Highly selective NIR fluorescent probe for acetylcholinesterase and its application in pesticide residues detection
Shengui He, Shufang Zhang, Xin Zhao, Xinyue Zhu, Lisen Chen, Jingnan Cui
State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116024, China
ABSTRACT A series of near-infrared (NIR) fluorescent substrates (NDRO-1∼8) derived from fluorophore NDRH with different volumes of ester bond as the recognition group were designed and synthesized for the detection of acetylcholinesterase (AChE), among which NDRO-1 with the smallest acetate group displayed the highest activity toward AChE.The detection limit of NDRO-1 for sensing AChE was 0.32 μg/mL, and Km was 6.40 μmol/L, indicating ultra-sensitivity and good affinity of NDRO-1 toward AChE.NDRO-1 was used to detect the inhibitory of four kinds of pesticides including methamidophos, dichlorvos, and the detection limit was lower than 50 μg/L, which was further used in pesticide residues detection.
Keywords:NIR fluorescent probe Acetylcholinesterase Enzyme-activated Pesticide residue detection
Pesticides are widely used in agricultural production, while pesticide residues on food can pose a health threat to people at the same time [1–3], including nausea, vomiting, numbness in the extremities, high blood pressure, heart disease, pancreatitis, stroke,and even cancer [4–6].Currently, there are many reports on pesticide residue detection technology which are divided into traditional detection and rapid detection.Rapid detection is mainly based on the principle of enzyme inhibition, which is fast, low cost and can real-time monitor [7–10].Enzyme inhibition principle refers to the fact that organophosphorus or carbamate pesticides can inhibit the cholinesterase activity in animals, and pesticides can be quantified indirectly by detecting the cholinesterase inhibition rate toward pesticides.
Acetylcholinesterase (AChE) belong to the serine hydrolysis family [11,12], and mainly hydrolyzes the neurotransmitter acetylcholine specifically.AChE has a very important function in the organism, and its physiological function is affected by many toxic substances, such as organophosphorus compounds.Metabolic abnormalities are also associated with many diseases, such as Alzheimer’s disease (AD) and myasthenia gravis, diabetes, and liver damage [13–18].Many pesticides act by inhibiting acetylcholinesterase, so pesticide residues can be detected indirectly by detecting the inhibition of acetylcholine activity in samples.
The development of chemical tools that can detect the microscopic changes of substances has always been a goal pursued by researchers.Small molecule fluorescent probe is one of the powerful detection tools, which has significant advantages such as high sensitivity, excellent specificity, strong anti-interference ability, high resolution simple operation, and can realize highthroughput screening andin-situdetection [19–22].It converts microscopic changes in the environment into optical signals that can be easily observed, such as the pH value, water content, viscosity,ion concentration, enzyme activity and other properties in the system [23–33].Compared with the visible light emitting dyes, nearinfrared (NIR) fluorescent probes have relatively longer emission wavelengths (650–900 nm), excellent tissue penetration, low autofluorescence background, and reduced biological damage [34–37].NDRH is a NIR fluorophore, as a derivative of rhodamine and fluorescein, also possesses good photophysical properties of fluorescein and rhodamine, such as high fluorescence quantum efficiency, good photostability and good solubility [38–40].
From the perspective of fluorescent probe and based on the principle of enzyme inhibition, we designed and synthesized a series of Rodin fluorescent probes, for the detection of acetylcholinesterase (AChE), among which NDRO-1 with the smallest acetate group displayed the highest activity toward AChE.NDRO-1 was further used to detect the inhibitory of four commonly used pesticides, and the standard recovery rate of pesticides in several common vegetables.
The fluorophore NDRH and probes NDRO-1∼8 were synthesized followed the synthetic route in Scheme 1, and the details were described in Supporting information.NDRH displayed a near-infrared(NIR) fluorescence emission with maximum emission at 650 nm and the relationship between fluorescence intensity at 650 nm and the activity of NDRO-1∼8 toward AChE was shown in Fig.1.Among the fluorescent substrates NDRO-1∼8, NDRO-1 with acetyl
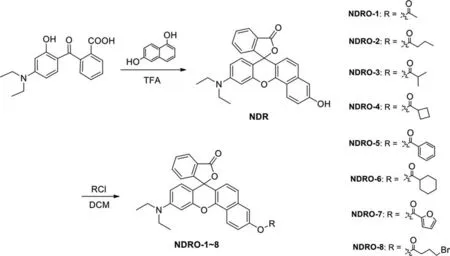
Scheme 1.Synthesis of fluorescent substrates NDRO-1∼8.
