (g) P, and (h) W of C-Fe3O4/P2W18.(i) EDX spectrum of C-Fe3O4/P2W18.
The morphology characteristics and crystalline structures of samples are determined.As shown in Fig.1a, the length and width of MIL-88A(Fe) nanorods with smooth and dense surface are about 2 μm and 500 nm, respectively.After surface modification and etching of MIL-88A(Fe), the hollow capsule shell-shaped MIL-88A(Fe)/P2W18composite can be obtained (Fig.S1a in Supporting information).In Fig.S2 (Supporting information), it can be observed from Fourier transform infrared spectroscopy (FTIR)that the main peak position of MIL-88A(Fe) do not change significantly after being modified by P2W18, demonstrating that the structure of MIL-88A(Fe) template is not destroyed after etching.The characteristic peaks of W−O group (910, 943, and 1075 cm−1) can be clearly observed from MIL-88A(Fe)/P2W18, which confirms that P2W18is modified on the surface of MIL-88A(Fe)shell.In order to detect the crystal structure and phase purity,X-ray diffraction (XRD) analysis of MIL-88A(Fe), P2W18, and MIL-88A(Fe)/P2W18is performed (Fig.S3 in Supporting information).As observed, the diffraction peaks of MIL-88A(Fe) are identical with the data published in references [42], suggesting that a relatively pure MIL-88A(Fe) template.For MIL-88A(Fe)/P2W18, the characteristic diffraction peaks of P2W18are clearly displayed, which further illustrates that P2W18is successfully modified on MIL-88A(Fe).Moreover, the loading amount of P2W18is calculated to be 30.25 wt%viainductively coupled plasma optical emission spectrometer (ICP-OES) (Table S1 in Supporting information).Subsequently,MIL-88A(Fe)/P2W18composites are carbonized at 300, 400, 500,and 600 °C.Due to the insufficient carbonization at 300 °C, one can see from the XRD patterns (Fig.1b) that there are no obvious changes compared with MIL-88A(Fe)/P2W18, and the resulting material can be marked as MIL-88A(Fe)/P2W18–300.When the calcination temperature is 400 °C, the diffraction peaks of P2W18and Fe3O4can be clearly observed (marked as C-Fe3O4/P2W18).The peaks at 6.44°, 9.35°, 24.69°, 25.38°, 27.35°, 29.07°, and 35.93°correspond to the index numbers of P2W18(100), (−111), (223),(204), (115), (−404), and (−307), respectively.Meanwhile, diffraction peaks at 18.27°, 30.09°, 35.42°, 43.05°, 53.39°, 56.94°, and 62.52° correspond to the index numbers of Fe3O4(110), (220),(311), (400), (422), (511) and (440), respectively.Furthermore, as shown in Fig.S4 (Supporting information), the characteristic peaks of MIL-88A(Fe) cannot be found from FTIR of C-Fe3O4/P2W18, suggesting the sufficient carbonization of MIL-88A(Fe) at 400 °C.Fig.S5 exhibits that the content of C in C-Fe3O4/P2W18composite is calculated to be 6.8 wt%.When the temperature continues to rise to 500 °C and 600 °C (Fig.1c), no diffraction peaks of P2W18are observed due to the higher carbonization temperature, and the diffraction peaks of FeWO4are existent, which indicates the disintegration of P2W18, thus the obtained materials are marked as C/FeWO4-500 and C/FeWO4-600, respectively.Furthermore, MIL-88A(Fe)/P2W18-300 and C-Fe3O4/P2W18maintain the original hollow capsule shell structure with uniform distribution (Fig.1d and Fig.S1b in Supporting information).With the temperature increasing, the surface of C/FeWO4-500 is slightly fragmented, and the framework of C/FeWO4-600 is completely fractured and collapsed(Figs.S1c and d in Supporting information).Based on the points discussed above, C-Fe3O4/P2W18presents the optimizational carbonization and retains the original hollow capsule shell structure.When employed as the sulfur host, C-Fe3O4/P2W18hollow capsule can relieve the volume change of sulfur cathode and achieve high sulfur loading.The high resolution transmission electron microscopy (HRTEM) images in Fig.1d demonstrate that the lattice fringe spacings of 0.38 nm and 0.25 nm agree well with (001)plane of P2W18and (311) plane of Fe3O4, which proves the high crystallinity of P2W18and Fe3O4.Moreover, the elemental mapping and energy-dispersive X-ray spectroscopy (EDX) of C-Fe3O4/P2W18show that Fe, O, P, and W elements are uniformly distributed in the shell layer (Figs.1e–i).
