Octacyclic and decacyclic ent–abietane dimers with cytotoxic activity from Euphorbia fischeriana steud.
Yulin Peng, Yibo Chng, Chengpeng Sun, Weiyi Wng, Cho Wng, Yn Tin,Bojing Zhng, S Deng, Wenyu Zho, Xiohi M
a College of Pharmacy, College of Integrative Medicine, Dalian Medical University, Dalian 116044, China
b Second Affiliated Hospital, Dalian Medical University, Dalian 116023, China
c Key Laboratory of Marine Biogenetic Resources, Third Institute of Oceanography, Ministry of Natural Resources, Xiamen 361005, China
ABSTRACT A novel Diels-Alder adduct possesses a 6/6/6/5/6/6/6/6 octacyclic skeleton featured with bicyclo[2.2.2]octane moiety, biseupyiheoid A (1), along with another decacyclic 6/6/6/3/5/6/5/6/6/6 fused diterpenoid dimer, bisfischoid C (2), were isolated from Euphorbia fischeriana.Their structures were determined by spectroscopic, X-ray crystallographic approaches, and quantum mechanical calculations.The structural features of 1 and 2 were hypothesized to involve intramolecular Diels-Alder reactions with different coupling patterns.Dimer 1 showed antiproliferative activity through apoptosis activation in LoVo cells.
Keywords:Ditrpenoid dimers Euphorbia fischeriana ent-Abietanes Diels-Alder adduct Cytotoxic effect
Diterpenoids originated from geranylgeranyl pyrophosphate(GGPP) are a large group of highly diversified terpenoids with typical skeletons including acyclic, mono-, bi-, tri-, and tetracyclic systems [1].A small subset of diterpenoids comprises dimeric diterpenoids featured with at least 40 carbons in their structures, in which the two monomeric blocks are linked together directly or through an oxygen atom [2].Diterpenoid dimers possess increased complex ring systems and more chiral centers compared to their monomeric analogues, which poses a great challenge for their structural determination.
The genusEuphorbia(Euphorbiaceae) has been regarded as a valuable resource of bioactive diterpenoids with unique structural diversity [3].For example, euphopias A−C isolated fromE.helioscopiaare rearranged jatrophane diterpenoids with antiinflammatory potentials [4]; pedrolide is a polycyclic diterpene obtained fromE.pedroishowed the inhibition effects on Pglycoprotein [5].Some plants ofEuphorbiagenus, includingE.ebracteolateandE.fischeriana, have been used as traditional Chinese medicines for thousands of years.With the aim of discovering more intriguing diterpenoids from medicinal plants of the genusEuphorbia[6,7], biseupyiheoid A (1, Fig.1), an unprecedented dimericent–abietane containing spirocyclic 6/6/6/5/6/6/6/6 ring systems, and a decacyclic 6/6/6/3/5/6/5/6/6/6 skeletalent–abietane dimer, bisfischoid C (2), were identified from the roots ofE.fischeriana.The biological assay revealed that 1 showed the significant cytotoxic activity by apoptosis-induced effect in human colon carcinoma LoVo cells.
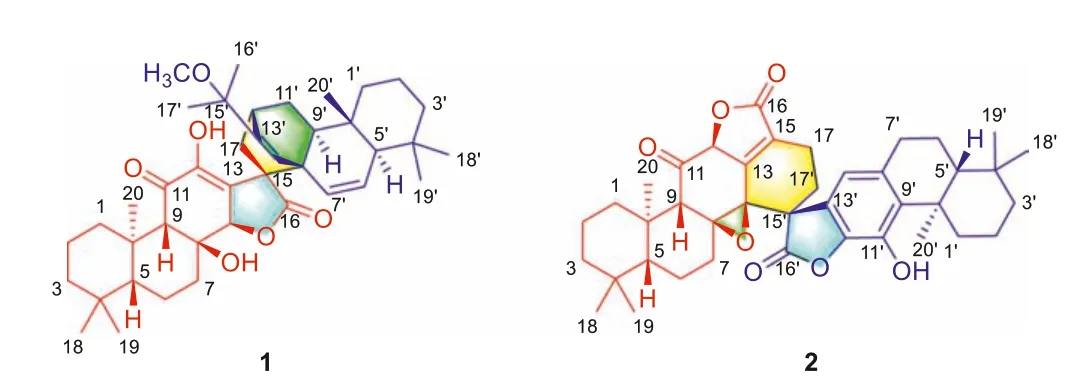
Fig.1.Structures of dimeric diterpenoids 1 and 2.
