Real-time quantification for sulfite using a turn-on NIR fluorescent probe equipped with a portable fluorescence detector
Fei Yn, Jingnn Cui, Cho Wng, Xingge Tin, Dwei Li, Yn Wng,Bojing Zhng, Lei Feng, Shnshn Hung, Xiohi M
a College of Pharmacy, Dalian Key Laboratory of Metabolic Target Characterization and Traditional Chinese Medicine Intervention, Academy of Integrative Medicine, Dalian Medical University, Dalian 116044, China
b Second Affiliated Hospital, Dalian Medical University, Dalian 116044, China
c State Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian 116024, China
d School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang 453007, China
ABSTRACT Sulfur dioxide and its derivative sulfite widely existed in air, water as the environment pollutant.Sulfite is also commonly used as preservative and additive in fresh fruits, vegetables, wines and pharmaceutical materials.Due to sulfite is closely related with human diseases, it is very urgent for the sensitive and rapid quantification of sulfite in various samples.In our study, a turn-on near infrared (NIR) fluorescent probe (MDQ) was developed for sulfite detection based on a Michael addition reaction, with high sensitivity (LOD 4.16 nmol/L), selectivity and fast response time (400 s).Using MDQ, a quantify method for sulfite in traditional Chinese medicines (TCMs) was developed with the advantages of high precision, accuracy and convenient operation.Furthermore, according to the photophysical property of MDQ, a portable fluorescence detector is designed to quantify sulfite for TCMs and surface water in Dalian city of China.Therefore, the developed fluorescent probe MDQ and portable fluorescent detector as a rapid inspection instrument were successfully used to real-time monitor the sulfite in various complex samples.
Keywords:Sulfite Fluorescent probe Portable fluorescence detector Traditional Chinese medicine Surface water
Sulfur dioxide (SO2) and its derivatives (e.g., sulfite) are widely distributed in environment and biology, which are probable to cause serious diseases, such as difficulty in breathing, some respiratory illnesses, lung cancer, and wheezing [1,2].Therefore, the amounts of sulfite in foodstuffs, water, and pharmaceutical products have been rigorous controlled and limited in many countries.The Joint FAO/WHO Expert Committee on Food Additives has issued that an acceptable daily intake of sulfite should be lower than 0.7 mg/kg of body weight for human [3].The United States Food and Drug Administration (FDA), UK, and European Union require sulfite warning on the label of foods in concentrations of 10 mg/kg sulfite or more.In China, several Chinese herbal medicines have been restricted the SO2as no more than 400 mg/kg and the SO2level inDioscoreae Rhizomaslices should be no more than 10 mg/kg.
Thus, the quantification of SO2and its derivatives in various samples is performed in the safety control for food, pharmaceutical products, and water.On the basis of electrochemistry, spectrophotometry, chromatography,etc., several methods have been extensively used for the determination of sulfites [4–6].However,these conventional methods for sulfite determination usually require troublesome sample pretreatment, sophisticated instrumentations, and longtime analysis.As now, benefited from the advantages of high sensitivity and selectivity, the activatable fluorescent probes have been widely used to sense target molecules [7–12].Thus, several colorimetric and fluorescent probes have been developed for sulfites detection on the basis of the coordination to metal ions [13], selective deprotection of levulinate group [14,15], and Michael-type additions [16–18].In contrast to the existing ratiometric or turn-off fluorescent probes [19–21], a near-infrared (NIR)fluorescent probe with large Stokes shift is designed for sensing sulfite in our present study.
In this study, dicyanoisophorone was used for the designation of the fluorescent probe, as a chromophore with the hopeful near infrared fluorescence emission (λem>650 nm), which is beneficial to reduce background fluorescence [22,23].On the other hand, 1-methylquinolinium cation was used as the active moiety for the assumed Michael addition with sulfite.Furthermore,compared with some lipid soluble fluorescent probes for detecting SO2[14,24], the quaternary ammonium salt increases the water solubility of designed probe, improving the application for sulfite detection.Finally, the conjugate of dicyanoisophorone and 1-methylquinolinium cation was synthesized (MDQ), which was assumed the Michael addition with sulfite as well as the turn-on NIR fluorescence signal based on the intramolecular charge transfer (ICT) process (Fig.1A).