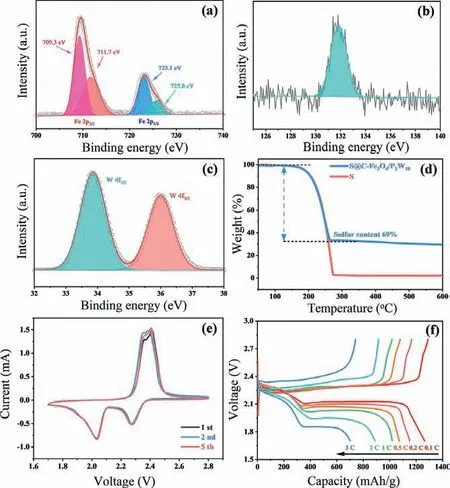
Fig.2.XPS analysis of C-Fe3O4/P2W18: (a) Fe 2p spectra, (b) P 2p spectra, and (c)W 4f spectra.(d) TGA curves of bare S and S@C-Fe3O4/P2W18.(e) CV curves of S@C-Fe3O4/P2W18 electrode at a scan speed of 0.1 mV/s.(f) Galvanostatic charge–discharge curves of S@C-Fe3O4/P2W18 electrode at current densities from 0.1 C to 3 C.
The surface chemistry environment of C-Fe3O4/P2W18is further analysed by X-ray photoelectron spectroscopy (XPS).Fig.S6 (Supporting information) presents typical survey spectra, strong W, P, C,Fe, and O signals can be observed.The high resolution XPS spectra of Fe 2p are revealed in Fig.2a, the peaks at 709.3 eV/711.7 eV and 723.1 eV/725.8 eV belong to the Fe 2p3/2and Fe 2p1/2orbital double peaks, respectively, confirming the formation of Fe3O4.The peak position at 131.9 eV in Fig.2b is in accord with binding energy of P 2p, and the characteristic peaks at 33.9 eV and 36.0 eV are corresponding to W 4f7/2and W 4f5/2(Fig.2c), respectively, suggesting P2W18can exist stably after the etching and carbonization process.Subsequently, C-Fe3O4/P2W18is used as a sulfur host material, the sulfur can diffuse into C-Fe3O4/P2W18by melt diffusion method (marked as S@C-Fe3O4/P2W18), and the XRD pattern shows the obvious diffraction peaks of sulfur (Fig.S7 in Supporting information).As shown in Figs.S8 and S9 (Supporting information), the S@C-Fe3O4/P2W18retains the original capsule shell structure.The sulfur particles accumulate inside the hollow thin shell with the large voids, and partial sulfur can be absorbed on the capsule shell surface.Moreover, the Brunauer-Emmett-Teller (BET) surface areas of C-Fe3O4/P2W18and S@C-Fe3O4/P2W18are obtained by nitrogen isothermal adsorption-desorption curve analysis (Fig.S10 in Supporting information).Compared with CFe3O4/P2W18(214 m2/g), S@C-Fe3O4/P2W18presents a relatively small specific surface area (2.26 m2/g), suggesting the high surface utilization rate of C-Fe3O4/P2W18as well as the uniform loading of sulfur.
