Antiviral spirooliganones C and D with a unique spiro[bicyclo[2.2.2]octane-2,2′-bicyclo[3.1.0]hexane] carbon skeleton from the roots of Illicium oligandrum
Shunggng M, Ruing Wng, Rongmei Go, Xiojing Wng, Yuno Liu,Yong Li, Li Li, Yuhun Li, Jing Qu,∗, Shishn Yu,∗
a State Key Laboratory of Bioactive Sub stance and Function of Natural Medicines, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100050, China
b Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100050, China
ABSTRACT Two unprecedented polycyclic spirooliganones C and D (1 and 2) with a novel spiro[bicyclo[2.2.2]octane-2,2′-bicyclo[3.1.0]hexane] carbon skeleton, one known dimeric prenylated C6–C3 compound (3), and a pair of new enantiomeric prenylated C6–C3 compounds (+)-5 and (−)-5 together with their direct precursors (+)-4 and (−)-4 were isolated from the roots of Illicium oligandrum.Their structures and absolute configurations were elucidated by spectroscopic analysis, single crystal X-ray diffraction data, and electronic circular dichroism calculations.A possible biosynthetic pathway for compounds 1 and 2 involving the Diels-Alder reaction between (−)-sabinene and cyclic prenylated tetrahydropyrano-type C6–C3 compounds was proposed.The characteristic prenylated C6–C3 compounds (+)-4 and (−)-4 were separated on a chiral stationary phase and their absolute configurations were determined by calculated ECD for the first time.In the antiviral bioassays, compounds 1 and (+)-5 exhibited significant inhibitory activity against CVB3 with IC50 values of 11.11 μmol/L and 1.11 μmol/L, respectively.Compounds 1 and 2 also showed moderate inhibition against influenza A (H1N1) virus.
Keywords:Illicium Illicium oligandrum Spirooliganone Prenylated C6-C3 compound Antiviral activity
Many infections in humans and animals are caused by RNA viruses, including Coxsackie viruses, which are important human pathogens.Coxsackie viruses belong to the genus Enterovirus (Picornaviridae) and are divided into subgroups A and B based on their pathological effects on newborn mice.Coxsackie virus B3(CVB3) has been reported to induce acute and chronic viral myocarditis in children and young adults, which is associated with aseptic meningitis, hepatitis, and meningoencephalitis [1].and chronic dilated cardiomyopathy [2].Due to the lack of approved antiviral drugs and vaccines for Coxsackie viral infections, there is an urgent need for new antiviral agents.
Plants continue to be an extraordinary reservoir of novel molecules of medicinal value, including the discovery of antivirals.Among the 61 antiviral small-molecule drugs approved from 1981 to 2019, 26 of them are synthetic drugs with natural product pharmacophones and 6 of them are derived from natural products [3],including 3 anti-influenza drugs derived from shikimic acid [4,5],which was first isolated in 1885 fromIllicium religiosum[6,7].As a rich source for bioactive natural products, a number of antiviral diterpenes [8,9], sesquiterpenes [10,11], and prenylated C6–C3compounds [12] have been isolated from plants of the Illicium genus.In particular, prenylated C6–C3compounds have always been a notable research topic in the field of natural products and synthetic chemistry due to their complex structures, diverse biological activities, and synthetic challenges [13–19].Equally notable is that natural products in dimerization patterns biosynthetically derived from Diels-Alder cycloaddition have been increasingly attracted the attention of scientists due to their complex skeletons, biological activities, biosynthetic pathways and synthetic challenges [20–26].We recently reported the isolation and antiviral activities of some structurally diverse prenylated C6–C3compounds and their complex Diels-Alder adducts with monoterpene or sesquiterpenes fromI.oligandrum[19,27,28].Herein, as a part of an investigation into the bioactive compounds from Chinese Illicium plants, the phytochemicals in the ethanol extract ofI.oligandrumwere explored.Two unprecedented polycyclic spirooliganones C and D (1 and 2)(Fig.1) were isolated from the roots ofI.oligandrum, which features a novel spiro[bicyclo[2.2.2]octane-2,2′-bicyclo[3.1.0]hexane]carbon skeleton.We also purified and structurally characterized a pair of new enantiomeric prenylated C6–C3compounds (+)-5 and(−)-5, together with their direct precursors (+)-4 and (−)-4.Moreover, illicidione D (3) and compound 4 were identified by comparing their spectroscopic data with previous literature data [29,30].The structural elucidation of the new compounds 1, 2, and 5, the absolute configurations of (+)-4 and (−)-4, and the antiviral activity evaluation of all compounds are described herein.
