β-1,2-Mannan-based glycoconjugates as potential antifungal vaccines
Jun Lio, Bo Pn, Xioin Zhuo, Guocho Lio, Yun Go, Zhenzhen Yo,Linghu Wng, Qiuye Wu, Weihu Pn, Binghu Jio,∗, Qingjie Zho,∗
a Department of Biochemistry and Molecular Biology, College of Basic Medical Sciences, Second Military Medical University, Shanghai 200433, China
b School of Pharmacy, Second Military Medical University, Shanghai 200433, China
c Department of Dermatology, Changzheng Hospital, Second Military Medical University, Shanghai 200003, China
d International Institute for Translational Chinese Medicine, Guangzhou University of Chinese Medicine, Guangzhou 510006, China
ABSTRACT Phosphorylated di-, tri- and tetra-saccharides of β-1,2-mannan antigen derived from Candida albicans (C.albicans) cell wall were synthesized and covalently conjugated with keyhole limpet hemocyanin (KLH)and human serum albumin (HSA) via a bifunctional linker under mild conditions.The semi-synthetic β-1,2-mannoside–KLH conjugates were evaluated for the immunization of BALB/c mice.The ELISA results revealed that all three conjugates could elicit high levels of specific IgG antibodies and the acquired antisera could effectively identify the β-1,2-mannan epitope.Furthermore, the immunofluorescence and flow cytometry assays also uncovered that the induced antibodies, especially that obtained from immunization with β-1,2-mannotriose–KLH conjugate (1b), could bind well to fungi cell.Eventually, the structure–immunogenicity relationship analysis of β-mannan showed that the length of oligo-β-mannoses had a big impact on their immunogenicity and β-1,2-mannotriose showed the strongest immunogenicity.The results suggested the great potential of β-1,2-mannotriose–KLH conjugate as an antifungal vaccine candidate.
Keywords:β-1,2-Mannan Oligosaccharide Antifungal Glycoconjugate Vaccine Antigen
Candida albicans(C.albicans), a yeast and a common opportunistic pathogen, is one of the major causes of rising morbidity and mortality of fungal infections especially among individuals with organ transplant, cancer, and human immunodeficiency virus (HIV) infections, as well as those undergoing immunosuppressive therapy or prolonged treatment with antibiotics [1–3].In clinic, azoles, polyenes, and echinocandins are three major classes of anti-Candidadrugs; especially, triazoles are often used as firstline antifungal agents [4–7].However, with continuous abuse, fluconazole, a representative triazole, has become ineffective against invasive aspergillosis and is suffering from severe drug resistance[8–10].Thus, it is of great significance to find new strategies or methods for the treatment ofC.albicansinfection.Compared to traditional chemotherapies, vaccination is more cost-effective.For example, many vaccines against bacteria have significantly reduced the morbidity and mortality of related infectious diseases [11–14].
For the design of anti-Candidavaccines, theβ-1,2-mannan epitope representing the carbohydrate part ofCandidacell wall phosphomannan–protein complex (PMC) [15–18] is an attractive antigen.In general, two forms ofβ-1,2-mannan can be found inCandidaspecies, which include the acid-labileβ-1,2-mannan oligosaccharides, known as antigenic factor 5, attached toα-(1,2)-mannan sidechainsviaa phosphodiester linkage and the acidstableβ-1,2-mannan, known as antigenic factor 6, as sidechains attached toα-(1,6)-mannan backbone (Fig.1) [19–24].The acidlabile phosphorylatedβ-1,2-oligomannosides are a common structure inC.albicansserotype A and B strains, whereas acid-stableβ-1,2-oligomannosides is unique to serotype A strains [25–29].These oligosaccharides are exposed on the fungal cells and function as an adhesin in the recognition of mammalian cells [19].Besides,they are highly conserved on the cell surface of pathogenic fungi[30] and thus are excellent target antigens for the development of potentially broadly useful antifungal vaccines.In the past decades,vaccines candidates that combined factor 5 or 6 or their mimics with immunogenic carrier proteins [tetanus toxoid (TT) or bovine serum albumin (BSA)], T cell peptides found inC.albicanscell wall proteins or other immunologic stimulants were synthesized, and their immunological studies indicated that both epitopes can act as antigens to induce effective and specific humoral immune responses and produce protective antibodies [31–35].Despite these great progresses, the correlations between the structures of antigenic factors, particularlyβ-1,2-mannan, and their immunogenicity are ambiguous.