Apparently, the hollow capsule shell of C-Fe3O4/P2W18not only can be used as the host with high sulfur content, but also can be beneficial to accelerate rapid transport of lithium ions due to its porous conductive network.Furthermore, P2W18on the surface of the capsule shell exhibits strong adsorption ability and favorable electrochemical catalysis for LiPSs (will be further discussed).Elemental mapping results (Fig.S11 in Supporting information) further display that Fe, W, O, P, C, and S elements are evenly distributed in S@C-Fe3O4/P2W18.Thermogravimetric analysis (TGA) is carried out to ascertain the active sulfur content in S@C-Fe3O4/P2W18.One can see from Fig.2d that the sulfur loading in S@C-Fe3O4/P2W18is 69 wt%.Fig.2e reveals cyclic voltammetry (CV) curves of S@C-Fe3O4/P2W18cathode in the potential range of 1.7–2.8 V at 0.1 mV/s.Clearly, two couple of redox characteristic peaks emerge in the cathode scan and anode scan, suggesting that there are multiple reaction mechanisms in LSBs.The cathodic characteristic peaks at 2.31 V and 2.03 V correspond to the formation of higher order Li2Sx(4 ≤x≤8) and further electrochemical reduction to insoluble Li2S2/Li2S, respectively.The anodic scans reveal two peaks near 2.37 V and 2.42 V, representing the oxidation of Li2S2/Li2S to long-chain Li2Sxand the final oxidation to elemental sulfur, respectively.Galvanostatic discharge/charge experiments of S@C-Fe3O4/P2W18electrode at the current densities of 0.1–3 C are studied (Fig.2f).Clearly, the discharge/charge profiles at each current rate display two pairs of redox peaks, which matches the test results of CV.Moreover, it delivers reversible capacities of 1289.7, 1151.5, 1071.3, 1019.4, 889.4, and 701.7 mAh/g at 0.1, 0.2, 0.5, 1, 2, and 3 C, respectively, showing excellent electrochemical performance.
S@MIL-88A(Fe)/P2W18–300, S@C/FeWO4–500, and S@C/FeWO4–600 electrodes are prepared by the same method for S@CFe3O4/P2W18.Fig.3a exhibits the initial galvanostatic discharge/charge profiles of as-prepared products at 0.1 C.S@CFe3O4/P2W18presents the lowest polarization value (ΔV) of 176 mV than that of S@MIL-88A(Fe)/P2W18–300 (263 mV),S@C/FeWO4–500 (244 mV), and S@C/FeWO4–600 (252 mV).The low polarization demonstrates the fast electrochemical reaction kinetics and increased catalytic activity, accelerating the facile conversion of LiPSs and guaranteeing the sufficient utilization of active sulfur [43].Fig.3b compares the cycling performance of S@MIL-88A(Fe)/P2W18-300, S@C-Fe3O4/P2W18, S@C/FeWO4-500,and S@C/FeWO4-600 electrodes at current density of 2 C in a voltage range of 1.7–2.8 V.Note that S@MIL-88A(Fe)/P2W18–300 exhibits poor cycle life and it falls to 414 mAh/g after 100 cycles.The poor performance of S@MIL-88A(Fe)/P2W18-300 results from the insufficient carbonization, which cannot guarantee outstanding conductivity of sulfur cathode.Whereas, with the increasing of carbonization temperature, the reversible capacities of S@C/FeWO4-500 and S@C/FeWO4-600 are obviously enhanced, which remain stable at around 534 and 558 mAh/g when the test is prolonged to 100 cycles.However, as mentioned above, the higher carbonization temperature results in the collapse of hollow capsule shell structure, causing the low sulfur utilization.Meanwhile, the large volume change of sulfur cathode during lithiation–delithiation cycles cannot be restrained.Fortunately, the reversible capacity of S@C-Fe3O4/P2W18continuously decreases from the 1stcycle to the 25thcycle, while its discharge capacity tends to be stable from the 26thcycle to the 160thcycle.The discharge capacity in the 160thcycle (710 mAh/g) accounts for a retention by 91% in comparison to that of the 25thcycle (783 mAh/g).Therefore, S@CFe3O4/P2W18with optimizational carbonization presents the best cycling performance for all as-prepared products because of its integrity of hollow capsule shell structure, excellent conductivity,and the higher sulfur loading.For comparison, the C-Fe3O4composites are synthesized by carbonizing MIL-88A(Fe) at 400 °C for 2 h The morphological and structural features are displayed in Figs.S12 and S13 (Supporting information), and it can be noticed that C-Fe3O4presents the rod-like structure with diameter of 500 nm and length of 2 μm.BET surface area and pore size distribution curves on C-Fe3O4/P2W18and C-Fe3O4are shown in Fig.S14 (Supporting information).One can see that the specific surface area of C-Fe3O4/P2W18is greater than that of C-Fe3O4(130 m2/g).Besides, the pore size of approximately 20 nm is determined for CFe3O4/P2W18.The mesoporous structure with large specific surface area not only furnishes a more active pathway for sulfur adsorption and conversion, but also effectively relieves sulfur cathode expansion/shrinkage upon cycling.Based on above results, electrochemical impedance spectroscopy (EIS) tests are determined to research the interfacial charge transfer processes in the fresh cells.The Nyquist plots present a semicircle in the high-frequency region and one sloped line at low-frequency location, as depicted in Fig.3c.One can see that S@C-Fe3O4/P2W18(37Ω) shows the low charge transfer resistance compared with S@C-Fe3O4(54Ω) and S@C/FeWO4-600 (46Ω), confirming the high conductivity of S@CFe3O4/P2W18and the rapid electron transport by P2W18catalyst(will be further discussed) [44,45].