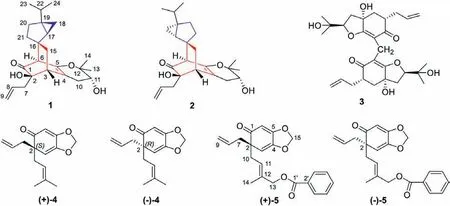
Fig.1.The structures of compounds 1–5.
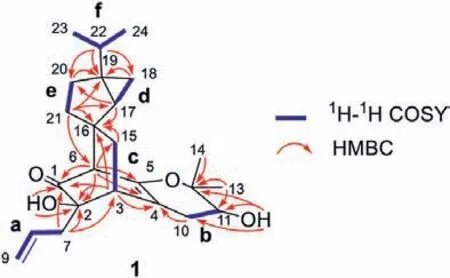
Fig.2.Key 1H–1H COSY and HMBC correlations for 1.
Spirooliganone C (1) was obtained as a crystal.Its molecular formula was deduced to be C24H34O4by HRESIMS and NMR(Table 1) data, suggesting 8° of unsaturation.The IR spectrum of 1 displayed characteristic absorbances for hydroxyl (3307 cm−1)and carbonyl (1721 cm−1) functionalities.The13C NMR and DEPT spectra showed 24 resonances attributable to four methyls, seven methylenes (one sp2carbon atδC118.3), six methines (one sp2carbon atδC135.0), six quaternary carbons (two sp2carbons atδC107.3 and 146.3), and one ketone carbonyl atδC210.1.The aforementioned four sp2carbons and a carbonyl accounted for 3° of unsaturation; thus, the remaining 5° of unsaturation required spirooliganone C (1) to possess a pentacyclic skeleton.
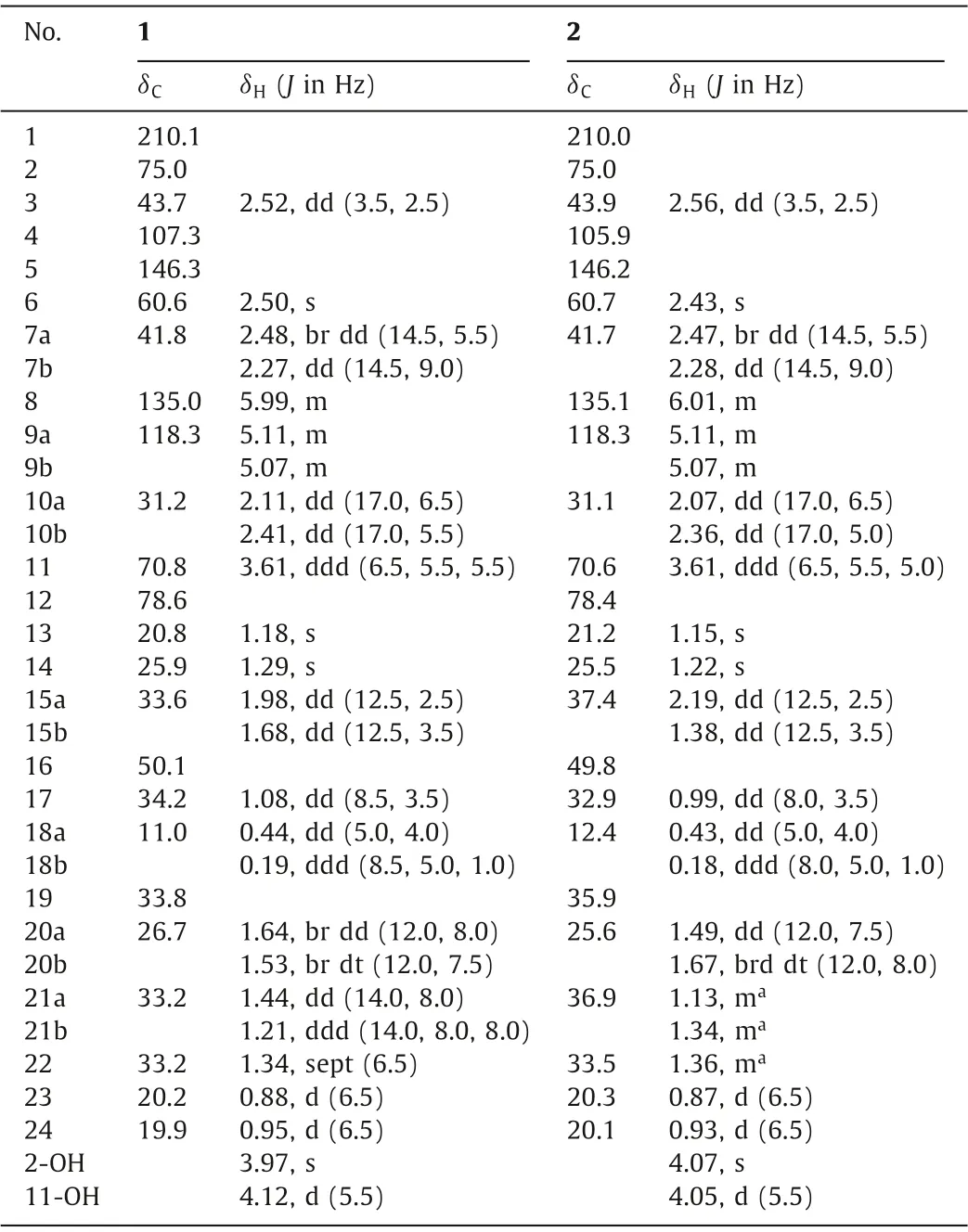
Table 1 1H (500 MHz) and 13C (125 MHz) NMR spectroscopic data for compounds 1 and 2 in acetone-d6.
In the1H NMR spectrum (acetone-d6), one doublet atδH4.12(1H,J=5.5 Hz) and one singlet atδH3.97, which did not display any correlations in the HSQC spectrum, were assignable to the exchangeable protons of two hydroxyls.The1H NMR spectrum of 1 also displayed two methyl singlets atδH1.18 and 1.29 and a singlet atδH2.50, which correlated with an intense13C signal atδC60.6 in the HSQC spectrum.The remaining