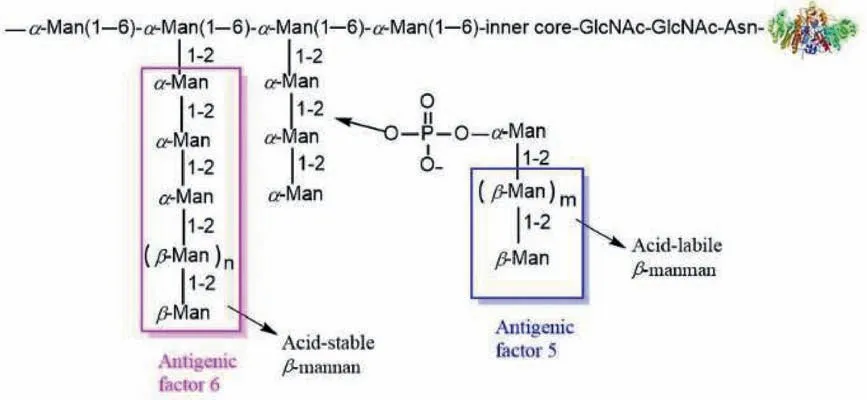
Fig.1.Two structural forms of β-1,2-mannan on the cell wall of Candida species.
In the present work, a series of phosphorylatedβ-1,2-mannan were synthesized and covalently coupled with KLH to form glycoconjugates 1a–1c (Fig.2) as antifungal vaccine candidates.The phosphorylatedβ-1,2-mannan oligosaccharides were mimics of antigenic factor 5.Furthermore, the selectedβ-1,2-mannobiose,triose and tetraose were used to systematically investigate the structure–antigenicity relationship of linearβ-1,2-mannan.The bifunctional linker of disuccinimidyl glutarate (DSG) was used not only for efficient coupling but also as the “extended arm” anchored to the carrier protein.In the meantime, this linker was proved to not induce linker-specific antibodies to interfere with the immunological properties of the resultant conjugates.The KLH, which is derived from a circulating glycoprotein of the marine molluskMegathura crenulata, is an effective and highly immunogenic stimulant that is usually used as hapten-carrier for glycoconjugate vaccine development.In addition, the oligosaccharide–HSA conjugates 2a–2c (Fig.2) were also synthesized and used as capture reagents for detectingβ-1,2-mannan-specific antibodies.
Synthesis of phosphorylatedβ-1,2-oligomannosides (3a–3c,Scheme 1) comprising two to fourβ-1,2-linked mannose residues are crucial for assembling the designed glycoconjugate vaccines.Herein, the methodology based on initialβ-glucosylation followed by inversion of configuration at C-2 position in the glucose residue[36–38], rather than direct mannosylation engaging conformationally rigid mannosyl donors of thioglycosides or glycosyl sulfoxides [39–41], was chosen for the consideration of highly efficient and large-scale preparation.The synthesis route was depicted in Scheme 1 and the building blocks 3a–3c were prepared according to our previous study [42].Withβ-1,2-oligomannosides 3a–3c in hand, the global deprotected compounds 4a–4c were then achieved by hydrogenolysis in the presence of Pd(OH)2/C (10 wt%)with excellent yields ranging from 88.7% to 91.5%.Thereafter, reaction of 4a–4c with a large excess of disuccinimidal glutarate 5 afforded the corresponding activated esters 6a–6c, separately, which were readily purified by filtration, precipitation with large amountof EtOAc, and washing of the precipitate several times with EtOAc in sequence.Subsequently, 6a–6c were coupled with KLH or HSA in a slightly alkaline atmosphere using phosphate buffer solution(PBS, 0.1 mol/L) to eventually provide desired glycoconjugates 1a–1c or 2a–2c, respectively, which were purified by passing through a Biogel A 0.5 column to remove remaining free sugars, dialyzing against distilled water to get rid of salts, and lyophilization.The mannose content of the glycoconjugates were analyzed by a well-known protocol of phenol-sulfuric acid method [43].The mannan-loading of KLH and HSA conjugates were displayed in Table 1, implying that the coupling reactions were efficient and in average about fifteen molecules of mannosyl were bound to each molecule of HSA.In addition, the results of mannose loadings of HSA-conjugates were also confirmed by MALDI-TOF mass spectrometry (see Supporting information).