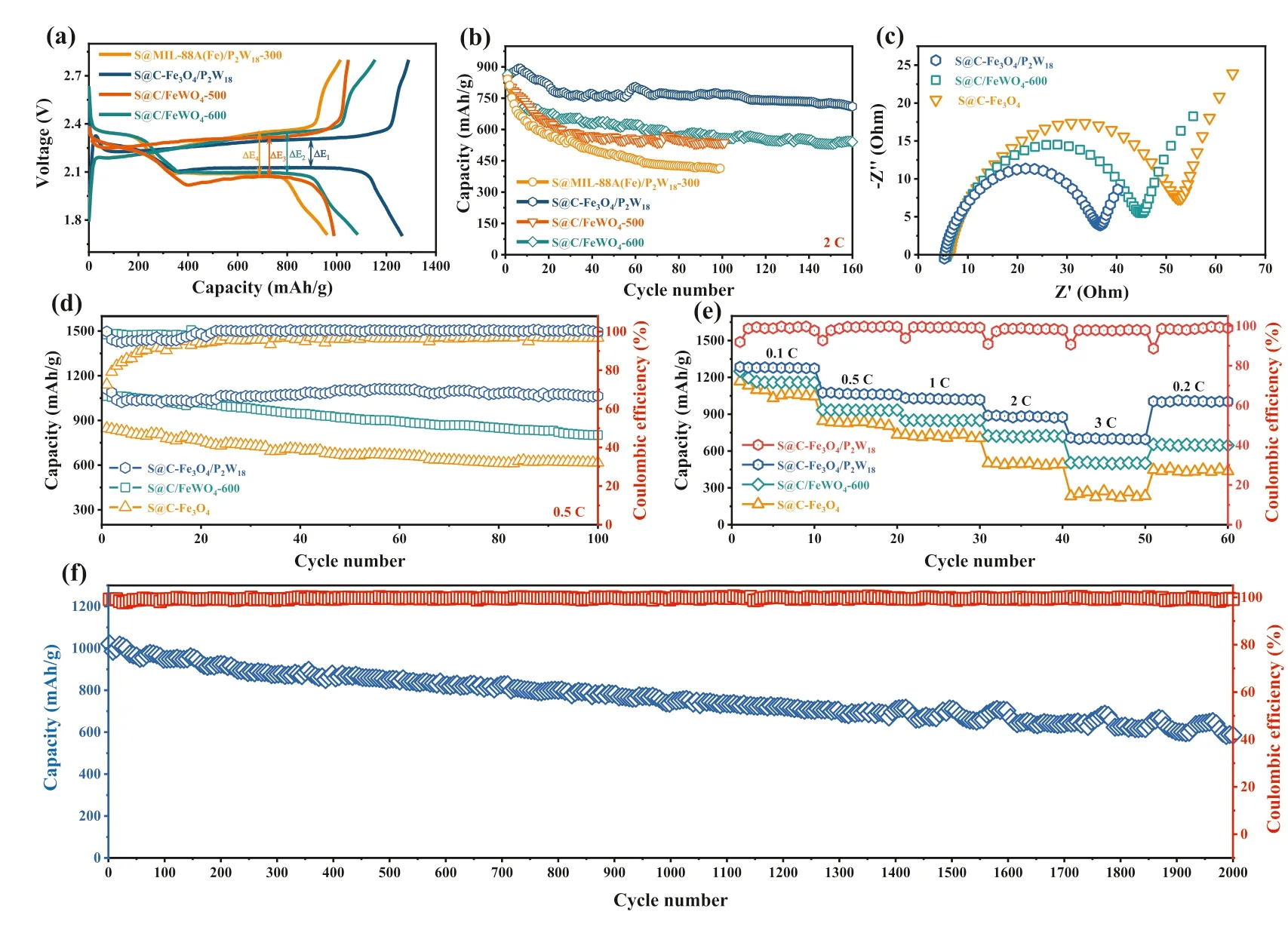
Fig.3.(a) The initial charge–discharge curves of S@MIL-88A(Fe)/P2W18-300, S@C-Fe3O4/P2W18, S@C/FeWO4-500, and S@C/FeWO4-600 electrodes at 0.1 C.(b) Cycle performance of S@MIL-88A(Fe)/P2W18-300, S@C-Fe3O4/P2W18, S@C/FeWO4-500, and S@C/FeWO4-600 electrodes at 2 C.(c) Nyquist plots of S@C-Fe3O4/P2W18, S@C/FeWO4-600,and S@C-Fe3O4 electrodes.(d) Cycle performance of S@C-Fe3O4/P2W18, S@C/FeWO4-600, and S@C-Fe3O4 electrodes at 0.5 C.(e) Rate performance of S@C-Fe3O4/P2W18,S@C/FeWO4-600, and S@C-Fe3O4 electrodes at current densities from 0.1 C to 3 C.(f) Long-life cycling performance of the S@C-Fe3O4/P2W18 at 1 C.
Moreover, the cycle performance of S@C-Fe3O4/P2W18,S@C/FeWO4-600, and S@C-Fe3O4is conducted at 0.5 Cviagalvanostatic measurements (Fig.3d).Capacity of S@C/FeWO4-600 fades from 1067 mAh/g to 801 mAh/g upon 100 cycles.In addition, the S@C-Fe3O4also displays poor cycle life.As expected, S@C-Fe3O4/P2W18achieves a superior discharge specific capacity of 1108 mAh/g in the 1stcycle.More strikingly, for S@C-Fe3O4/P2W18electrode, the retentive capacity of 1063 mAh/g up to 100 cycles is 96% of the initial cycle capacity, showing outstanding electrochemical stability.While capacity retention values of S@C/FeWO4-600 and S@C-Fe3O4are 75% and 72%, respectively.Besides, the average Coulombic efficiency of S@C-Fe3O4/P2W18electrode is more than 99% in the process of cycling, which is higher than that of S@C/FeWO4-600 (96%).More obviously, the Coulombic efficiency of S@C-Fe3O4fluctuates greatly, and the average Coulombic efficiency is only 