Cell-penetrating riboflavin conjugate for antitumor photodynamic therapy
Chunlei Wu, Ynyn Li, Zhehong Cheng, Pengxin Wng, Zhilong M, Ke Liu,Yulin Cheng, Yimin Zhou, Xin Lin, Ximing Sho, Yong Yng, Hongchng Li,b,∗∗,Lijing Fng,b,∗∗
a Guangdong Key Laboratory of Nanomedicine, Institute of Biomedicine and Biotechnology, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen 518055, China
b University of Chinese Academy of Sciences, Beijing 100049, China
c Department of Biomedical Engineering, Southern University of Science and Technology (SUSTech), Shenzhen 518055, China
ABSTRACT Riboflavin (RF, vitamin B2) is an essential vitamin and has been considered as a promising natural photosensitizer for photodynamic therapy (PDT).However, further exploration of RF in antitumor application was limited by its poor cellular uptake.In this study, using cell-penetrating peptides Arg8, (Cha-Arg)3 and small molecule triphenylphosphine (TPP) as delivery compounds, three RF conjugates were prepared to increase the accumulation of RF in cells, termed as Arg8-RF, (Cha-Arg)3-RF and TPP-RF, respectively.Compared with TPP-RF and Arg8-RF, (Cha-Arg)3-RF exhibited better cell internalization and stronger cytotoxicity against HeLa cells upon exposure to blue light.Further researches proved that (Cha-Arg)3-RF generated reactive oxygen species (ROS) under irradiation, which could indiscriminately destroy endogenous proteins and mitochondria, ultimately inducing cell death.This work provides a new approach to explore RF as a natural photosensitizer for antitumor photodynamic therapy.
Keywords:Riboflavin Antitumor Photodynamic therapy Cell-penetrating peptides
Photodynamic therapy (PDT) has been widely applied in the clinical treatment of multifarious solid tumors due to the potential advantages such as excellent spatiotemporal precision, noninvasiveness, localized treatment and the absence of drug resistance[1,2].PDT relies on the application of a photosensitive molecule to generate reactive oxygen species (ROS) in the presence of light and oxygen to kill tumor cells.Therefore, photosensitizers play a key role in the PDT process.To overcome the limitations of conventional photosensitizers with poor solubility, photostability and bioavailability, various newly developed photosensitizers such as small organic molecules, transition metal coordination compounds and semiconducting polymers, have been designed and utilized for multimodal imaging and therapy [3,4].However, syntheses of these kinds of photosensitizers are costly and time consuming because of multi-step procedures.In this respect, naturally derived photosensitizers are preferred and explored for their full biodegradability,low cost and nontoxicity.
Riboflavin (RF), commonly known as vitamin B2 (VB2), is an essential water-soluble vitamin to maintain the body’s homeostasis by playing vital roles in many metabolic processes [5,6].RF exists in all aerobic cells and acts as a precursor of flavin-based redox cofactors, such as flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD).They are all involved in various redox processes that regulate a huge number of biotransformations.RF is a promising naturally available photosensitizer with excellent biocompatibility [7,8].It is well established that RF undergoes reaction of photodynamic oxidation by both type I and type II mechanism.Upon exposure to blue light or UV-A light, RF is excited to be short lived singlet-excited state1Rib∗.Through intersystem crossing long-lived triplet-excited state (3Rib∗) generates efficiently from1Rib∗.In type I mechanism,3Rib∗interacts directly with oxidizable substrates containing amino acids, proteins, nucleotides,lipids and vitamins.This process will form ionic free radicals such as O2•−and OH•through one-electron or hydrogen atom transfer.In type II mechanism, energy-transfer from3Rib∗to molecular oxygen occurs and results in singlet oxygen.It has been reported that the singlet oxygen formation quantum yield of RF (0.51) is close to the commonly used porphyrin photosensitizer (0.85) [9].Edwards and Silva reported that the deactivation of deglycosylated HRP (horse radish peroxidase) under light irradiation in the presence of riboflavin was much more efficient than in the presence of methylene blue [10].