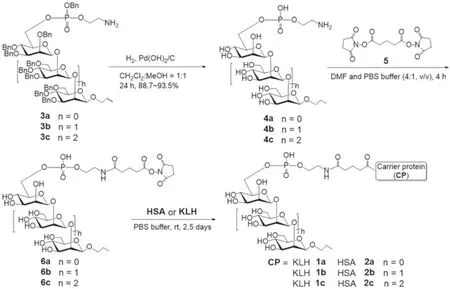
Scheme 1.Synthesis of glycoconjugates 1a–1c and 2a–2c.
The immunological properties ofβ-1,2-mannan–KLH conjugates 1a–1c were evaluated in six female BALB/c mice per group.Each conjugate was combined with complete Freund’s adjuvant (CFA) as initial emulsions and then injected subcutaneously (s.c.) into mice.The next three injections of the corresponding conjugate mixed with incomplete Freund’s adjuvant were used as boost immunizations with an interval of 14 days (Fig.S1 in Supporting information).Sterile PBS and KLH were vaccinated as negative control groups, separately.One week after the final immunization, the sera from the immunized mice were collected and analyzed by enzymelinked immunosorbent assays (ELISA) for detection of the induced antibodies [44].The microtiter plates were coated with the corresponding HSA conjugates 2a–2c as capture reagents to detect theβ-1,2-mannan-specific antibodies that aimed to avoid the interference of anti-KLH antibodies.The plates were blocked with blocking buffer (5% non-fat milk in Tris-buffered saline with 0.05% Tween-20) for 2 h at 37 °C.After washing with phosphate buffered saline Tween-20 (PBST), the plates were incubated with serially diluted antisera and placed in a 37 °C incubator for 1 h.Color development of each well was achieved by secondary antibody (goat antimouse IgG-HRP, Invitrogen).The optical density (OD) at 450 and 630 nm were read using a Universal Microplate Reader (Bio-Tek Instruments, Inc.).The antibody titers were defined as the dilution number yielding an observed OD value of 0.2, and the results were shown in Fig.3.
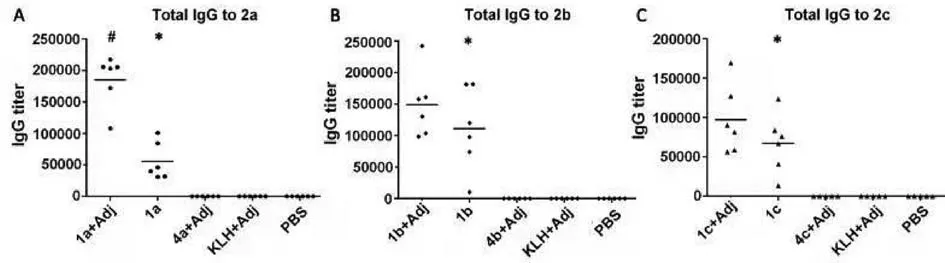
Fig.3.Specific IgG antibody responses of individual mouse immunized with conjugates 1a (A), 1b (B), and 1c (C), respectively.The antisera from immunized mouse with each conjugate were analyzed by ELISA with HSA conjugates 2a, 2b, and 2c as capture antigens and HRP-conjugate anti-mouse IgG antibody as the secondary antibody.#P < 0.05 vs. 1a–1c no adjuvant (Adj) group.∗P < 0.05 vs. 4a–4c with adjuvant group.
Obviously, all of the KLH-conjugates 1a–1c immunized with Freund’s adjuvant provoked higher titers ofβ-1,2-mannan-specific total IgG antibodies than that of the corresponding conjugate injected alone.It was noted that conjugates 1a–1c could elicit significant immune responses and produce high levels and antigenspecific IgG antibodies even in defect of external adjuvant, indicating that these conjugates exhibited self-adjuvanting property and may furnish the possibility of acquiring adjuvant-free vaccination.The immune response induced by 1b (average antibody titers>100,000) was notably stronger than that of 1a and 1c,which suggested thatβ-1,2-mannotriose antigen was of greatest immunogenicity.On the other hand, only very weak immune responses were elicited by freeβ-1,2-mannan oligosaccharides 4a–4c even when being combined with the Freund’s adjuvant.The similar results were also observed when administrated with the emulsion of KLH and Freund’s adjuvant.Therefore, the immunological resultants above uncovered that both the KLH and adjuvant could strengthen the immunogenicity of correspondingβ-1,2-mannan antigens to induce robust and effective immune responses.