multiplet signals in the1H NMR spectrum belonged to six discrete spin systems, a (C7–C9),b (C10–C11), c (C3–C15), d (C17–C18), e (C20–C21), and f (C22–C24) (drawn with thick blue bonds in Fig.2), as concluded from1H–1H correlation spectroscopy (COSY) and HSQC analysis.
In the HMBC spectrum, correlations (Fig.2) were observed from H-3 and H-6 to C-1, C-2, C-4, and C-5; from H-3 to C-16; from H-6 to C-15; and from H2-15 to C-2, C-3, C-4, C-6, and C-16,which indicated that C1-C6, C15-C16, and fragment c constitute a bicyclo[2.2.2]oct–5-en-2-one moiety.The HMBC correlations from H-17 to C-16 and C-20; from H2-18 to C-19; from H2-20 to C-18 and C-19; and from H2-21 to C-16, C-17, C-19, and C-20, as well as the aforementioned two fragments d and e, allowed for the establishment of a bicyclo[3.1.0]hexane moiety.Furthermore, key HMBC correlations from both H-17 and H2-21 to C-6 and from H2-15 to C-16 and C-17 were observed and suggested that the bicyclo[2.2.2]oct–5-en-2-one moiety and the bicyclo[3.1.0]hexane moiety were connected through a spirocarbon C-16, constructing an unprecedented carbon skeleton for 1.Additionally, the allyl group (fragment a) and one hydroxyl group were connected to a quaternary C-2 carbon (δC75.0) by means of HMBC correlations from H2-7 and 2–hydroxyl protons (δH3.97) to C-1, C-2, and C-3.The isopropyl group (fragment f) was connected with C-20 based upon HMBC correlations from H-22 to C-18, C-19, and C-20.