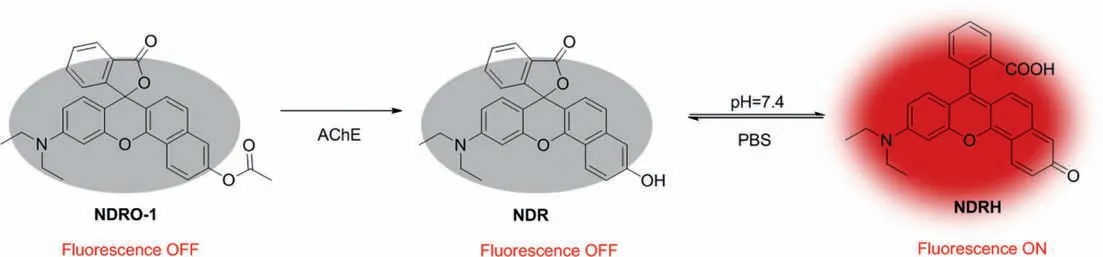
Scheme 2.Proposed reaction mechanism of NDRO-1 with AChE.
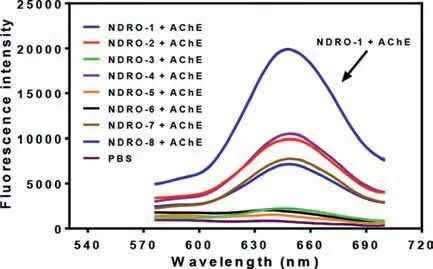
Fig.1.Fluorescence spectrum of NDRO-1∼8 toward AChE after incubation for 0.5 h.(λex=550 nm)
Available online 15 February 2022 group as the recognition site showed the highest reactivity toward AChE, NDRO-2 containing butyryl group, NDRO-4 containing cyclobutyryl group, NDRO-7 with bulky furyl methyl ester group and NDRO-8 with bromo substituted butyl ester group displayed moderate activity, NDRO-3 with isobutyl ester group, NDRO-6 with cyclohexyl methyl ester group and NDRO-5 with phenyl methyl ester group barely respond to AChE.From these results, it was speculated that the higher reactivity of NDRO-1, NDRO-2, and NDRO-3 was due to the fact that their acyl groups were alkyl chains with smaller spatial structure, which may be suited to the catalytic cavity of AChE, while NDRO-5 and NDRO-6 were basically not hydrolyzed by the enzyme due to their larger spatial structure.However, from the point of view of reactivity, the reactivity of compound NDRO-1 toward AChE is about twice as high as that of NDRO-2 and NDRO-4, so NDRO-1 was selected as a tool to detect AChE in subsequent experiments Scheme 2.shows the proposed reaction mechanism of NDRO-1 that AChE catalyzes hydrolysis of NDRO-1 and releases fluorophore NDRH.
AChE in the experiment was extracted from duck blood.The enzyme activity was determined to be 2.5 × 105U (μmol min−1mL−1) by Ellman-absorbance method, and the protein concentration of AChE proenzyme solution of duck blood was determined to be 4.6 mg/mL by Bradford protein concentration method (the test methods were shown in Supporting information).
From Fig.2a, it can be seen that the probe NORO-1 itself displayed no fluorescence, and after the addition of AChE a remarkable fluorescence signal was generated at 650 nm, indicating that the fluorophore NDRH was generated, which was further demonstrated by HPLC in Fig.2b, a new chromatographic peak was also detected and identified as NDRH.
Generally, solvents have a great influence on the fluorescence performance and enzyme activity, which will affect the application range of the detection reaction.As shown in the Fig.3, it could be seen that DMSO has a small impact on the reaction, and the residual activity of enzyme in 2% DMSO was about 90%, so the percentage content should be best controlled within 2% (v/v).The effect of methanol on the reaction system was similar to that of DMSO, and the residual activity of enzyme in 2% methanol was about 90%.Acetone had the greatest effect on the reaction system, the residual activity of enzyme in 10% acetone was lower than 20%.