To further explore whether the antisera induced by 1a–1c could recognize the naturalβ-1,2-mannan antigen epitopes on the surface ofC.albicanscell, the binding affinity experiments were carried out by immunofluorescence (IF) and flow cytometry (FC).The heat-killedC.albicanscells were firstly pretreated with 3%BSA blocking buffer to exclude latently nonspecific protein binding sites on the cell surface and subsequently incubated with antisera of 1a–1c, separately.Eventually, cells were covered in Fluor 488-labeled goat anti-mouse IgG antibodies and inspected with IF and FC.As shown in Fig.4, the resultants of IF assays disclosed that the obtained antiserum from mice immunized with 1b exhibited the best binding affinity and evenly attached to cell surface of both hyphal and germtube phenotype of theC.albicansSC5314 strain whereas the negative results, hardly combining with the fungi cells, were observed for immunized antisera obtained from 1a and 1c, as well as the control group.Furthermore, we also tested the binding ability of immunized antiserum from 1b to three clinical isolates ofC.albicans(Fig.S2 in Supporting information), which were obtained from Shanghai Key Laboratory of Molecular Medical Mycology (Shanghai, China) and identified by internal transcribed spacer sequencing.The results showed that the antiserum could bind to all tested isolates, indicating that the recognition ofC.albicansis not strain-specific.
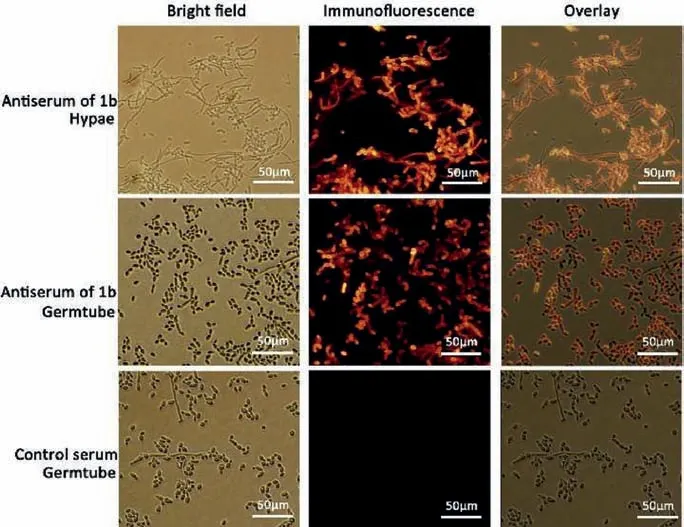
Fig.4.Indirect immunofluorescence analysis confirmed that IgG antibodies in antisera from mice immunized with 1b could bind to the cell surface of different forms of C.albicans SC5314 strain.Antisera from mice immunized with 1a, 1c, 4a–4c, and KLH control group could not react with C.albicans SC5314 strain (Figs.S3 and S4 in Supporting information).Scale bar: 50 μm.
The IF determinations were illustrated in Fig.5.The collected antisera from mice immunized with 1b combined with or without adjuvant exhibited satisfactory binding ability toC.albicanscell.However, the IF results of 1a and 1c were completely different from 1b.Negatively, the antiserum from mice immunized with 1c demonstrated slightly reactivity to fungus cell while the immune antisera of 1a or control group showed no binding activity toC.albicans.The IF consequences were mainly in accord with the resultants of FC suggested that the antibodies originated from immunization ofβ-1,2-mannotriose conjugate showed prominently higher reactivity toC.albicanscells than that fromβ-1,2-mannodiose conjugate, whereas the binding affinity of the induced antisera byβ-1,2-mannotetraose conjugate decreased sharply.Therefore, we may draw a simple conclusion that the length of oligo-β-mannoses plays an important role in immunogenicity andβ-1,2-mannotriose has the strongest and most effective immunogenicity.The elicited antibodies byβ-1,2-mannotriose–KLH conjugate 1b could recognize not only the syntheticβ-1,2-mannotriose oligosaccharide but also the naturalβ-1,2-mannan antigen epitope on theC.albicanscell, indicating thatβ-1,2-mannotriose rather thanβ-1,2-mannodiose or tetraose represented the unique structural motif for antigenic factor 5.We imaged that larger oligomers could provide more epitopes to induce antibodies that response toC.albicans.However, it is not a simple linear relationship between the length of manno-oligomers and immunogenicity.Only proper length of oligomers which has a reasonable spatial structure could be sufficient to induce antibodies that can recognizeC.albicans.