Based on the photophysics and photochemistry effects, riboflavin and its derivatives have been therapeutically useful as photosensitizers in pathogen destruction or inactivation against many viruses and bacterium [11].However, there are only a few studies using RF for antitumor application.Yuanet al.revealed that RF was able to recognize G-T mismatch specifically and induce single strand breaks in duplex DNA targets efficiently under irradiation [12].Taniguchiet al.demonstrated Aβ1–42 could be oxidized using a riboflavin catalyst and visible light irradiation,leading to the attenuation of its aggregation potency and neurotoxicity [13].Feiet al.used the assembled RF nanocrystals with optical waveguiding and photosensitizing properties for anticancer photodynamic therapy [14].As an endogenous small biomolecule,RF is nontoxic, excellently biocompatible and biodegradable,and widely available at low cost, and thus has great potential to be a natural photosensitizer for antitumor photodynamic therapy.
Although RF is endogenous, its average concentration in a cell is too low for its photochemical activity as a photosensitizer [8,15,16].To obtain a significant photosensitive toxicity for antitumor therapies, a high intracellular concentration of RF is needed.We found the cellular uptake of RF was very weak in many cell lines including those which overexpress riboflavin receptors [17,18].Therefore, to develop RF as an efficient antitumor photosensitizer, it was crucial to enhance the cellular uptake of RF.For this purpose,we anticipated that the intracellular concentration of RF could be increased by conjugating it with delivery compounds such as cell-penetrating peptides or small molecules.After great accumulation of RF in cancer cells, cell death would be induced upon blue light irradiation due to the non-selective destruction of endogenous proteins by ROS produced in the PDT process.In this work, cell-penetrating peptides Arg8and (Cha-Arg)3, as well as small molecule triphenylphosphine (TPP) were used to conjugate with RF, providing Arg8-RF, (Cha-Arg)3-RF and TPP-RF, respectively.Among them, (Cha-Arg)3-RF showed better cell internalization and generated ROS efficiently under irradiation, which could indiscriminately destroy endogenous proteins and mitochondria, ultimately inducing cell death.
RF consists of a D-ribityl chain and an isoalloxazine core which is responsible for its electronic and photoelectronic properties.The presence of the isoalloxazine moiety provokes significant absorption around 375 and 445 nm with a maximum fluorescence emission peak at 550 nm.Exposure to blue light or UV-A light, RF undergoes complex photochemical reactions with proteins, causing loss of protein functions and inducing protein degradation.These proteins generally incorporate amino-acid residues which can be easily oxidized, such as tryptophan, histidine and tyrosine.Previous researches have demonstrated the flavin-mediated light-induced degradation of these easily oxidized amino acids and elaborated the reaction pathways, but detailed insights involved the peptides and proteins containing these amino-acid residues have not been studied [19,20].Regarding this, we compared the degradation efficiency of tetrapeptides WIII, HIII and YIII by RF under the irradiation of blue light (LED lamp, 13 W, 450–475 nm).After irradiation for 20 min, WIII and HIII were completely degraded while 40% of YIII remained, suggesting that WIII and HIII were more sensitive to RF than YIII (Fig.S12 in Supporting information).Based on the crystallographic data of some native proteins, tyrosine was found with the surface abundance of 4.8%, which was higher than histidine (2.2%) and tryptophan (<2%) [21].Therefore, we investigated the degradation of peptides and proteins rich in tyrosine residue.As indicated in Fig.1A, tetrapeptide AcYQRL was degraded completely in the presence of RF upon blue light irradiation, but the control sequence AcFQRL remained stable under the identical experimental conditions, suggesting that tyrosine is the main factor that induces the degradation of the peptide.Similarly, random tyrosine-containing hexapeptides SFPKYV and YSPIYL could also be fully degraded in this system (Fig.1B).Moreover, tyrosine-rich insulin and green fluorescent protein (GFP) disappeared completely in the presence of RF under the irradiation of blue light (Fig.1C).Based on these results, we speculated that this photodamage effects on peptides and proteins could occur to intracellular proteins and kill cancer cells if RF was abundant in cells.As identified by ESI-MS, the photoproduct of RF was lumichrome (Rt 9.4 min inthe HPLC chromatogram, Fig.1D), which was in accordance with the reported photosensitive mechanism of RF [8,20].
