Efficient dye-sensitized solar cells based on concerted companion dyes: Systematic optimization of thiophene units in the organic dye components
J iaxin Luo, Zhengli Xie, Jiazhi Zou, Xinyan Wu, Xueqing Gong, Chengjie Li,Yongshu Xie
Key Laboratory for Advanced Materials and Institute of Fine Chemicals, Centre for Computational Chemistry and Research Institute of Industrial Catalysis,School of Chemistry and Molecular Engineering, East China University of Science and Technology, Shanghai 200237, China
ABSTRACT To develop efficient concerted companion (CC) dyes for fabricating high-performance DSSCs, three organic dyes XL1-XL3 have been designed by varying the position and number of the β-hexylthiophene (HT)bridges, and these organic dye units are covalently linked with our previously reported porphyrin dye XW10 to construct the corresponding CC dyes XW74-XW76.Among the organic dyes, XL3 contains two β-hexylthiophene units at both the donor and acceptor parts and thus possesses stronger light-harvesting capability in the green light region.Because of the most complementary absorption between XL3 and XW10 as well as the excellent photovoltaic behavior of the individual XL3 dye, the corresponding CC dye XW76 affords the best PCE (10.78%) among all the CC dyes.Upon coadsorption with CDCA, XW76 affords a highest PCE of 11.35%, which outperforms the previous cosensitization system of XW10+WS-5.This work provides an approach for developing efficient DSSCs based on CC dyes composed of an organic dye unit with suitable π spacers inserted at appropriate positions.
Keywords:Dye sensitized solar cells Concerted companion dyes Porphyrin dyes Cosensitization
Among the third-generation photovoltaic devices, dyesensitized solar cells (DSSCs) have aroused considerable attention owing to their easy fabrication and colorful appearance since the first report by Grätzel and O’Regan in 1991 [1–3].The power conversion efficiencies (PCE) have been dramatically promoted with the development of various mesoscopic semiconductors [4],photosensitizers [5], redox shuttles [6,7] and device designs [8,9].Among them, the photoactive sensitizers act as the electron pumps to harvest light and transfer the excited electrons into the semiconductor in the DSSCs.In this respect, various types of sensitizers have been developed.For example, ruthenium-based complexes[10], metal-free organic dyes [11–13] and zinc porphyrin dyes[14–18] have been designed to enhance the PCEs [19–25].
Porphyrin sensitizers, as the analogues of chlorophylls in photosynthesis, possess the properties of intense and broad absorption, convenient modification and remarkable light-thermal stability, which enable them to be effective in fabricating efficient DSSCs[26,27].However, the absorption valley for porphyrins in the green light region limits their light-harvesting capability and the related photovoltaic performance [28,29].
To fill up the absorption valley and improve the PCEs, various organic sensitizers have been designed and used as the cosensitizers for porphyrins to fabricate efficient DSSCs [18,30].However, a number of factors need to be optimized for fabricating the cosensitized DSSC devices, and the distribution of the dyes on the semiconductor is rather difficult to control, which is unfavorable for further improving the photovoltaic performance [31].To address these problems, we have recently developed a novel class of concerted companion (CC) dyes to achieve the “intramolecular cosensitization” effect by covalently linking the organic dye and porphyrin dye units through long chains [14,32].Remarkably, such dyes feature panchromatic absorption, and endow the corresponding DSSCs with high-efficiency and long-term photostability.Based on this background, we herein report the optimization of the organic dyes to systematically modulate the absorption and thus improve the performance of CC dyes.With respect to the design of organic dyes, the thiophene unit has been extensively used as theπspacer [33], and its number [34,35] and position [36,37] in the dye framework dramatically affect the photophysical properties of the sensitizers.Thus, three organic dyes XL1-XL3 have been synthesized by incorporating theβ-hexylthiophene (HT) unit as theπspacer, the electron-rich phenothiazine unit as the donor [38,39],and benzothiadiazole as the auxiliary acceptor [40].Then, these organic dye units are covalently linked with our previously reported porphyrin dye XW10 [20] to construct the corresponding CC dyes XW74−XW76 (Fig.1).