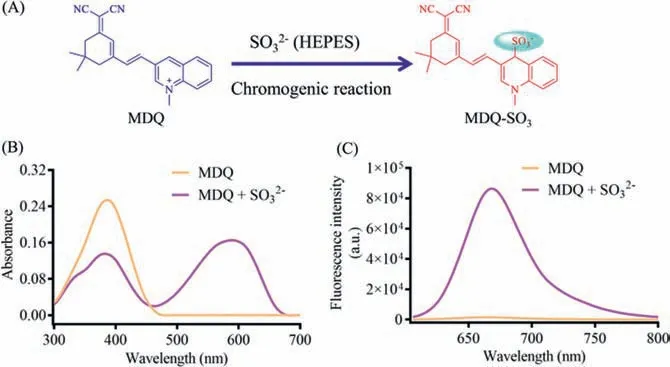
Fig.1.(A) Illustration for the reaction between MDQ and sulfite.(B) The absorption spectra of MDQ in presence/absence of SO32−.(C) The fluorescence spectra of MDQ in presence/absence of SO32−.[SO32−]=6 μmol/L, λex=580 nm.
As shown in Fig.1B, the addition reaction between MDQ and sulfite afforded a new absorbance peak (λmax=590 nm) in comparison with the original absorption of MDQ (λmax=386 nm), which was induced by the supposed product MDQ-SO3from the Michael addition reaction between MDQ and sulfite, and accompanied by a strong NIR fluorescence signal (λem=670 nm).In contrast, no fluorescence signal was observed for MDQ excited by laser at 580 nm(Fig.1C).So, MDQ as a substrate can be activated by sulfite along with distinct turn-on fluorescence signal, showing the potential application for sulfite detection.
As a designed fluorescent probe, the fluorescence behavior of MDQ and responses of MDQ toward sulfite was evaluated in the presence of various interference factors.For the pH values of HEPES buffer, significant fluorescence signal was observed for MDQ in alkalinous HEPES buffer (pH>8).Meantime, the addition reaction of MDQ and sulfite displayed strong fluorescence signal in HEPES buffer with pH values more than 7 (Fig.S7 in Supporting information).Above all, HEPES buffer was optimized as pH 8 for the detection of sulfite using MDQ.In contrast to the pH values, the detection of sulfite by MDQ is insensitive to temperature(Fig.S8 in Supporting information), which indicated its broad application for SO32−detection (20–40°C).Additionally, the specificity of MDQ toward sulfite was also investigated in the presence of various species, such as amino acids, metal ions, and common anions.As shown in Fig.S9 (Supporting information), the similar fluorescence intensities suggested a good selective detection of sulfite by MDQ, which means that the presence of amino acids,ions display little interference on the fluorescence signals.Finally,the time-dependent stability and fluorescence response of MDQ toward sulfite has been studied (Fig.S10 in Supporting information).First, MDQ showed a good stability with no fluorescence signal in HEPES buffer in 1000 s.Secondly, the fluorescence intensity (λem=670 nm) of the addition reaction between MDQ and sulfite promptly increased and reached a plateau at about 400 s, indicating a fast response of MDQ toward sulfite.As the incubation time ranged to 1000 s, the fluorescence intensity exhibited negligible changes, suggesting the stability of addition product, along with the maneuverability of the sulfite detection.
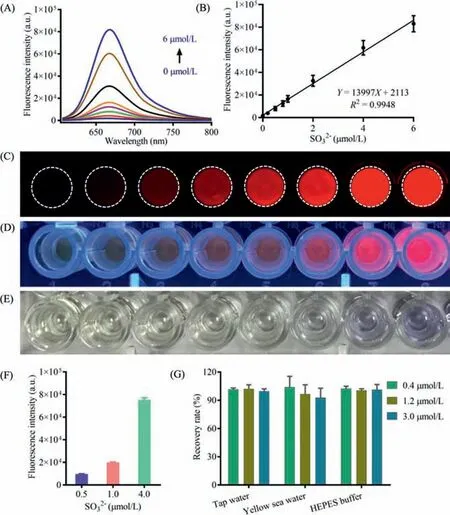
Fig.2.(A) Fluorescence spectra of MDQ toward SO32−at different concentrations(0–6 μmol/L). λex=580 nm, λem=670 nm.(B) Linear relationship between fluorescence intensities and SO32−concentrations. n=3.Imaging of MDQ toward SO32−under fluorescence imager (C, λex=532 nm, λem=670±15 nm), UV (D, 365 nm),and sun light (E).The quantification repeatability of SO32−by MDQ.(F) Precision(n=6) and (G) accuracy evaluation (n=3) for the quantification of SO32−by MDQ in different solvent system.All data points are mean ± SD.