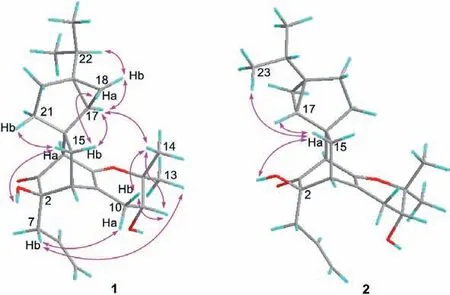
Fig.3.Selected NOEs/ROESY correlations of compounds 1 and 2.
The remaining 5 carbon resonances, including two geminal methyl carbons, indicated the presence of a prenyl unit (C10–C14),as evidenced by HMBC correlations from H3-13 and H3-14 to C-11(δC78.6) and C-12 (δC70.8) and the above-mentioned fragment b.The remaining one degree of unsaturation and the HMBC correlations from H2-10 to C-3, C-4, and C-5 (an oxygenated sp2carbon)indicated that the prenyl unit in a dihydropyran ring system was fused to the allylated 3-cyclohexenone moiety (a C6–C3unit, C1–C9) at C-4 and C-5.Another hydroxyl group was attached at C-11 by the HMBC correlations from 11–hydroxyl protons (δH4.12) to C-10, C-11, and C-12.
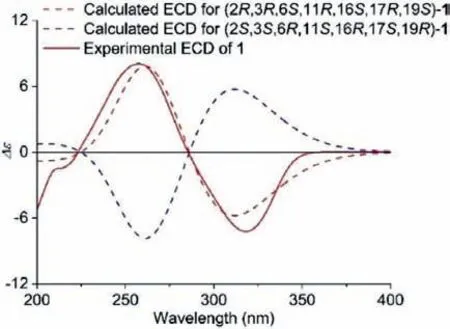
Fig.4.Experimental ECD spectrum of 1 (red line) and the calculated spectra of 1(red dashed line and blue dashed line).
The relative configurations of the seven chiral centers, including one spirocarbon present in the structure of 1, were assigned by employing nuclear Overhauser effect (NOE) and ROESY experiments (Fig.3).NOEs were observed for Ha-10 and H3-13 after irradiation of Hb-7 (δH2.27), indicating that the allyl group, Ha-10, and Me-13 were on the same face of the dihydropyranocyclohexaenone (DHPC) pseudoplane, as depicted in Fig.2.When Hb-10 was irradiated, strong NOEs for H-11, weak NOEs for 11–hydroxyl protons, and obvious NOEs for Me-14 were observed (Fig.S10 in Supporting information).Similarly, strong NOEs for H-11,weak NOEs for 11–hydroxyl protons, and obvious NOEs for Hb-10 were observed after irradiation with Me-14 (Fig.S10).These data and the ROESY cross peaks of Hb-10/H3-14 suggested that Hb-10 and Me-14 were on the same face of DHPC and theα-orientation for 11–hydroxyl.On the other hand, the stereochemistry of the spirocarbon in the molecule was also unambiguously assigned by NOE experiments.Diagnostic NOEs for 2–hydroxyl protons, H-3,and Hb-21 were observed after irradiation with H-15a (δH1.98).Moreover, irradiation with H-17 enhanced the signals for Me-14,Hb-15, and Hb-18.These results indicated that the three-member ring in the bicyclo[3.1.0]hexane moiety and Hb-15 were on the same side of the vertical surface formed by C3-C15-C16-C6 and that the three-member ring and CH2-15 were on the same side of the five-membered ring (C16-C17-C19-C20-C21).Furthermore, the observed NOEs for Hb-15, Hb-20, and Hb-21 after irradiation of H-18a and NOEs for H-22 and H-17 after irradiation of H-18b demonstrated that the isopropyl group and H-17 were on another side of the five-membered ring, opposite the three-membered ring and CH2-15, as depicted in Fig.3.