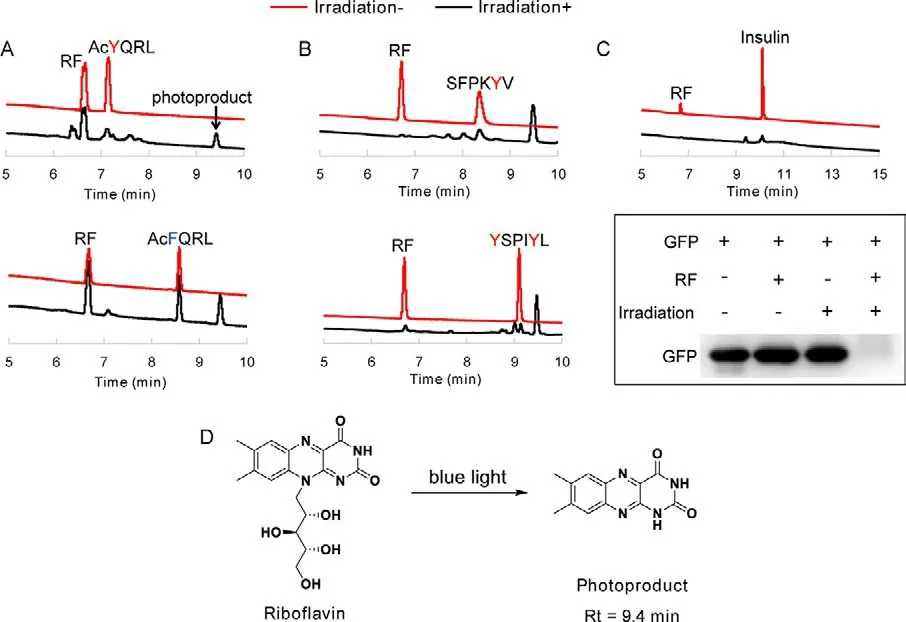
Fig.1.The degradation of tyrosine-containing peptides and proteins by RF.(A, B) HPLC analysis of the degradation of peptides in the presence of RF and blue light.(C) The degradation of tyrosine-containing proteins by RF under the irradiation of blue light.(D) The photoproduct of RF.
PDT is a O2-dependent process, in which triplet-excited state of photosensitizers leads to the formation of either singlet oxygen(1O2)viaenergy transfer to environmental oxygen (Type II mechanism), or hydrogen peroxide and hydroxyl radicalviaradicalisation (Type I mechanism).Thus, we studied the photodegradation of tetrapeptide AcYQRL in the presence or absence of O2with RF as a photosensitizer.Upon blue light irradiation, AcYQRL disappeared quickly in the presence of air, while this tetrapeptide remained intact under N2atmosphere (Fig.2A).These results manifested the photooxidation of RF is truly O2-dependent.Subsequently, we investigated the influence of both the equivalent of RF and the irradiation time on the degradation efficiency.For hexapeptide YSPIYL,more than 50% of it was degraded after irradiation for 5 min, and 20 min was needed to ensure the complete degradation (Fig.2B).At the same time point, treatment of the peptide with greater amount of RF resulted in the higher degradation ratio, indicating that increasing the intracellular concentration of RF is crucial for its photochemical activity.Combined, these data provided an experimental basis for the following cytotoxicity test.
RF is important in metabolic process occurring in living organism.However, the average concentration of RF in a cell is very low, so the photoactivity of RF is undetectable at the cellular level.We chose the following cancer cell lines, PC3, HeLa, MDA-MB-231,A549 and U-87, to investigate the cellular uptake of RF.Although riboflavin transporters and the riboflavin carrier protein are overexpressed in many tumor types, tumor stem cells and tumor neovasculatures, no fluorescence signal of RF could be detected in all tested cell lines (data not shown), including PC3 cells which were reported to have high expression level of riboflavin receptors [17].To develop RF as a photosensitizer for PDT, it is necessary to increase the intracellular level of RF.We considered that it was possible to improve the cellular uptake of RF through coupling a functional ligand to it.Arg-rich peptides such as Arg8and TAT are commonly used delivery vehicles for drugs and nanomaterials.In the previous study, we discovered that (Cha-Arg)3composed of three cyclohexylalanines (Cha) and three arginines (Arg)alternately was an effective cell-penetrating peptide.(Cha-Arg)3is mainly distributed in the cytoplasm, which is different from the mitochondria-penetrating peptide (Cha-D-Arg)3developed by Hortonet al.[22,23].Thus, using two cell-penetrating peptides,Arg8and (Cha-Arg)3to mediate the delivery of RF, we synthesized two RF conjugates, termed as Arg8-RF (1), (Cha-Arg)3-RF(2), respectively.Considering that ROS can be readily produced in mitochondria where the oxygen levels are high, we also conjugated a mitochondria-targeting small molecule triphenylphosphine(TPP) with RF to synthesize TPP-RF (3).Their structures and synthetic routes were indicated in Fig.3.In brief, RF was reacted with adipic anhydride to generate carboxylated riboflavin RF-COOH(4).Treatment of 4 with 2-maleimido-ethanamine in the presence of HATU and DIEA provided RF-Ma (5).Subsequently, Arg8-RF (1)and (Cha-Arg)3-RF (2) were prepared by coupling RF-Ma (5) bearing a maleimide group with HS-Arg8(6) or HS-(Cha-Arg)3(7) under mild conditions, respectively.For the synthesis of TPP-RF (3),(3-carboxypropyl)triphenylphosphonium bromide was transformed to amine-terminated TPP-NH2(8)viacoupling withN,N-bis(3-aminopropyl)methylamine.Then TPP-RF (3) was obtained by the reaction of TPP-NH2(8) with carboxylated riboflavin 4 in the presence of HATU and DIEA.