The number and position of the HT units dramatically affect the photovoltaic behaviors of XL1−XL3.When it is shifted from the donor (XL1) to the acceptor side (XL2), the anti-aggregation and recombination suppressing capability are obviously improved,accompanied with the elongation of the electron lifetime, giving rise to an improvedVOC(819 mV) for XL2.On the other hand, dye XL3 contains two HT units at both sides of the benzothiadiazole unit, and it not only shows stronger absorption in the green light region, but also keeps the well-defined anti-aggregation character.As a result, broadened IPCE profile, improvedJSC, highVOCand a relatively high PCE of 8.69% have been obtained for XL3.Because of the most complementary absorption character between XL3 and XW10 as well as the good photovoltaic behavior for the individual XL3 dye, the corresponding CC dye XW76 affords the highestJSC,VOCand PCE (10.78%) among the CC dyes.Notably, further coadsorption with chenodeoxycholic acid (CDCA) affords a high PCE of 11.35%.This work illustrates the importance of tailoring the position and number ofπspacers in the organic dye components for regulating the spectral response and thus elevating the photovoltaic performance of the CC dyes.
The synthetic procedures for XL1−XL3 and XW74−XW76 are illustrated in Scheme S1 (Supporting information).The dye precursors were obtainedviaSonogashira and Suzuki coupling reactions.Subsequent hydrolysis reactions under alkali conditions afforded the final dyes.The structures of all new compounds have been characterized by NMR and mass spectra, see details in Supporting information.
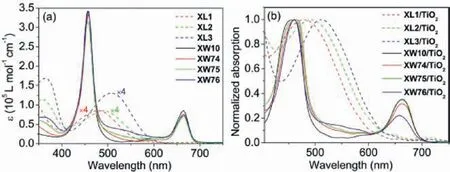
Fig.2.Absorption spectra of the sensitizers (a) in THF solutions, and (b) adsorbed on TiO2 films (2 μm).
Fig.2 presents the absorption spectra of the dyes in THF and adsorbed on thin TiO2films, and Table S1 (Supporting information)summarizes the corresponding data.The different positions of the HT unit in the isomeric dyes XL1 and XL2 induce a 16 nm red shift of the low energy transition band for XL2 (494 nm) relative to that of XL1 (478 nm), demonstrating that the thiophene moiety linked neighboring to the acceptor unit may induce less distortion and better conjugation of the molecule [41], as indicated by the calculated smaller dihedral angles in the optimized structures (vide infra).Different from these two dyes, XL3 contains two HT units on both sides of the benzothiadiazole unit, exhibiting a further bathochromically shifted absorption peak at 507 nm with a higher molar extinction coefficient.Compared with XL1 and XL2, XL3 is expected to be a better cosensitizer for porphyrin dye XW10, considering its better compensation of the absorption valley of XW10.Indeed, by combining XL3 with XW10, the CC dye XW76 presents a better panchromatic response compared to XW74 and XW75, especially in the wavelength range from 500 nm to 600 nm (Fig.2a).
Upon adsorption onto the TiO2films (Fig.2b), XL1−XL3 and XW74−XW76 show broadened bands, which facilitate the light harvesting in the DSSCs.Notably, the absorption spectra exhibit negligible blue shift upon adsorption, compared with those in the solutions, indicating the absence of notable aggregation on the TiO2films [32].
Electrochemical behavior of the organic dyes and CC dyes adsorbed on TiO2films were measured by cyclic voltammetry (CV)and differential pulse voltammetry (DPV) (Table S2, Figs.S2 and S3 in Supporting information) to evaluate the feasibility of electron transfer.The highest occupied molecular orbital (HOMO) levels of XL1−XL3 and XW74−XW76 are 0.95∼0.99 Vvs.NHE, more positive than the I−/I3−redox potential (∼0.4 Vvs.NHE), indicating the possibility for dye regeneration.In addition, their lowest unoccupied molecular orbital (LUMO) levels are calculated to be −1.26∼−0.94 VversusNHE, well above the conduction band edge of TiO2(−0.5 Vvs.NHE), implying the feasibility for electron injection.