As in bicyclo[2,2,2]oct–5-en-2-one, the orientation of theβ,γunsaturated carbonyl moiety determines the sign of the Cotton effect.The CD spectrum of 1 shows a negative Cotton effect at 318 nm for the n→π∗transition of theβ,γ-unsaturated carbonyl moiety.Based on the extended octant rule [31,32] and the aforementioned relative configuration, the absolute stereochemistries of all the stereogenic centers in 1 were determined to be 2R, 3R, 6S,11R, 16S, 17R, and 19S, which were also supported by the calculated CD spectrum (Fig.4).It was noted that 1 and its spirocarbon epimer 2 could not be separated by reversed-phase C18 column chromatography, but their separation was achieved on an ADH chiral column usingn-hexane-isopropanol as the mobile solvent.Fortunately, single crystals of 1 were obtained in acetone-H2O containing residual isopropanol.A single-crystal X-ray diffraction experiment of 1 was performed by using Cu Kαradiation, and the X-ray crystallography data indicated that one molecule of 1 and one isopropanol molecule coexisted in a crystal lattice in the ratio of 1:1, as shown in the stacking diagram (Fig.5).The refinement of Flack’s parameter [0.01(19)] further corroborated the absolute configuration of 1 [33,34].
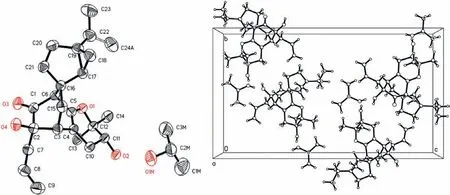
Fig.5.ORTEP drawing (left) and stacking diagram (right) of 1 and isopropanol.
Spirooliganone D (2), obtained as a colorless oil, had a molecular formula of C24H34O4as deduced from HRESIMS and NMR data(Table 1), which was the same as 1.The1H and13C NMR data were very similar between 1 and 2.The correlations observed in the COSY and HMBC spectra were also nearly identical between 1 and 2, suggesting that the same carbon-carbon skeleton is present in 1 and 2.The most substantive differences between these compounds are the NMR data for C-15 and the bicyclo[3.1.0]hexane moiety, indicating that the bicyclo[3.1.0]hexane moiety located at the spirocarbon (C-16) in 2 had the opposite orientation, as depicted in Fig.3.In NOE experiments, when H-15a was irradiated, diagnostic NOEs for 2–hydroxyl protons, H-17, and H3-23 were observed.These results suggested that the three-membered ring in the bicyclo[3.1.0]hexane moiety and Ha-15 were on the same side of the vertical surface formed by C3-C15-C16-C16 in 2 and that isopropyl,CH2-15 and H-17 were on the same side of the five-membered ring(C16-C17-C19-C20-C21), as shown in Fig.2.The relative configurations of the remaining chair carbons were determined by a series of NOE and ROESY experiments, which resulted in the same configuration as that of the corresponding chair carbons in 1.Moreover,the CD spectrum of 2 had a high degree of similarity to that of 1 (Fig.S21 in Supporting information).Thus, the absolute configuration of 2 was determined to be 2R, 3R, 6S, 11R, 16R, 17R, and 19S.