To confirm the modification of RF did not alter its photodynamic effect, the superoxide and ROS generation efficiency of the synthesized RF conjugates were examined and compared with RF using nitroblue tetrazolium (NBT) and 1,3-diphenylisobenzofuran(DPBF) assay, respectively [24,25].We observed that the absorbance of NBT at 560 nm was enhanced and the absorbance of DPBF at 415 nm was disappeared in the presence of RF or its conjugates upon exposure to blue light (Figs.S13 and S14 in Supporting information), indicating that RF and its conjugates could generate superoxide and ROS under irradiation, and the modified compounds maintained the photodynamic effect of RF.
The cellular uptake of the synthesized RF conjugates was evaluated in human cervical carcinoma cell line HeLa in comparison with RF.The cells were incubated with Arg8-RF, (Cha-Arg)3-RF,TPP-RF and RF for 2 h at 37 °C, and subsequently examined by fluorescent microscopy.As shown in Fig.4A, for RF-treated HeLa cells,no obvious fluorescent signal of RF could be detected, confirming that the cellular uptake of RF was poor despite it was an essential nutrient for human.Deduced from the fluorescence intensity, TPPRF could hardly enter HeLa cells and Arg8-RF displayed low cellular uptake.To our delight, (Cha-Arg)3-RF displayed distinct green fluorescence predominantly localized in the cytoplasm, indicating the high accumulation of (Cha-Arg)3-RF in HeLa cells compared with RF, TPP-RF and Arg8-RF.Moreover, the flow cytometry analysis also showed that the cellular uptake of (Cha-Arg)3-RF was much higher than RF, Arg8-RF and TPP-RF (Fig.4B).
The photocytotoxicity of RF and the synthesized RF conjugates were then evaluated against HeLa cells by CCK8 assays.For compounds RF, TPP-RF, and Arg8-RF with weak internalization, negligible or only small changes in cell viability were observed in the presence or absence of blue light at a concentration of 50 μmol/L for each compound (Fig.5A).As expected, (Cha-Arg)3-RF with high cellular uptake displayed strong cytotoxicity upon exposure to blue light.Then we examined the effects of drug dose and light dose on the cell viability for (Cha-Arg)3-RF.As shown in Fig.5B, the cell viability gradually decreased when the drug concentration and irradiation time increased.At 10 μmol/L, the cell viability decreased distinctly after 20 min irradiation, while remarkable cell death was observed with 25 μmol/L of (Cha-Arg)3-RF.Consistent with the degradation of peptides and proteins, the cell viability was also negatively correlated with the irradiation time, 20 min irradiation resulted in the lower cell viability compared with 10 min irradiation.The IC50value of (Cha-Arg)3-RF was 11.59 ± 0.38 μmol/L under 20 min irradiation.The photocytotoxicity of (Cha-Arg)3-RF was further studied through propidium iodide (PI) staining assay.PI is a dye for labeling dead cells since it can stain the late apoptotic and necrotic cells but cannot pass through the plasma membrane of the viable cells.After irradiation, the cells treated by (Cha-Arg)3-RF showed red fluorescence signal in the PI channel, indicating the occurrence of cell death.In contrast, (Cha-Arg)3-RF could not induce cell death without irradiation (Fig.5C).In the RF-treated groups,the fluorescence signal of PI could hardly be observed, indicating RF was noncytotoxic with or without irradiation.These results demonstrated that (Cha-Arg)3-RF was able to produce photocytotoxicity against cancer cells through increasing the cellular uptake of RF and would be a promising photosensitizer for PDT.