93%.To further illustrate the structure of S@C-Fe3O4/P2W18upon cycling, the electrochemical cells after 100 discharge–charge cycles at 0.5 C are disassembled in the argon-filled glovebox, and their morphologies are observed by transmission electron microscopy (TEM) and scanning electron microscopy (SEM) (Figs.S15 and S16 in Supporting information).As expected, the robust capsule shell structure is basically preserved, guaranteeing the excellent electrochemical performance.Moreover, EIS tests are carried out after cycling at 0.5 C for 100 cycles (Fig.S17 in Supporting information).The charge transfer resistance of S@C-Fe3O4/P2W18is still significantly lower than that of S@C-Fe3O4and S@C/FeWO4-600, which suggests the smallest electrochemical reaction polarization and excellent conductivity during repeated lithiation-delithiation cycles.S@C-Fe3O4/P2W18also displays the better rate performance under different current densities than that of S@C/FeWO4-600 and S@C-Fe3O4(Fig.3e).When the current densities increase from 0.1 C to 0.5, 1, 2, and 3 C, the specific capacities of S@C-Fe3O4/P2W18cathode decrease slightly from 1286 to 1075, 1032, 889, and 709 mAh/g.The lower capacity fading rate is comparable to the recent reports for other multi-function sulfur hosts (Fig.S18 in Supporting information).Specifically, when the current density is recovered to 0.2 C, the discharge specific capacity could be restored to 1014 mAh/g, which indicates the high electrochemical reversibility.However, the cells based on S@C/FeWO4-600 and S@C-Fe3O4cathodes show significantly fast capacity decay