Biogenetically spirooliganones C-D (1 and 2), a pair of spirocarbon epimers, are the first hybrid examples of monoterpene(−)-sabinene and cyclic prenylated tetrahydropyrano-type C6–C3compounds, compared with the previously reported hetero Diels-Alder adducts spirooliganones A and B formed by (−)-sabinene and spirocyclic prenylated tetrahydrofurano-type C6–C3derivatives.Compounds 1 and 2 share a novelspiro[bicyclo[2.2.2]octane-2,2′-bicyclo[3.1.0]hexane] 6/6/5/3 tetracyclic ring carbon skeleton.A plausible biosynthetic pathway for 1 and 2 is illustrated in Scheme 1.4-Allylresorcinol derivatized from phenylalanine [30] can be proposed as the precursor of cyclic prenylated tetrahydropyranotype C6–C3compound derivatives (vi).Initially, the presumed precursor 4-allylresorcinol undergoes prenylation, 1,3-σmigration[35] of prenyl, and oxidation steps, followed by key epoxyprenyl side chain cyclization and dehydration to afford intermediate iv.Subsequently, iv would be converted to active diene viviaaromatization-dearomatization [36], followed by a selective Diels-Alder reaction with (−)-sabinene from the direction of small steric hindrance to form a pair of [4+2] cycloaddition adducts 1 and 2.
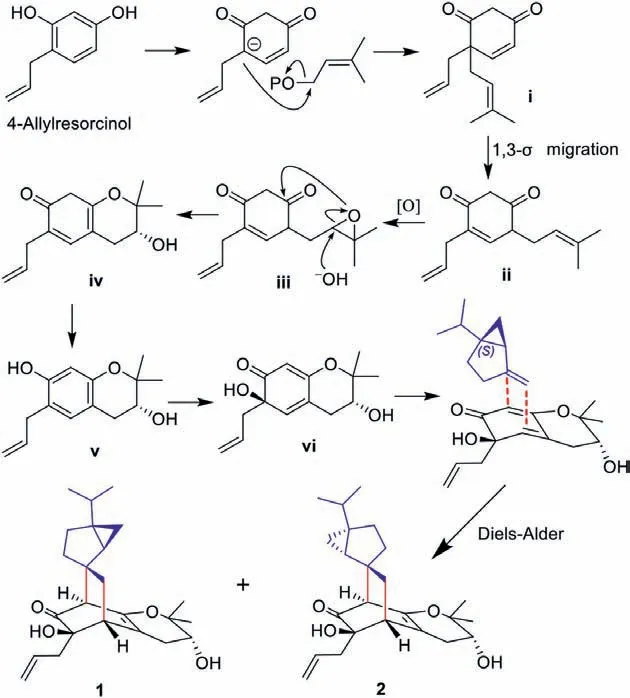
Scheme 1.Plausible biogenetic pathway of 1 and 2.
(±)-2-Allyl-2-(3-methylbut-2-enyl)-4,5-methylenedioxy-cyclohexa-3,5-dienone (4) was isolated as a light yellow oil from the roots ofI.oligandrumin a previous investigation [30], which also occurred in plants in theIlliciumgenus, includingI.verum[37],I.burmanicum[38],I.henryi[39], andI.simonsii[40].This compound was first obtained from 2-allyl-4,5-methylenedioxyphol by chemical reaction in 1983 [41].However, only the planar structure of 4 was documented, and the absolute configuration of the chair center in 4 has not yet been resolved.The baseline ECD curve and the low specific rotation showed that 4 was a racemate.Compounds(+)-4 and (−)-4 were separated on a chiral-phase column (Fig.S22 in Supporting information), and the theoretical calculation of ECD spectra of (2S)-4 and (2R)-4 was performed in methanol using the time-dependent density functional theory (TD-DFT) method at the B3LYP/6-311++G(d,p) level [42] and compared with the experimental spectra to determine the absolute configuration in 4.As shown in Fig.6, the calculated ECD curve of 2S matched the experimental ECD curve of (+)-4, and 2Rmatched the (−)-4 ECD curve with good agreement.Thus, the absolute configurations of(+)-4 and (−)-4 were determined to be 2Sand 2R, respectively.Moreover, the (2S) absolute configuration of illioliganone C [43], a dihydroxylated derivative previously isolated from the stem bark ofI.oligandrum, was revised to 2Ron the basis of a comparison between the experimental ECD spectra of (−)-4 and illioliganone C.