Then, the effect of incubation time to the reaction was investigated.As can be seen in Fig.S4 (Supporting information), the fluorescence intensity changes showed that the enzymatic reaction was almost balanced at 60 min.The fluorescence intensity showed a good linear relationship within 0–35 min.Therefore, the subsequent quantitative and kinetic experiments should be terminated within a linear range, and the reaction should be terminated within 35 min.
Next, the influence of different metal ions and the amino acids to the enzyme activity was investigated.As shown in Fig.4, it could be found that these amino acids and ions showed little interference to the fluorescence intensity of the reaction, while glutamic acid (Glu), cysteine (Cys) and metal ion Cu2+or Zn2+etc.had a slight effect on reaction.In summary, the ions and amino acids have little effect to the reaction between NDRO-1 and AChE,thus the probe could be applied to complex systems.
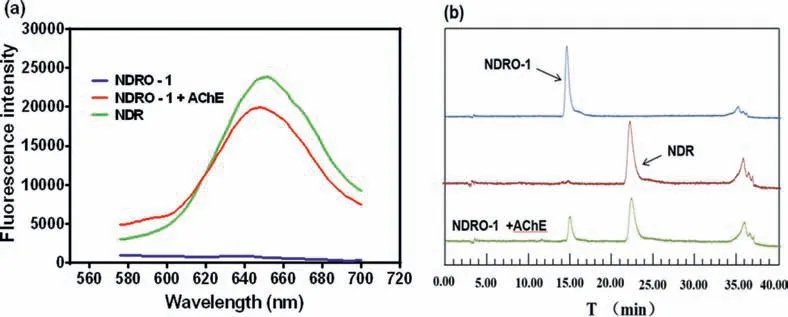
Fig.2.(a) Fluorescence spectrum before or after the addition of AChE (λex=550 nm).(b) High performance liquid chromatogram of NDRO-1, NDR and the reaction mixture(detection wavelength 550 nm).
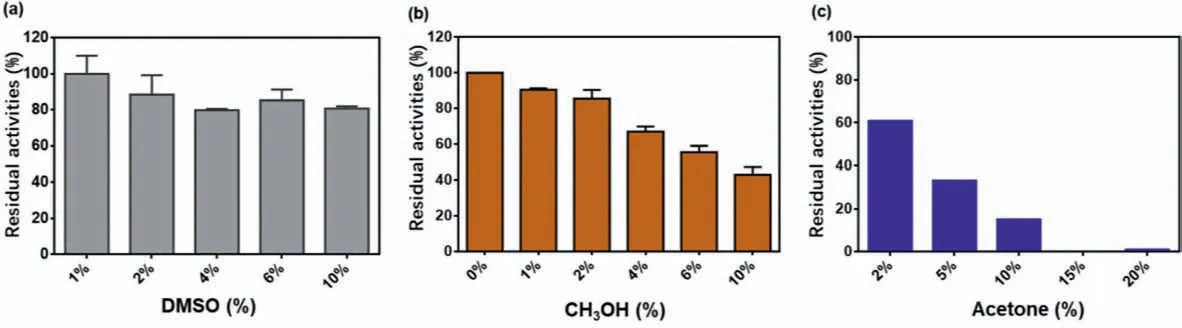
Fig.3.Effect of three solvents (DMSO, CH3OH, acetone) on the reaction system respectively.
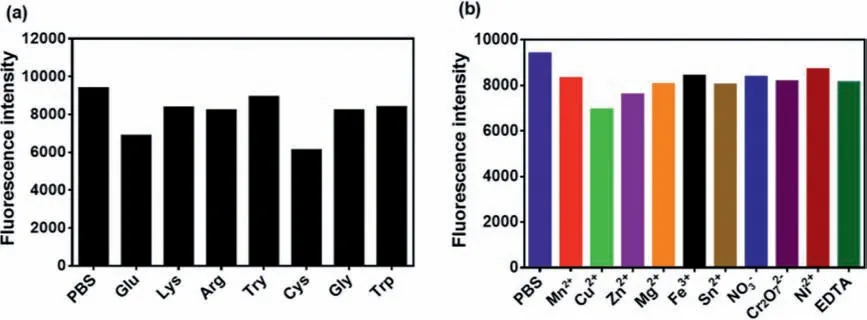
Fig.4.(a) Effect of amino acids on the reaction (PBS, Glu, glutamic acid; Lys, lysine; Arg, arginine; Try, tyrosine; Cys, cysteine; Gly, glycine; Trp, tryptophan).(b) Effect of common ions (Mn2+, Cu2+, Zn2+, Mg2+, Fe3+, NO3−, Cr2O72−, Ni2+) and EDTA on the reaction.