rates and maintain the specific capacities of only 511 and 296 mAh/g at 3 C, respectively.Based on superior cyclic capability and rate performance of S@C-Fe3O4/P2W18,long-term cycle stability is indispensable for practical applications of LSBs.In Fig.3f, the discharge specific capacity decreases slowly when S@C-Fe3O4/P2W18electrodes are cycled at 1 C, retaining a capacity of 585 mAh/g even after 2000 deep cycles.The ultralow capacity decay of 0.021% per cycle is obtained, which is better than many other sulfur cathodes with metal-based hosts (Table S2 in Supporting information).Meanwhile, the Coulombic efficiency of S@C-Fe3O4/P2W18electrode is more than 98% in the process of cycling.These results indicate that S@C-Fe3O4/P2W18has excellent ultra-long cycle performance as cathode materials of LSBs.
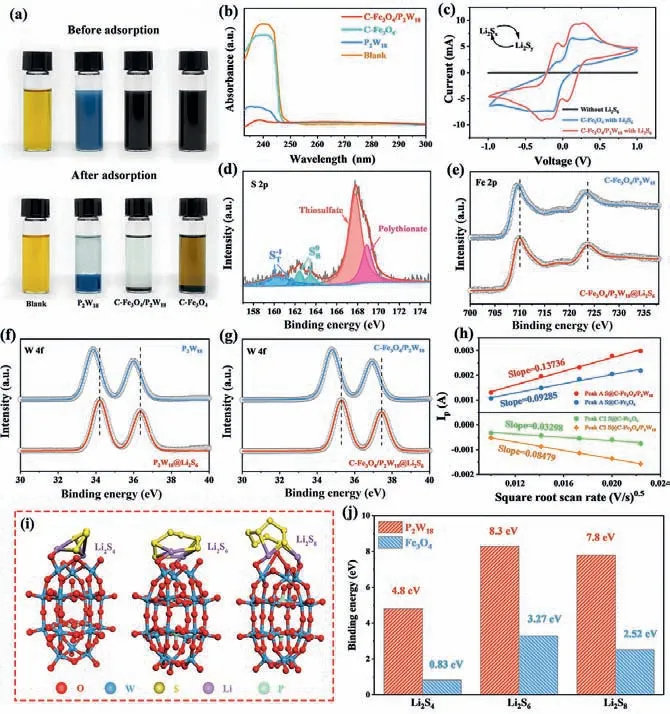
Fig.4.(a) Optical observation and (b) UV–vis spectra of Li2S6 solution adsorbed by C-Fe3O4/P2W18, C-Fe3O4, and P2W18.(c) CV curves of Li2S6 symmetrical cells with C-Fe3O4/P2W18 and C-Fe3O4 working electrodes.High-resolution XPS analysis of (d) S 2p spectra for P2W18@Li2S6, (e) Fe 2p spectra for C-Fe3O4/P2W18 and C-Fe3O4/P2W18@Li2S6, (f) W 4f spectra for P2W18 and P2W18@Li2S6, and (g) W 4f spectra for C-Fe3O4/P2W18 and C-Fe3O4/P2W18@Li2S6.(h) Plots of the peak current versus the square root of scan rate for S@C-Fe3O4/P2W18 and S@C-Fe3O4.(i) Optimized geometries of Li2S4, Li2S6, and Li2S8 adsorbed on terminal oxygen of P2W18.(j) Binding energy values for Li2S4, Li2S6, Li2S8 on P2W18 (terminal oxygen) and Fe3O4 (311) surface, respectively.