Using MDQ as a fluorescent molecular tool, the quantify method for environmental sulfite was developed in this study.In HEPES buffer, the fluorescence responses of MDQ (10 μmol/L)toward sulfite with concentrations ranged from 0 μmol/L to 6 μmol/L were investigated, giving successive fluorescence spectra corresponding to the sulfite amount (Fig.2A).An excellent linear relationship was obtained between the fluorescence intensity(λem=670 nm) and sulfite amount (Fig.2B), together with the highly sensitive LOD (4.16 nmol/L, 3σ/k), suggesting the quantify concentrations for sulfite determination in tested samples.Furthermore, the fluorescence behavior of MDQ toward the concentration changes of sulfite could be observed by imaging experiments.As can be shown in Fig.2C, imaging results exhibited gradually increasing fluorescence signal along with the increased sulfite amount.Under the portable ultraviolet lamp (365 nm), the wells with the existence of sulfite displayed distinct red fluorescence signals in comparison with the colorless image of MDQ without sulfite (Fig.2D).Even under the naked eye, a noticeable color change from yellow to purple was observed for these wells in the presence of different sulfite amounts (Fig.2E).The distinct color alteration of MDQ solutions induced by different sulfite levels displayed the potential application of MDQ for preliminary sulfite detection by naked eye.
For the quantify method of sulfite by MDQ, it was validated systematically based on various indicators.As shown in Fig.2F, the fluorescence intensities corresponding three sulfite amounts were measured, which indicated a good repetitiveness.The intra precision and inter day precision about the sulfite determination were evaluated by the fluorescence intensity measurement.As a result,the sulfite determination method by MDQ displayed an excellent precision about both intra-day (RSD<1%) and inter day (RSD<3%) (Table S1 in Supporting information).The accuracy about sulfite determination by MDQ was also investigated by recovery evaluation.In different tested medium (tap water, sea water and HEPES buffer), good recoveries (95%−105%) were achieved for these sulfite spiked samples (0.4, 1.2 and 3.0 μmol/L) (Fig.2G, Table S2 in Supporting information), which indicated that the concentration of sulfite in real samples could be accurately determined by MDQ.Therefore, using MDQ as the fluorescent probe, a sensitive and reliable quantify method was established for sulfite.