Compound 1, obtained as a yellow oil, has a molecular formula of C41H58O6with 13° of unsaturation, as deduced from its HRESIMS (m/z669.4129 [M+Na]+, calcd.669.4126).The1H NMR data (Table S1 in Supporting information) exhibited eight tertiary methyls atδH0.56 (s, 3H), 0.80×3 (s, 9H), 0.85 (s, 3H), 0.93(s, 3H), 1.33 (s, 3H), and 1.36 (s, 3H), and a methoxy group atδH3.26, three olefinic signals atδH5.43 (br s), 5.87 (dd,J=10.2,2.9 Hz) and 5.99 (dd,J=10.2, 1.7 Hz).In addition, a proton associated with the oxygenated carbon atδH5.41 (s) and a number of protons atδH1.0−3.0 were also observed, suggesting the existence of a terpenoid backbone.The13C NMR and DEPT spectra (Table S1 in Supporting information) displayed 41 carbon resonances, including nine methyls, ten methylenes, nine methines (three olefinic and one oxygenated), and thirteen nonprotonated carbons (two oxygenated, three olefinic, and two carbonyls).Three double bonds and two carbonyl moieties accounted for five degrees of unsaturation, suggesting the existence of eight rings in the structure of 1.
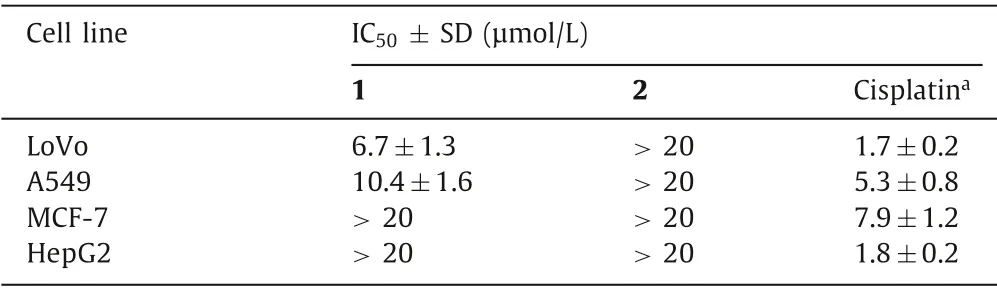
Table 1 Cytotoxic activities of 1 and 2.
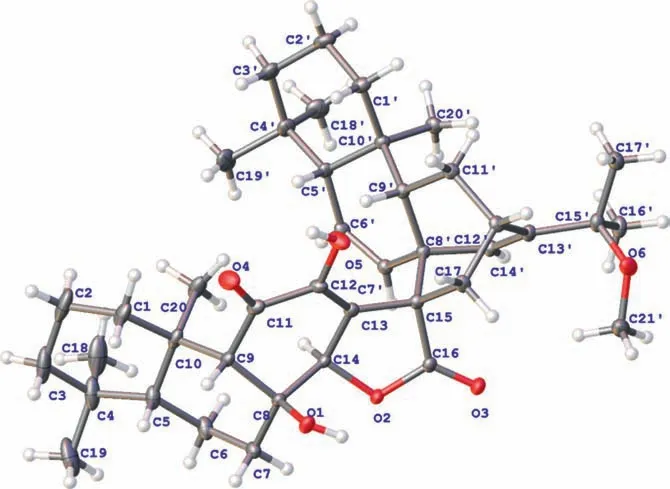
Fig.2.X-ray crystallographic structure of 1 (CCDC number: 2121291).
The planar structure of 1 was determined by its 2D NMR spectra (Fig.S1 in Supporting information).The1H–1H COSY spectrum shows H2–1/H2–2/H2–3 and H-5/H2–6/H2–7 correlations, together with long-range correlations from H3–20 to C-1, C-5, C-9, and C-10,from H-9 to C-8, C-10, C-11, C-12, and C-14 in the HMBC spectrum,allowing in three fused six-membered rings as shown in Fig.1.In addition, HMBC correlations of H-14/C-12 and C-13, H2–17/C-12, C-15, and C-16 were observed, indicating that a block of abietane(part A) was present in 1.The cross-peaks of H2–1′/H2–2′/H2–3′,H-5′/H-6′/H-7′, and H-9′/H2–10′/H-11′ in the1H–1H COSY spectrum, as well as cross-peaks from H3–20′ to C-1′, C-5′, C-9′, and C-10′, from H-9′ to C-8′ and C-14′, from H3–16′ to C-13′, from OCH3–15′ to C-15′ in the HMBC spectrum, confirmed the presence of another abietane part with a methoxy at C-15′ (part B) in the structure of 1.The1H–1H COSY correlation of H-12′/H2–17 and HMBC correlations of H-14′/C-15 and H-9′/C-15 suggested that part A was linked with the part Bviatwo single bonds of C-17 and C-15 with C-12′ and C-8′.Taking together, compound 1 was confirmed to be an abietane dimer with a bicyclo[2.2.2]octane moiety.