Li2S6with high solubility in the electrolyte is employed as the representative LiPSs intermediate to examine the polysulfide absorption by the C-Fe3O4/P2W18, P2W18, and C-Fe3O4.As shown in Fig.4a, the Li2S6and three as-prepared powders are sealed in vials, and then mix the compounds for 6 h, respectively.Clearly,for C-Fe3O4/P2W18and P2W18, the solution fades to be almost colorless, while no obvious color change is observed for C-Fe3O4,proving the strong adsorption ability of P2W18.The interaction between Li2S6and three as-prepared samples is further verified by the ultraviolet–visible (UV–vis) spectra (Fig.4b).According to the analysis of supernatant, a strong peak near 240 nm before adsorption is found.The peak intensity decreases slightly after Li2S6solution is adsorbed by C-Fe3O4, while the peak intensity decreases significantly after Li2S6solution is adsorbed by C-Fe3O4/P2W18and P2W18, testifying that P2W18has stronger adsorption ability for LiPSs.The catalytic effect of C-Fe3O4/P2W18on accelerating the kinetics of polysulfide conversion is investigated using CV tests.As observed in Fig.4c, the Li2S6symmetric cell based on the CFe3O4/P2W18electrode presents six distinct peaks, while the CFe3O4electrode shows inconspicuous redox peak pairs and the lower polarization current.These results suggest that the P2W18sites in C-Fe3O4/P2W18could effectively facilitate the reversible conversion of LiPSs, ensuring the superior redox kinetics and high electrochemical reversibility of C-Fe3O4/P2W18.
To sufficient assess the chemical interaction between P2W18and polysulfides, XPS analyses are performed on the pristine CFe3O4/P2W18, P2W18and C-Fe3O4/P2W18@Li2S6, P2W18@Li2S6composites after the adsorption experiment (Fig.4a).Fig.4d shows the XPS spectra of S 2p in P2W18@Li2S6, the peaks centered at 160.5 eV and 161.8 eV are in accord with terminal sulfur), the peaks at 162.6 eV and 163.7 eV correspond to the bridging sulfur (and signals detected at 167.9 eV and 169.1 eV peaks can be matched to thiosulfate (formed by the oxidation of sulfur in polysulfides)and polypolysulfate complex (formed primarily by the reaction between the anchored polysulfide and thiosulfate, as shown in Eq.1),respectively [46–48].

The soluble LiPSs (Li2Sx,x≥4) can be converted into low solubility polysulfate medium and short-chain polysulfide (Li2Sy, y <3), which is indicative of the catalytic role of P2W18to actively promote a series of redox reactions of LiPSs.Fig.4e is the characteristic spectra of Fe 2p in C-Fe3O4/P2W18and C-Fe3O4/P2W18@Li2S6.It can be observed that there is no obvious shift in the peak position.Notably, as an active component in the composites, when P2W18interacts with Li2S6, the binding energy of the two W 4f peaks can be significantly increased.For P2W18(Fig.4f), the W 4f peaks shift from the original 33.8 eV/35.9 eV to 34.2 eV/36.3 eV after adsorption of Li2S6, while the W 4f peaks in C-Fe3O4/P2W18shift from the original 34.8 eV/36.9 eV to 35.2 eV/37.4 eV after adsorption of Li2S6(Fig.4g).These phenomena suggest that the active P2W18can provide suitable polar sites on the capsule shell, facilitating the rapid transfer of electrons through the P2W18to the exposed sites and finally to the S−S bonds of polysulfides, which is consistent with EIS results shown in Fig.3c.More importany,the chemical binding formed by the P2W18can be used as a Lewis acid site to greatly improve the sulfur cathode retention ability and chemically restrict the dissolution and migration behavior of LiPSs,thus restraining the shuttle effect and prolonging the cycle life of the LSBs.