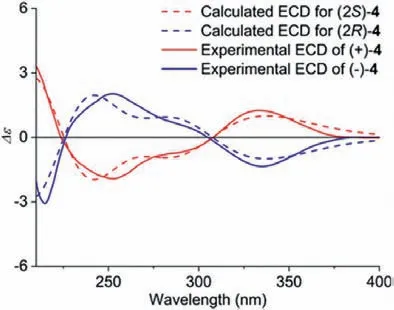
Fig.6.Experimental ECD spectra of (+)-4 and (−)-4 and the calculated spectra of(S)-4 and (R)-4.
(±)-Illioliganone J (5) was isolated as a colorless oil with=0 (c0.05, MeOH).The IR spectrum provided evidence for the carbonyl (1719 and 1632 cm−1) and phenyl (1413 cm−1)groups.From the ESIHRMS spectrum, the identification of a sodium adduct ion atm/z389.1382 [M+Na]+allowed for us to infer a molecular formula of C22H22O5(calcd.for C22H22O5Na, 389.1365),along with a degree of unsaturation of 12.The1H and13C NMR data (Table 2) of 5 closely resembled those of illioliganone D [30],which indicated that they shared the same carbon skeleton of a noncyclic prenylated C6–C3compound.Nevertheless, a detailed comparison of their 1D NMR data revealed that the main differences were the presence of carbon resonances for one methyl group atδC21.7 and one oxygenated methylene group atδC63.9 in illioliganone D, which were replaced byδC14.3 and 70.2 in 5,respectively.These data indicated that 5 is a geometric isomer of illioliganone D with an E configuration isopentenyl unit, which was confirmed by the NOE correlations betweenδH5.35 (H-11) andδH4.60/4.64 (H2-13).The baseline ECD curve and the optical rotatory data of=0 suggested that 5 is a racemate, which was confirmed by chiral analysis (Fig.S33 in Supporting information).Subsequently, the chiral HPLC separation of 5 afforded (+)-5 and (−)-5.The absolute configurations of (+)-5 and (−)-5 were assignedas 2Sand 2R, respectively, by a comparison of their experimental spectra with those of 4.
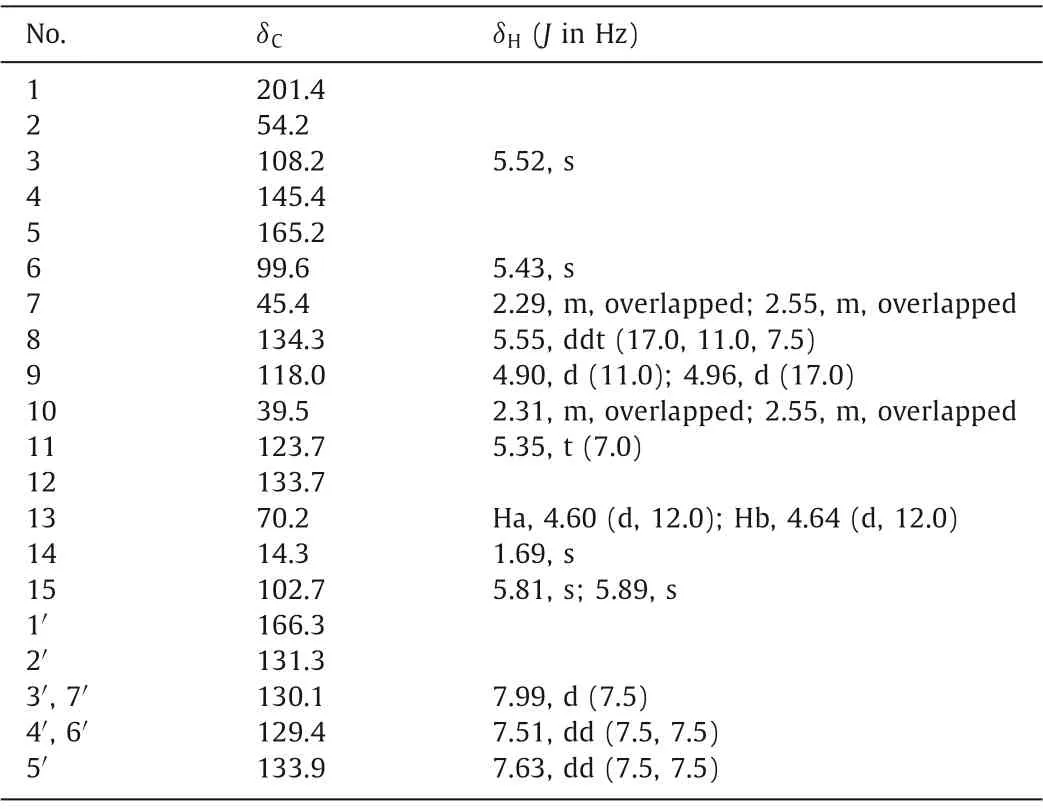
Table 2 1H (500 MHz) and 13C (125 MHz) NMR spectroscopic data for compound 5 in acetone-d6.