The relative configuration of 1 was partially fixed by the NOESY spectrum (Fig.S1).The correlation of H3–20/H-14 indicated their same orientation.The relative configuration of the rigid bicyclo[2.2.2]octane was established by correlations of H-9′/H-17 and H3–20′/H-14′.As for the C-8 and C-15, their configuration was a challenge, due to the lack of corresponding signals.Fortunately, a signal crystal of 1 was successfully obtained from a mixed solvent system (MeOH/CH2Cl2, 5:1), and X-ray diffraction was carried out with Cu Kαradiation (Fig.2), which not only confirmed the proposed structure but also determined the absolute configuration of 1 as 5R, 8S, 9S, 10R, 14R, 15S, 5′R, 8′R, 9′S, 10′S, 12′S.Finally, 1 was conducted as the first example of a novel class of diterpenoid dimer based onent–abietane, and it was named biseupyiheoid A.
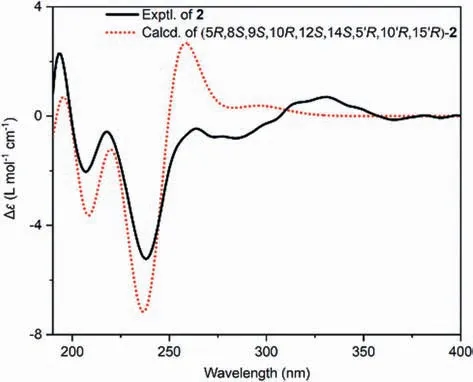
Fig.3.Calculated and experimental ECD spectra of 2 at the CAM-B3LYP/def2-tzvp level.
The molecular formula of compound 2 was deduced as C40H48O7by analysis of the HRESIMS (m/z641.3477 [M+H]+,calcd.641.3473),13C NMR, and DEPT spectra (Table S1).Compound 2 was also a dimeric diterpenoid that shares the same skeleton with bisfischoids A and B [8], as evidenced by its detailed 2D NMR analysis (Fig.S2 in Supporting information).The relative configuration of C-12 in 2 was determined by the cross-peak of H-12/H3–20 in the NOESY spectrum (Fig.S2).Although the correlations of H2–17′/H-14 were observed, the relative configuration of C-15′ and C-8 (C-14) could not be clearly assigned.Therefore, four possible isomers of 2 that differed in the configurations of C-8 (C-14) and C-15′ (Fig.S3 in Supporting information) have resorted to quantum chemical calculations of NMR and DP4+ analysis [9–11].The results show that isomer 8S∗,14S∗,15′R∗−2 with DP4+ possibility of 100% agrees well with the experimental data.Finally, the structure of 2 was elucidated as shown in Fig.1 by ECD calculations (Fig.3).
Although 1 and 2 have different backbones, it should be noted that the linkage connecting the two blocks of dimers 1 and 2 are both cyclohexene rings.It is reasonable to speculate that the dimerization reaction in the formation of 1 and 2 was the Diels-Alder-like [4+2] cycloaddition [12].A biosynthetic pathway starting from GGPP is proposed in Scheme 1.The first key intermediate produced from GGPP was i with a triene moiety in theent–abietane framework.Enzyme-dependent oxidation and esterification reactions in plants could yield caudicifolin, a major diterpenoid monomer exited inE.fischeriana[6], from intermediate i.The oxidation at C-12 and the dehydration at C-17 would be able to give ii with a diene fragment.The highly reactive epoxy moiety could be cleaved and oxidized to form intermediate iii.Further dehydration reactions can give iv with an aromatic ring.The [4+2]cycloaddition between iii and iv affords v that readily to form 2[13,14].The key intermediate iv may undergo hydrolysis and oxidation to give vi.The oxidation of OH-12 and the subsequent ketoenol tautomerism and esterification reactions formed viii.Another[4+2] reaction between i and viii produced ix, which produced 1 eventually.