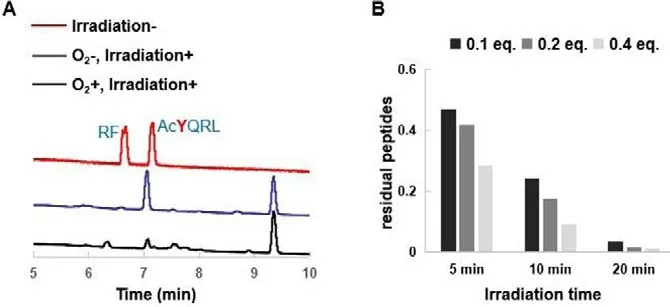
Fig.2.(A) O2-dependent photodegradation of peptides in the presence of RF.The PBS (10 mmol/L, pH 7.4) solution of 1.3 mmol/L AcYQRL and 0.52 mmol/L RF was flushed with N2 to remove O2.After irradiation for 20 min, the solution was analyzed by HPLC.(B) The degradation efficiency of YSPIYL with different irradiation time and RF equivalent.The samples of YSPIYL (1.3 mmol/L) in PBS containing 0.1,0.2 and 0.4 equiv.RF were irradiated for 5 min, 10 min and 20 min, respectively,and were subsequently analyzed by HPLC.
To explore the PDT effects of (Cha-Arg)3-RF, intracellular ROS production upon light irradiation was then tested using a commercially available ROS probe, RedoxSensorTMRed CC-1.Red CC-1 is nonfluorescent and can be oxidized by ROS in the cytosol to be a red-fluorescent product (absorption/emission maxima∼540/600 nm).As shown in Fig.6, for (Cha-Arg)3-RF-treated HeLa cells, strong red fluorescence signal was observed after irradiation indicating the cellular ROS generation.Without irradiation, (Cha-Arg)3-RF-treated HeLa cells showed no fluorescence signal.As a control group, ROS signal was hardly observed for RF-treated HeLa cells due to the poor cellular uptake of RF.Consistent with the fluorescence images, the flow cytometry analysis also indicated that(Cha-Arg)3-RF with high accumulation in HeLa cells could generate ROS under irradiation (Fig.S15 in Supporting information).Based on the primary photosensitive studies against peptides and proteins by RF, we speculated that the resulting ROS reacted indiscriminately with proteins in cytoplasm, causing loss of protein functionality and resulting in cell death.
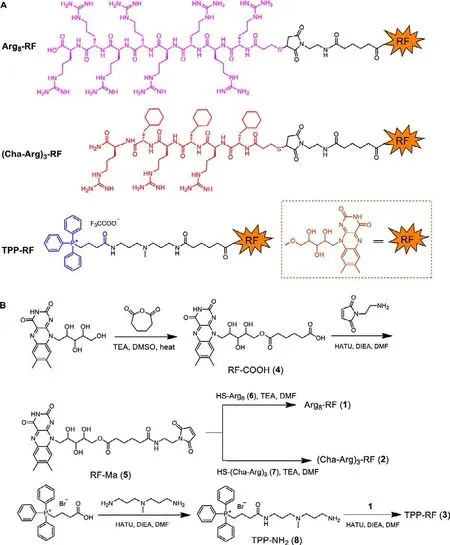
Fig.3.(A) The structure diagram of RF conjugates.(B) The synthetic routes of RF conjugates.
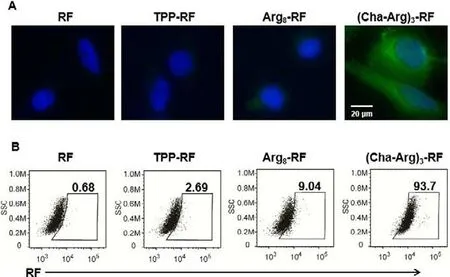
Fig.4.The cellular uptake of RF and its conjugates in HeLa cells analyzed by fluorescence imaging (A) and flow cytometry (B).HeLa cells were incubated, respectively with 25 μmol/L of RF, Arg8-RF, (Cha-Arg)3-RF and TPP-RF for 2 h, and then stained with Hoechst (nucleus stain,λex = 405 nm,λem = 420–490 nm) for 30 min.RF and its conjugates were excited at 488 nm, and their fluorescence was monitored at green channel.For flow cytometry analysis, the cells were harvested by trypsin enzymic digestion after the same incubation, and the fluorescence intensity of RF was recorded by Cyto FLEX.