In addition, the previous studies [45,46] showed that the mannans onC.tropicaliscell surface shared structural homology toC.albicansand theβ-1,2-linked oligomannosyl chains attached to the phosphate group served as the major common epitope throughoutC.albicansandC.tropicalis.Hence, it would be worthwhile to investigate the binding mode of IgG antibodies in antiserum toC.tropicalis.The IF results reflected that the antisera from mice immunized with 1b contained the antibodies that could react withC.tropicaliswhereas antibodies in antisera from mice immunized with 1a, 1c, and 4a–4c could not bind toC.tropicalis(Fig.6).
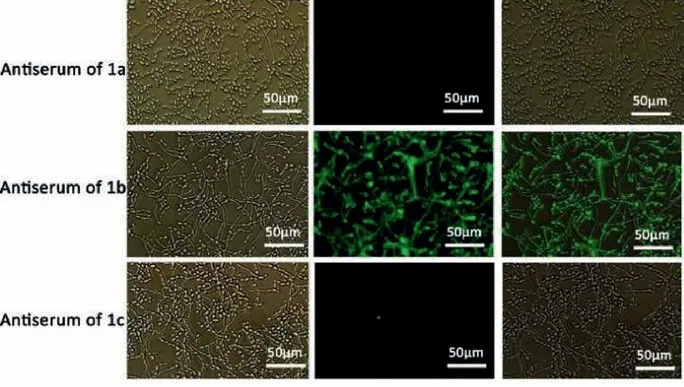
Fig.6.Indirect immunofluorescence analysis of IgG antibodies in antisera from mice immunized with 1a–1c binding ability to C.tropicalis.Bright field (left), immunofluorescence (middle), and overlay (right).Scale bar: 50 μm.
In summary, a series of phosphorylatedβ-1,2-mannan oligosaccharides as mimics of antigenic factor 5 were prepared in a highly convergent and effective way, which were subsequently coupled with KLH or HSA to generate corresponding glycoconjugates.The immunological properties of 1a–1c were evaluated in BALB/c mice and the ELISA resultants revealed that all KLH-conjugates could induce robust T cell-dependent responses and produce high levels ofβ-1,2-mannan specific IgG antibodies.Furthermore, these KLHconjugates displayed self-adjuvanting property that could promote robust antibody responses even in absence of an external adjuvant.Additionally, we investigated the affinity between the elicited antisera andC.albicanscells, and the immunofluorescence and flow cytometry assays clearly reflected that the order of binding affinity wasβ-1,2-(Man)3> β-1,2-(Man)2> β-1,2-(Man)4, which means that the antibody induced by 1b could react well with the oligosaccharide antigens on fungus cell and the length of linearβ-1,2-mannan had a significant impact on their antigenicity.Thus,it is concluded thatβ-1,2-(Man)3is an optimally antigenic target for designing antifungal vaccine and the semi-synthesizedβ-1,2-mannotrios–KLH conjugate 1b would be of great potential as a vaccine candidate.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
Acknowledgment
This work was financially supported by the Natural Science Foundation of China (Nos.21502223, 81773580).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2021.12.065.
 Chinese Chemical Letters2022年9期
Chinese Chemical Letters2022年9期
- Chinese Chemical Letters的其它文章
- A review on recent advances in hydrogen peroxide electrochemical sensors for applications in cell detection
- Rational design of nanocarriers for mitochondria-targeted drug delivery
- Emerging landscapes of nanosystems based on pre-metastatic microenvironment for cancer theranostics
- Radiotherapy assisted with biomaterials to trigger antitumor immunity
- Development of environment-insensitive and highly emissive BODIPYs via installation of N,N’-dialkylsubstituted amide at meso position
- Programmed polymersomes with spatio-temporal delivery of antigen and dual-adjuvants for efficient dendritic cells-based cancer immunotherapy