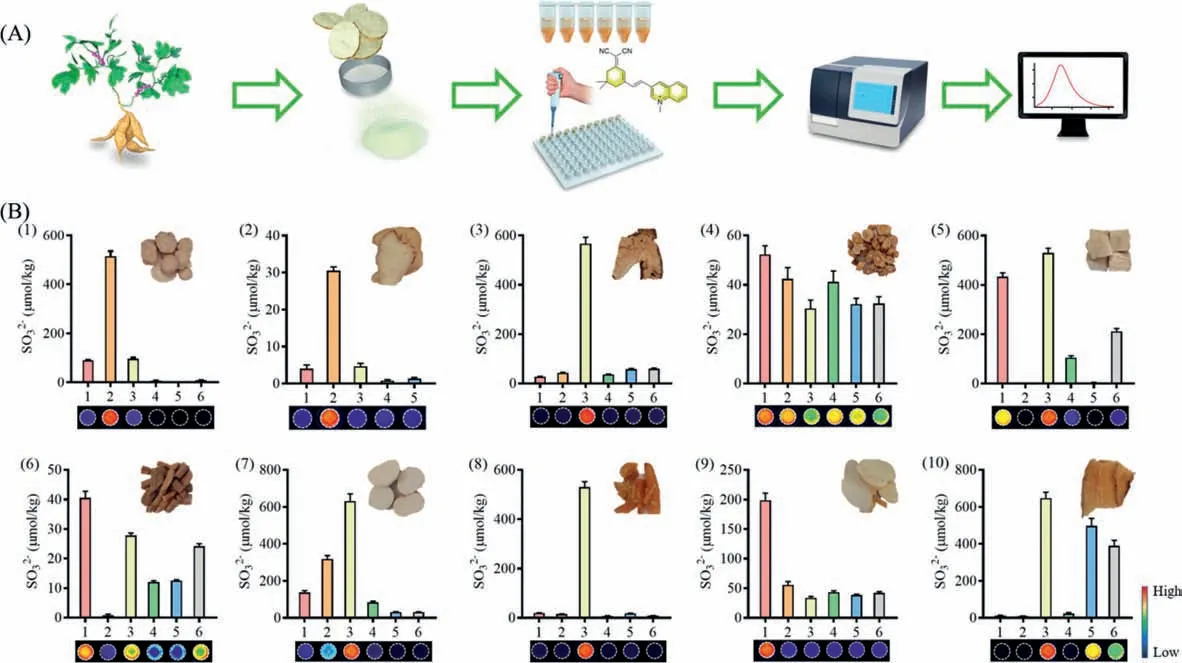
Fig.3.Sulfite determination in various TCMs using MDQ.(A) Illustration for the procedure.(B) Sulfite content of various TCMs: Paeoniae Radix alba (1), Bletillae Rhizoma(2), Atractylodis macrocephalae Rhizoma (3), Codonopsis Radix (4), Puerariae Thomsonii Radix (5), Achyranthis bidentatae Radix (6), Dioscoreae Rhizome (7), Asparagi Radix (8),Trichosanthis Radix (9), Gastrodiae Rhizoma (10). n=3 each group.All data points are mean ± SD.
For the safety control of traditional Chinese medicines (TCMs),sulfite should be determined accurately.In general, SO2in TCMs is determined by ion chromatography, gas chromatography and other analysis methods [25–27].Herein, ten TCMs (each 5–6 batches)were determined the sulfite levels using the present fluorescence method based on MDQ.A standard operation procedure was established for sulfite quantification of TCMs in combined with material preparation, Michael addition reaction, and fluorescence intensity measurement (Fig.3A).In general, the powdered TCMs were extracted with HEPES buffer, which were further incubated with MDQ for the determination of sulfite by the fluorescence intensity.Meantime, non-fluorescence signal was observed in these tested samples excited by laser at 532 nm, indicating little interference of endogenous molecules, such as peptides, secondary metabolites(e.g., flavones, saponins, and polyphenols).Analysis of the fluorescence intensity of tested samples and standard sulfite samples has been performed to determine the sulfite concentration.It can be shown that the sulfite levels of all tested samples were no more than 700 μmol/kg, most of which were lower than 100 μmol/kg(Fig.3B, Table S3 in Supporting information).In addition, the fluorescence images of tested samples were obtained, displaying different fluorescence intensity corresponding to the sulfite amounts in these TCMs.The fluorescence images reflected the sulfite amounts visually, which can be used to determine the sulfite levels in an intuitive way.Therefore, using MDQ as the sensitive probe, a validated method has been established for the quantification of sulfite in TCMs.
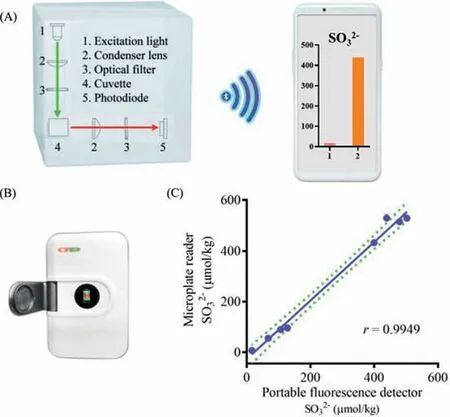
Fig.4.(A) Schematic diagram of the portable fluorescence detector for the sulfite determination.(B) Photograph of the portable fluorescence detector.(C) The correlation analysis of the sulfite determination in various TCMs by the microplate reader and the portable fluorescence detector.