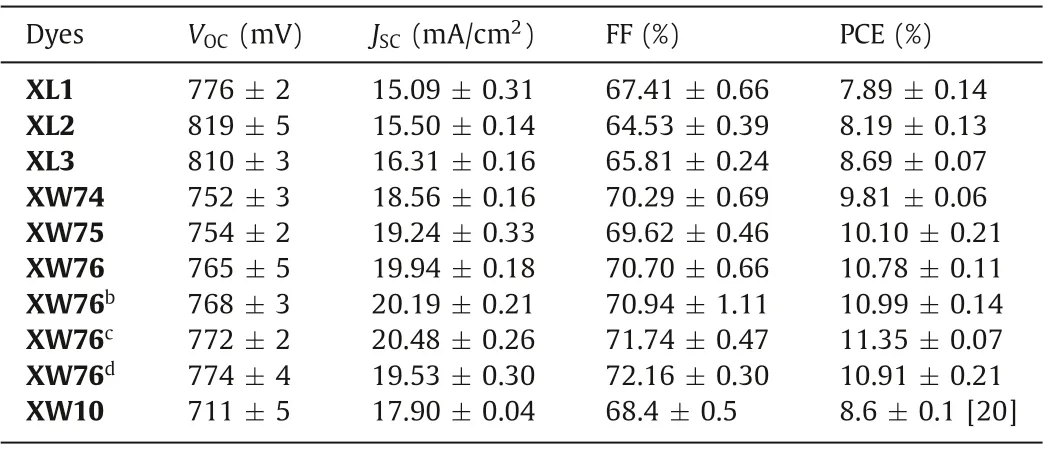
Table 1 Photovoltaic performance of DSSCs based on XL1-XL3, XW74-XW76 and the reference dye XW10.a
The two sub-dye units in the CC dyes are expected to be electronically independent, as have been revealed by the theoretical calculations in our previous work [32].Herein, we have investigated the optimized molecule structures and the frontier molecular orbitals of the XL dyes using DFT calculations [42].As presented in Fig.S4 (Supporting information), the XL dyes exhibit similar electron distribution in the frontier orbitals.The HOMOs are delocalized mainly over the phenothiazine donor, the neighboringπspacer and the auxiliary benzothiadiazole acceptor.Whereas, the LUMOs are distributed mainly from the acceptor to the benzothiadiazole moiety.For the isomeric dyes XL1 and XL2, the latter exhibits better overlapping between the HOMO and LUMO electrons with respect to the former, demonstrating that the HT unit neighboring to the acceptor terminal is favorable for the charge transfer and injection of the excited electrons into the TiO2semiconductor.With respect to the optimized geometry, the dihedral angles between the HT and benzothiadiazole units are smaller than 15°, irrespective of the position of the HT unit.On the other hand,the angles between the donors and the adjacent units increase in the order of XL2 (31.58°) Fig.3.(a) J−V characteristic curves, (b) IPCE spectra, plots of (c) Cμ and (d) τ versus the bias voltages of the DSSCs based on the sensitizers. To evaluate the photovoltaic behavior of the XL series dyes,they have been utilized in fabrication of DSSCs using the I−/I3−electrolyte (see Supporting information for more details).TheJ-Vcurves (Fig.3a) and IPCE spectra (Fig.3b) were studied under AM 1.5 global sunlight and the corresponding photovoltaic parameters are collected in Table 1.The solar cells based on XL1 afford theJSC,VOC, and PCE of 15.09 mA/cm2, 776 mV, and 7.89%, respectively,and XL2 exhibits an improvedJSCof 15.50 mA/cm2, which may be ascribed to the red-shifted absorption and better overlapping between the HOMO and LUMO orbitals as indicated by the theoretical calculations (vide supra).Meanwhile, XL2 exhibits an improvedVOCof 819 mV, which is among the highestVOCvalues achieved for the DSSCs based on the iodine electrolyte [18,43,44].The extraordinaryVOCvalue may be ascribed to the well balanced antiaggregation ability around both the donor and the acceptor [45].As a result of the improvedJSC,VOC, an improved PCE of 8.19% was achieved for XL2.Compared with XL2, XL3 affords a similarVOC,accompanied with a dramatically enhancedJSCof 16.31 mA/cm2,which may be ascribed to the stronger light-harvesting capability as a consequence of the larger conjugated skeleton.Finally, XL3 affords a highest PCE of 8.69% among the XL series of dyes, which may be ascribed to its most extensive absorption wavelength range as well as its highest molar extinction coefficient (vide supra), in spite of its smallest adsorption amount (Table S3 in Supporting information).To further understand the trend for the photocurrents,the incident photon-to-current conversion efficiency (IPCE) spectra have been recorded.As depicted in Fig.3b, XL1−XL3 display similar high IPCE plateaus exceeding 85% around 500 nm, with the band edges gradually extended in the sequence of XL1 Coadsorption of XL1−XL3 with various concentrations of CDCA was also investigated (Table S4 in Supporting information) to check the possibility of further improving theVOCby reducing the dyeaggregation [46].However, loweredVOC,JSCand PCE values were obtained in the presence of CDCA.The decreasedVOCvalues indicate that the inherent excellent anti-aggregation ability of the XL-sensitizers cannot be further improved by using CDCA, and the loweredJSCvalues may be attributed to the decrease in the dye loading amounts due to the competitive adsorption of CDCA [24]. On this basis, we continued to check the photovoltaic behavior of the CC dyes XW74−XW76, which contain both