Mitochondria play a central role in maintaining cell and organ function, but are vulnerable to ROS damage, consequently inducing cell death.To assess whether the ROS generated by (Cha-Arg)3-RF would cause mitochondrial damage, the mitochondrial membrane potential (MMP) change of HeLa cells was evaluated using a specific membrane-potential-dependent dye, tetramethylrhodamine ethyl ester (TMRE).TMRE is a non-fixable lipophilic cation that is actively pumped into and accumulates within normal mitochondria.When mitochondria is damaged, the fluorescence signal of TMRE is rapidly lost indicative of a loss of MMP [26].As shown in Fig.7, the treatment of HeLa cells with (Cha-Arg)3-RF and irradiation led to the significant reduction of red fluorescence signal from TMRE, indicating that the mitochondria membrane potential was changed.The strong red fluorescence of TMRE retained for the group treated with (Cha-Arg)3-RF without irradiation.No fluorescent signal changes of TMRE were detected for the RF treated group with or without light irradiation.Furthermore, flow cytometry was used to quantify the TMRE signal, and the results were consistent with the imaging data (Fig.S16 in Supporting information).These studies demonstrated that the ROS generated by (Cha-Arg)3-RF under irradiation could destroy mitochondria with a decrease in the mitochondrial membrane potential, which may eventually activate cell apoptotic death pathways.
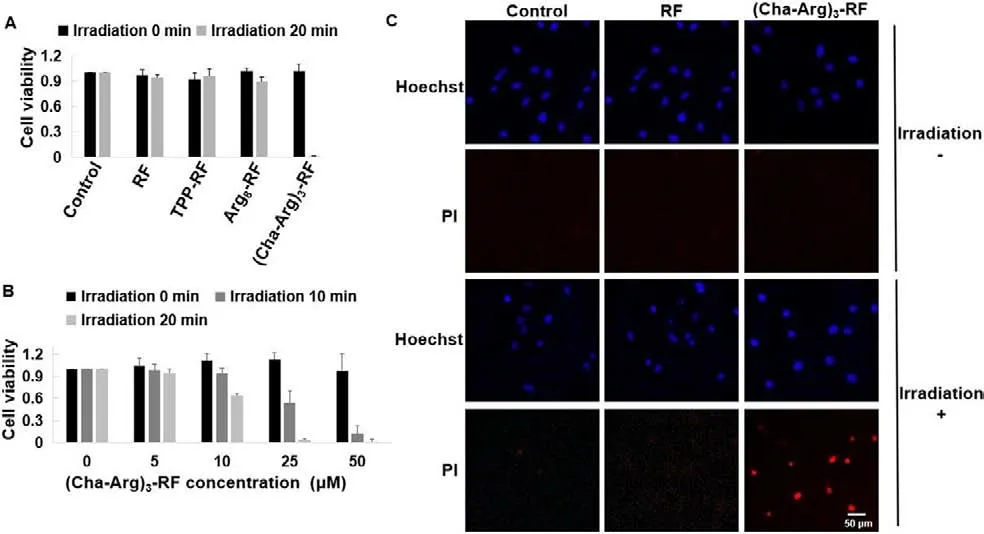
Fig.5.(A) The cytotoxicity of RF and its conjugates against HeLa cells with or without the irradiation of blue light.HeLa cells were incubated, respectively with 50 μmol/L of RF, TPP-RF, Arg8-RF and (Cha-Arg)3-RF for 4 h.After washing the drug mixture with PBS, the cells were then irradiated for 20 min using LED lamp (13 W, 450–475 nm).Then removing PBS and re-feeding the cells with culture medium for 48 h incubation, CCK8 was added to determine the cytotoxicity.(B) Survival of HeLa cells after treatment with (Cha-Arg)3-RF at different concentrations and irradiation time.HeLa cells were incubated with (Cha-Arg)3-RF (0, 5, 10, 25, 50 μmol/L) for 4 h, and then irradiated for 0, 10 and 20 min, respectively.After 48 h incubation, CCK8 was added to determine the cytotoxicity.For the cytotoxicity test, data are shown in mean value ± standard error of the mean value (SEM) of three independent experiments.The experiment was performed in quadruplicate.(C) Fluorescence images of HeLa cells treated with RF or(Cha-Arg)3-RF (25 μmol/L) for 4 h in the presence or absence of blue light irradiation for 20 min and then stained with Hoechst and PI.PI was excited at 550 nm and its fluorescence was monitored at red channel.