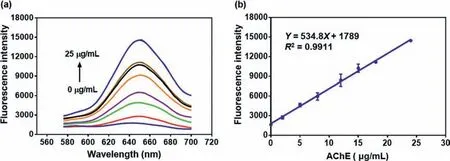
Fig.5.(a) Fluorescence intensity of NDRO-1 (10 μmol) toward AChE at different concentrations (0–25 μg/mL).(b) Linear relationship between the fluorescence intensity at 650 nm and AChE concentrations (λex=550 nm).
From the experimental results in Fig.5, it could be seen that the fluorescence intensity at 650 nm had a good linear relationship(R2=0.9911) with the AChE protein concentration.The detection limit (3σ/k) was 0.32 μg/mL, indicating that the probe could be used to detect AChE activity quantitatively and sensitively.
According to the experimental results in Fig.6a, the fluorescence intensity of the product NDRH at the wavelength of 650 nm has a strict linear relationship (R2=0.9936) with its concentration,indicating that the content of the product NDRH could be characterized by fluorescence intensity.After explored the experimental conditions of the reaction between the probe and the enzyme, the kinetic behavior of AChE hydrolysis probe NDRO-1 was further investigated.As shown in Figs.6b and c, the probe showed obvious Michaelis-Menten kinetic characteristics, and theKm=6.40 μmol/L,which indicated that the probe had a good affinity with the enzyme.
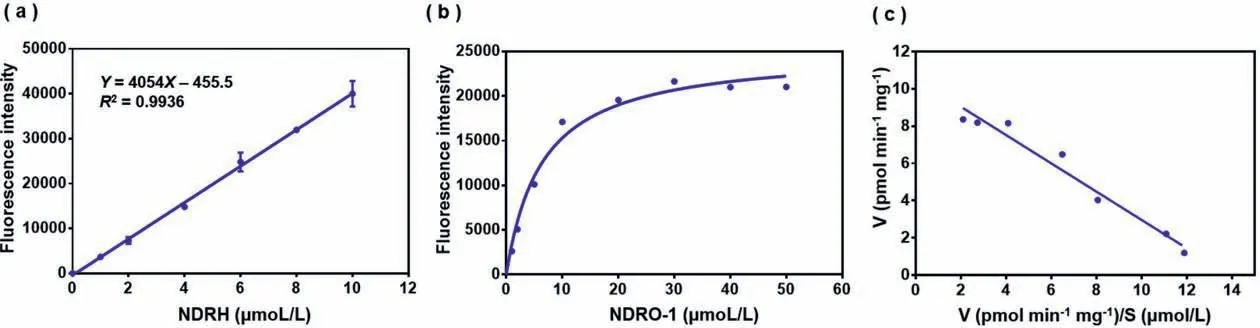
Fig.6.(a) The linear curve of the fluorescence intensity with the increasing NDRH concentration (The final concentration is 0–10 μmol/L.).(b) Michaelis-Menten kinetics curves.(c) Eadie-Hofstee curve.
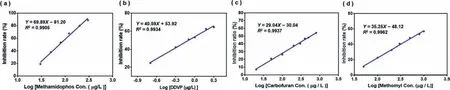
Fig.7.(a) Graph of linear relationship between methamidophos concentration logarithm and enzyme inhibition rate.(b) Linear plot of log concentration of dichlorvos and enzyme inhibition rate.(c) The linear relationship between carbofuran concentration logarithm and enzyme inhibition rate.(d) Linear relationship between logarithm of loganide concentration and enzyme inhibition rate.