the organic dye units of XL1−XL3 and a porphyrin dye unit of XW10 [20].As a result, higher IPCE plateaus over the 300 nm to 700 nm range and higherJSCvalues in the sequence of XW74 (18.56 mA/cm2) With the purpose of revealing the factors affecting theVOCvalues, electrochemical impedance characteristics under the dark condition have been monitored (Fig.S8 in Supporting information).The charge transport resistance (Rtr) and charge recombination resistance (Rrec) at a bias potential of −0.75 V are summarized in Table S6 (Supporting information), and the plots of chemical capacitances (Cμ) and electron lifetimes (τ)versusthe bias voltages are shown in Figs.3c and d.The results indicate that theRtrvalues are comparable for all the dyes (9.94∼15.28Ω).By contrast,theRrecvalues are dramatically different and positively related to theVOCvalues.Theoretically, for a certain electrolyte, thequasi-Fermi level of the TiO2semiconductor, which is associated with the conduction band edge level (Ec) and the free electron density,is a key factor affecting theVOC[48].As presented in Fig.3c, theEc-relatedCμvalues exhibit slight difference for all the dyes, indicative of the negligible effect of the conduction band shift on theVOC.By contrast, theτvalues, which are related positively to the free electron density in the TiO2films, raise in the order of XW10 Photostability is a crucial factor for practical applications of DSSCs [49].In this work, the long-term photovoltaic performance of the DSSCs soaked in visible light was monitored to evaluate the photostability.After 500 h of visible light soaking, the representative cells sensitized by XL3 and XW76 afforded efficiencies of 7.75% and 10.04%, which are 89% and 93% of the initial PCEs,respectively (Fig.S9 in Supporting information), further confirming that the DSSCs based on the CC dyes comprising two anchor groups display superior photostability with respect to those based on the single-anchor ones. In summary, three metal-free organic sensitizers XL1−XL3, featured with different numbers and positions ofβ-hexylthiophene units and a benzothiadiazole unit, have been synthesized and used as the organic components for covalently linking with a porphyrin dye XW10 to construct the corresponding CC dyes XW74−XW76.Notably, XL2 with theβ-hexylthiophene near the acceptor side affords a strikingVOCof 819 mV because of its superior antiaggregation effect.Dye XL3 comprising two HT groups on both sides of the benzothiadiazole unit not only shows intensified absorption bands in the green light region but also maintains the excellent anti-aggregation capability to give a relatively highVOC(810 mV).On this basis, the corresponding CC dyes show enhancedJSCvalues compared to XW10 because of their panchromatic absorption behavior.Among them, XW76 contains the XL3 subunit,which exhibits the best photovoltaic behavior among the XL series of dyes as well as the most complementary absorption with XW10,affording the highest PCE of 10.78%.Coadsorption of XW76 with CDCA affords a further improved PCE of 11.35%, which outperforms the previous intermolecular cosensitization system of XW10+WS-5(11.0%) [20].These results illustrate the importance of tailoring the position and number ofπspacers in designing high-performance organic dyes, which can be used as components for further linking with a porphyrin dye unit to construct CC dyes for fabricating efficient DSSCs. Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Acknowledgments This work was financially supported by the National Natural Science Foundation of China (Nos.22131005, 21772041, 21971063 and 22075077), the Program of Shanghai Academic Research Leader (No.20XD1401400), the Natural Science Foundation of Shanghai (No.20ZR1414100), and the Fundamental Research Funds for the Central Universities (No.222201717003). Supplementary materials Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.01.052.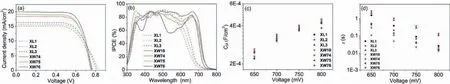
 Chinese Chemical Letters2022年9期
Chinese Chemical Letters2022年9期