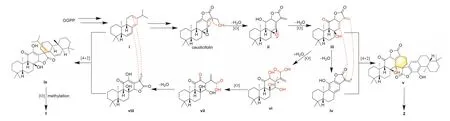
Scheme 1.Proposed biogenetic pathway for 1 and 2.
ent–Abietanes in the genusEuphorbiahave long been known for their promising cytotoxic activities [15,16].The cytotoxic activities of 1 and 2 against four human cancer cells derived from diverse organs were evaluated.The results (Table 1) revealed that dimer 1 (IC50=6.7 μmol/L) possesses an anti-proliferative effect against colon carcinoma LoVo cells, while dimer 2 displayed no cytotoxicity.Subsequently, the apoptosis-inducing activity for compound 1 was evaluated, and the result revealed that 1 could induce the apoptotic cell death of LoVo cells in a dose-dependent manner (Fig.4A).Dimer 1 was further investigated for the expression levels of apoptosis-related markers in LoVo cells.As shown in Fig.4B, compound 1 up-regulated levels of Bax, cleaved caspase 3, and cleaved caspase 9, and down-regulated the level of Bcl-2 dose-dependently.
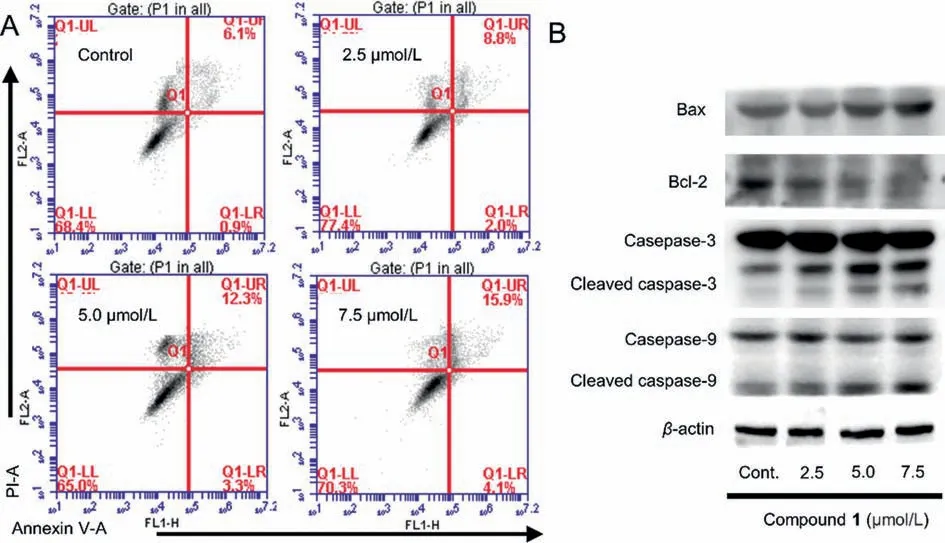
Fig.4.(A) Cell apoptosis induction effects of dimer 1 analyzed by annexin V-FITC/PI flow cytometry.(B) Compound 1 regulated the expression of apoptosis-related proteins in LoVo cells.
In conclusion, biseupyiheoid A (1) and bisfischoid C (2),two spiroent–abietane dimers with 6/6/6/5/6/6/6/6 and 6/6/6/3/5/6/5/6/6/6 fused ring systems, respectively, were obtained fromE.fischeriana.Dimeric diterpenoids 1 and 2 possessed the skeletons derived from Diels-Alder cycloaddition in different patterns, which further expanded the structural diversity of diterpenoids from the genusEuphorbia.Dimer 1 displayed prominent cytotoxicity and showed significant apoptosis-inducing activities in LoVo cells mediated by regulating Bax, caspase 3, caspase 9, and Bcl-2.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank the National Natural Science Foundation of China (No.81930112), Distinguished Professor Program of Liaoning Province (No.XLYC2002008), Natural Science Foundation of Liaoning Province (Nos.2020-BS-203 and 2020-MS-256), and Natural Science Foundation of Fujian Province (No.2021J01509).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.03.003.
 Chinese Chemical Letters2022年9期
Chinese Chemical Letters2022年9期
- Chinese Chemical Letters的其它文章
- A review on recent advances in hydrogen peroxide electrochemical sensors for applications in cell detection
- Rational design of nanocarriers for mitochondria-targeted drug delivery
- Emerging landscapes of nanosystems based on pre-metastatic microenvironment for cancer theranostics
- Radiotherapy assisted with biomaterials to trigger antitumor immunity
- Development of environment-insensitive and highly emissive BODIPYs via installation of N,N’-dialkylsubstituted amide at meso position
- Programmed polymersomes with spatio-temporal delivery of antigen and dual-adjuvants for efficient dendritic cells-based cancer immunotherapy