Structurally diverse prenylated C6–C3compounds have been reported to have inhibitory effects on RNA viruses.Therefore, compounds 1–3 and the isolated racemic mixtures 4 and 5 as well as their enantiomers [(+)-4, (−)-4, (+)-5, and (−)-5)] were tested for antiviral activity against CVB3, influenza A (H1N1), and influenza A (H3N2) [44].As shown in Tables S1 and S2, compounds 1 and(+)-5 exhibited significant inhibitory activity against CVB3 with IC50values of 11.11 μmol/L and 1.11 μmol/L and SI values of 2.1 and 4.3, respectively, compared with the positive control ribavirin(IC50=1173.56 μmol/L and SI=7.0) but less effective than the capsid inhibitor pleconaril.On the other hand, despite their structural similarity, compounds 2 and (−)-5 were much less active, and their IC50values were>33.33 μmol/L and>11.11 μmol/L, respectively,indicating that stereo configurations of 17Rin 1 and 2Sin (+)-5 might be favorable for anti-CVB3 activity.Additionally, a pair of epimers 1 and 2 showed moderate inhibitory activity against influenza A (H1N1) with IC50values of 11.11 μmol/L and 11.11 μmol/L,respectively.
In conclusion, one pair of unusual spirocarbon epimers(spirooliganones C-D, 1 and 2), one dimeric prenylated C6–C3compound (3), and two pairs of racemates [(+)-4/(−)-4 and (+)-5/(−)-5)] were isolated from the roots ofI.oligandrum.Spirooliganones C-D represent a novel family of cyclic prenylated tetrahydropyranotype C6–C3derivatives possessing a unique 6/6/5/3 tetracyclic ring carbon skeleton.A possible biosynthetic pathway involving the Diels-Alder reaction of (−)-sabinene and cyclic prenylated tetrahydropyran-type C6–C3compounds was proposed for compounds 1 and 2, which will inspire further biomimetic and total synthesis interests.The antiviral activity screening of all compounds showed that spirooliganone C and (+)-5 exhibited potent antiviral activities against CVB3.To a certain extent, the anti-CVB3 activities of a pair of spirocarbon epimers (1 and 2) and a pair of enantiomers [(+)-5 and (−)-5)] were related to their stereo configurations of C-17 and C-2, respectively.
Declaration of competing interest
The authors report no declarations of interest.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Nos.21732008 and 22177135).We are grateful to the Department of Instrumental Analysis of our institute for the acquisition of the IR and NMR spectra and the X-ray crystallographic measurements and analyses.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.02.050.
 Chinese Chemical Letters2022年9期
Chinese Chemical Letters2022年9期
- Chinese Chemical Letters的其它文章
- A review on recent advances in hydrogen peroxide electrochemical sensors for applications in cell detection
- Rational design of nanocarriers for mitochondria-targeted drug delivery
- Emerging landscapes of nanosystems based on pre-metastatic microenvironment for cancer theranostics
- Radiotherapy assisted with biomaterials to trigger antitumor immunity
- Development of environment-insensitive and highly emissive BODIPYs via installation of N,N’-dialkylsubstituted amide at meso position
- Programmed polymersomes with spatio-temporal delivery of antigen and dual-adjuvants for efficient dendritic cells-based cancer immunotherapy