A series of CV curves with scan speeds of 0.1, 0.2, 0.3, 0.4, and 0.5 mV/s are recorded to research the Li+diffusion coefficient of S@C-Fe3O4/P2W18and S@C-Fe3O4(Figs.S19a and b in Supporting information).The Li+diffusion kinetics can be acquired according to the Randles-Sevcik equation (Eq.2) [49]:

wherenrepresents electron number,IPis the peak current,DLiis the diffusion coefficient of Li+,Srepresents electrode area,νis the scan rate,C0is the Li+concentration in solution, and the slopes ofIp/v0.5curves have positive correlation withDLi[50].The slope values (Ip/v0.5) of Peak A, Peak C1, and Peak C2 are calculated in Fig.4h and Fig.S19c (Supporting information).Note that S@CFe3O4/P2W18cathode shows high Li+diffusion coefficients compared with S@C-Fe3O4, which corresponds to the fast conversion of long-chain LiPSs to Li2S2/Li2S catalyzed by P2W18, promoting the diffusion of lithium ions to sulfur species.The DFT calculations are conducted to further prove the interaction between P2W18and LiPSs.Fig.4i and Fig.S20 (Supporting information) show the adsorption situations of Li2S4, Li2S6, and Li2S8on P2W18(terminal oxygen) and Fe3O4(311) surface, respectively.The binding energy(Eads) of LiPSs adsorbing on the P2W18and Fe3O4molecules is calculated referring to the following Eq.3:

whereELiPSsis the surface energy of LiPSs,EP2W18/Fe3O4is the surface energy of P2W18or Fe3O4, and theEtotalis the total energy of P2W18or Fe3O4combined with LiPSs.Based on Eq.3, the positive value ofEadsindicates that the corresponding adsorption structure is energetically beneficial to form [51].As described in Fig.4j, the binding energies of Li2S4, Li2S6, and Li2S8on the P2W18are calculated to be 4.8, 8.3, and 7.8 eV, and the adsorption energies between polysulfides and Fe3O4are 0.83 eV for Li2S4, 3.27 eV for Li2S6, and 2.52 eV for Li2S8.The higher binding energy means the strong capture ability of P2W18on LiPSs, which is advantageous to the enhancement of electrochemical performance of LSBs.
In conclusion, the hollow capsule shell with P2W18, Fe3O4, and C components has been successfully synthesized by optimizing the carbonization temperature.When used as the sulfur host, the capsule shell can alleviate the volume change of sulfur cathode among lithiation–delithiation cycles and guarantee the higher sulfur loading.The strong carbon shell is favorable to increase electrical conductivity of sulfur cathode material, and abundant porous structure can accelerate the diffusion and transfer kinetics of lithium ions.What is more, the P2W18on the capsule shell has impregnable chemical bond and efficient catalytic activity for the LiPSs, which is conducive to facilitate the redox reaction of LiPSs and prevent the shuttle effect.Therefore, S@C-Fe3O4/P2W18cathode achieves high reversible capacity, outstanding rate performance, and ultralong stable life span.This study may stimulate the search for more advanced cathode structures, such as hollow structure doped with POMs, yolk shell or multi-shell particles through the molecular design, to increase the electrochemical property of LSBs with longlife cycle stability.
Declaration of competing interest
The authors declare no conflicts of interests.
Acknowledgments
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (No.21971085)and the Natural Science Foundation of Shandong Province (No.ZR2019MB004, China).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.11.043.
 Chinese Chemical Letters2022年9期
Chinese Chemical Letters2022年9期
- Chinese Chemical Letters的其它文章
- A review on recent advances in hydrogen peroxide electrochemical sensors for applications in cell detection
- Rational design of nanocarriers for mitochondria-targeted drug delivery
- Emerging landscapes of nanosystems based on pre-metastatic microenvironment for cancer theranostics
- Radiotherapy assisted with biomaterials to trigger antitumor immunity
- Development of environment-insensitive and highly emissive BODIPYs via installation of N,N’-dialkylsubstituted amide at meso position
- Programmed polymersomes with spatio-temporal delivery of antigen and dual-adjuvants for efficient dendritic cells-based cancer immunotherapy