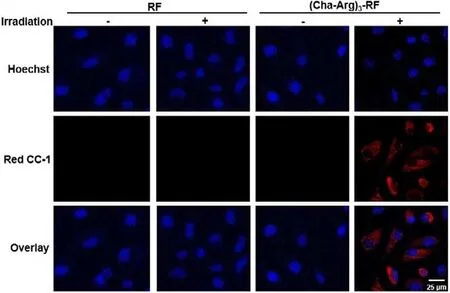
Fig.6.Fluorescence images of HeLa cells to indicate intracellular ROS production induced by (Cha-Arg)3-RF in the condition of blue light irradiation.HeLa cells were incubated with RF or (Cha-Arg)3-RF (10 μmol/L) for 4 h, and then incubated with Red CC-1 (5 μmol/L) for 1 h.After irradiation for 10 min, cells were stained with Hoechst and imaged.Red CC-1 was excited at 550 nm and its fluorescence was monitored at red channel.
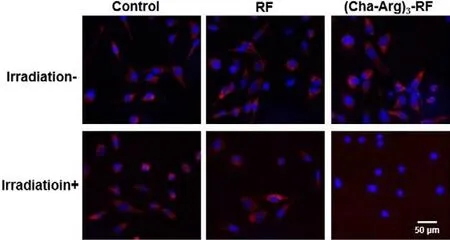
Fig.7.Fluorescence images of HeLa cells treated with RF or (Cha-Arg)3-RF(10 μmol/L) for 4 h in the presence or absence of blue light irradiation for 10 min and then stained with Hoechst and TMRE.TMRE was excited at 550 nm and its fluorescence was monitored at red channel.
RF is an essential vitamin and is a promising natural photosensitizer with nontoxicity, excellent biocompatibility and biodegradability.Upon exposure to blue light, RF undergoes complex photochemical reactions with peptides and proteins containing oxidizable amino-acid residues such as tryptophan, histidine and tyrosine, causing loss of protein functionality and inducing protein degradation.To achieve efficient cellular uptake of RF and explore its photosensitive properties in cancer cells, three RF conjugates were prepared by tethering RF to cell-penetrating peptide Arg8and (Cha-Arg)3, as well as a mitochondria-targeting small molecule TPP, respectively.Among them, (Cha-Arg)3-RF was able to enter HeLa cells more effectively than TPP-RF and Arg8-RF.Cytotoxicity evaluation and PI staining assay demonstrated that (Cha-Arg)3-RF had strong phototoxicity against HeLa cells upon blue light irradiation at 10 μmol/L, while RF, TPP-RF and Arg8-RF were noncytotoxic up to 50 μmol/L.The generation of ROS during the photodynamic process probably destroyed the endogenous proteins and the mitochondria, ultimately leading to cell death.Conclusively, as a promising photosensitizer for PDT, (Cha-Arg)3-RF has great potential for further exploration in cancer drug discovery.Moreover,the study provides a new strategy for the development of effective PDT drugs originating from natural RF.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.21977111 and 22007096), the National Key Research and Development Program of China (No.2021YFA0910000), the Natural Science Foundation of Guangdong Province (Nos.2019A1515012073 and 2018B030308001), and the Shenzhen Science and Technology Innovation Commission (No.JCYJ20210324120200001).The authors appreciate Peking University Shenzhen Graduate School for the assistance of Mass facility.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.01.036.
 Chinese Chemical Letters2022年9期
Chinese Chemical Letters2022年9期
- Chinese Chemical Letters的其它文章
- A review on recent advances in hydrogen peroxide electrochemical sensors for applications in cell detection
- Rational design of nanocarriers for mitochondria-targeted drug delivery
- Emerging landscapes of nanosystems based on pre-metastatic microenvironment for cancer theranostics
- Radiotherapy assisted with biomaterials to trigger antitumor immunity
- Development of environment-insensitive and highly emissive BODIPYs via installation of N,N’-dialkylsubstituted amide at meso position
- Programmed polymersomes with spatio-temporal delivery of antigen and dual-adjuvants for efficient dendritic cells-based cancer immunotherapy