According to the optical property of MDQ and the fluorescence behavior of the detection of SO32−by MDQ, a portable fluorescence detector was equipped for quantifying SO32−.As shown in the schematic diagram (Fig.4A), the portable detector was mainly equipped with a laser (λex=525 nm), a filter (λem>650 nm) and a photoelectric converter.When the tested solution was subjected into the tested cell, the fluorescence intensity could be measured under the excitation at 525 nm.Then, delivered by Bluetooth, the fluorescence signal was exhibited and stored in a mobile terminal.Thus, the present portable fluorescence detector can be carried and operated by one person for SO32−determination in real time and position.Using the portable detector (Fig.4B), SO32−was determined in eight TCMs, exhibiting excellent correlation with the quantification analysis by the commonly used microplate reader (Fig.4C), which suggested the application of the developed portable detector for accuracy quantification of SO32−.
Sulfite amount is an important factor for water quality, which mainly derived from the air pollution (SO2), domestic and industrial wastewater.Some methods have been developed (e.g., LCMS/MS method, spectrophotometric method, test strip method and spot test method) for sulfite determination in water, beverages,wine, and beer [6,16].In contrast to previous methods, we aimed to perform a rapid and portable determination of sulfite in water samples.Herein, ten surface water samples (river, lake and pool)collected from three districts in Dalian city were quantified sulfite using the present portable fluorescence detector together with the method based on MDQ.As a result, the sulfite level was no more than 30 μmol/L for all water samples (Fig.5).Especially, there were three water samples containing few sulfites (<5 μmol/L).Thus, the portable fluorescence detector together with a new fluorescence tool (MDQ) was developed for sulfite determination, with some advantages of convenience, maneuverability, and high sensitivity.It also displayed the potential application for field-test of sulfite amount in water sample.
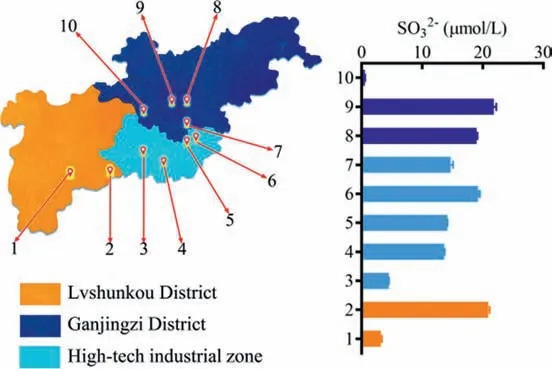
Fig.5.The SO32−content in surface water distributed in three districts of Dalian city (China) determined by the portable fluorescence detector. n=3.All data points are mean ± SD.
In summary, the conjugate of dicyanoisophorone and 1-methylquinolinium cation as a fluorescent probe (MDQ) was applied to develop a quantifying method for SO32−in real samples with high sensitivity and selectivity, fast response and low detection limit.Additionally, a portable fluorescence detector has been designed and equipped for SO32−detection based on the established method using MDQ.The present portable detector together with a sensitive and selective fluorescence tool (MDQ) displayed potential application for the rapid quality control of various samples, such as water, traditional Chinese medicines, foods, and wines.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported financially by Distinguished Professor Program of Liaoning Province (No.XLYC2002008), Dalian Science and Technology Leading Talents Project (Nos.2019RD15, 2020RJ09 and 2020RQ076), Liaoning Provincial Natural Science Foundation(No.20180550761), Liaoning Revitalization Talents Program (No.XLYC1907017) and the Open Research Fund of the School of Chemistry and Chemical Engineering, Henan Normal University (No.2021YB07).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2022.03.006.
 Chinese Chemical Letters2022年9期
Chinese Chemical Letters2022年9期
- Chinese Chemical Letters的其它文章
- A review on recent advances in hydrogen peroxide electrochemical sensors for applications in cell detection
- Rational design of nanocarriers for mitochondria-targeted drug delivery
- Emerging landscapes of nanosystems based on pre-metastatic microenvironment for cancer theranostics
- Radiotherapy assisted with biomaterials to trigger antitumor immunity
- Development of environment-insensitive and highly emissive BODIPYs via installation of N,N’-dialkylsubstituted amide at meso position
- Programmed polymersomes with spatio-temporal delivery of antigen and dual-adjuvants for efficient dendritic cells-based cancer immunotherapy