Probe NDRO-1 was further used to detect the inhibition rate of 4 kinds of pesticides (methamidophos, dichlorvos, furudan, and meidovir) and pesticide residues in some vegetables.According to the experimental results in Fig.S5 (Supporting information), the pre-incubation time of dichlorvos, methamidophos with the enzyme were fixed at 15 min, and the pre-incubation time of carbofuran, methomyl with the enzyme were fixed at 30 min.Then,according to the principle of enzyme inhibition, different gradient concentrations of pesticide standard solutions were set up, preincubated with the enzyme for 15 min, and then added the probe NDRO-1 to detect the activity of the remaining enzyme.The inhibition rate was calculated by the fluorescence intensity.Plot the linear relationship between the inhibition rate and the logarithm of the pesticide concentration.
From Fig.7a, it could be seen that the enzyme inhibition rate showed a good linear relationship (R2=0.9905) with the logarithm of the methamidophos concentration from 30 μg/L to 300 μg/L, and the minimum detection limit could reach 30 μg/L, which was about 65 times more sensitive than the detection limit (2 mg/L) of the state-regulated enzyme inhibition method [41].The linear correlation of the standard curve was good, which indicated that the system could be used to quantify methamidophos concentration in this range.As shown in Fig.7b, there was a good linear relationship (R2=0.9934) between the logarithm of concentration and the enzyme inhibition rate if the dichlorvos concentration within the range of 0.2–2 μg/L, and the minimum detection limit was up to 0.2 μg/L, which was about 500 times more sensitive than the detection limit (100 μg/L) stipulated by the national enzyme inhibition method.From Fig.7c, it can be seen that the enzyme inhibition rate shows a good linear relationship (R2=0.9937) with the logarithm of the furudan concentration within the range of 20–800 μg/L, and the LOD value is 20 μg/L which is lower than the state-stipulated detection limit of enzyme inhibition method(50 μg/L).From Fig.7d, it can be seen that the enzyme inhibition rate shows a good linear relationship (R2=0.9937) with the logarithm of the methomyl concentration in the range of 20–800 μg/L,and the lowest detection limit is 50 μg/L.
In the recovery experiment, methamidophos recoveries from green pepper in two groups were 104% and 102%, respectively (Table S1 in Supporting information), and the recovery rate of dichlorvos from cherry tomatoes were 102%, 114% and 101%, respectively(Table S2 in Supporting information).In general, in our final standard recovery experiment, from the results of methamidophos,dichlorvos, furudan and meidovir standard recovery in Tables S1–S4 (Supporting information), thePvalue (standard recovery rate)were within 80%–110%, which conforms to the national provisions on food testing.
In this paper, we successfully synthesized a series of NIR fluorescent probes and selected probe NDRO-1 which showed high selectivity and sensitivity toward AChE.The probe has the advantages of high sensitivity, low detection limit, rapid response, good affinity (Km=6.4 μmol/L) in the detection of AChE enzyme activity.Based on the inhibition principle of AChE, four pesticides were selected, and the linear relationship between pesticide concentration and enzyme inhibition rate were established.The sensitive detection of pesticide at μg/L grade was realized, which provided a powerful detection method for the detection of trace pesticide residues.Several vegetables suitable for surface pesticide extraction were selected to conduct the surface pesticide recovery experiment, which indicating that the system is suitable for detecting pesticide residues on the surface of vegetables.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgment
This work was supported financially by National Key R&D Program of China (No.2018YFC1603001).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.02.020.
 Chinese Chemical Letters2022年9期
Chinese Chemical Letters2022年9期
- Chinese Chemical Letters的其它文章
- A review on recent advances in hydrogen peroxide electrochemical sensors for applications in cell detection
- Rational design of nanocarriers for mitochondria-targeted drug delivery
- Emerging landscapes of nanosystems based on pre-metastatic microenvironment for cancer theranostics
- Radiotherapy assisted with biomaterials to trigger antitumor immunity
- Development of environment-insensitive and highly emissive BODIPYs via installation of N,N’-dialkylsubstituted amide at meso position
- Programmed polymersomes with spatio-temporal delivery of antigen and dual-adjuvants for efficient dendritic cells-based cancer immunotherapy
