Synthesis, structure and characterization of 2-pyrazinecarboxylic acid decorated CeIII-incorporated isopolyoxotungstate
NIU Jingyang, QIAN Shui, ZENG Baoxing, ZHAO Junwei
(College of Chemistry and Chemical Engineering, Henan University, Kaifeng 475004, Henan, China)
Abstract: A novel CeIII-incorporated isopolyoxotungstate [(CH3)2NH2]6KNa3[Ce2(H2O)6W22O74 (HPCA)2]●28H2O (1, HPCA = 2-pyrazinecarboxylic acid) was triumphantly prepared by step-by-step assembly strategy in aqueous medium and characterized by single-crystal X-ray diffraction, IR spectrum and thermogravimetric analysis. Structural analysis indicates that its polyoxoanion [Ce2(H2O)6W22O74(HPCA)2]10- is constituted by two [W11O38]10- segments and two metal-organic [Ce(H2O)3(HPCA)]3+ cations. In addition, each [Ce(H2O)3(HPCA)]3+ cation is not only grafted to the vacancy of two [W11O38]10- units through three oxygen atoms, but is combined with [W11O38]10- units to form a one-dimensional chain structure. Apparently, [Ce(H2O)3(HPCA)]3+ cations play an important bridging role in the structure construction of the one-dimensional chain structure.
Keywords: polyoxometalate; isopolyoxotungstate; rare-earth incorporated polyoxotungstate
Polyoxometalates (POMs), as a promising group of early transition metal-oxygen clusters, have extensively caught increasing attention benefiting from their characteristic architectures and capacious applications in adsorption[1-3], bioactivity[4-5], magnetism[6-8], electrochemistry[9-10]and materials science[11-12]. Within this domain, polyoxotungstates (POTs), as a spellbinding subset of POMs bearing striking structures and physicochemical properties, have been deeply studied in recent years[13]. Depending on the composition difference, POTs can be divided into two categories: heteropolyoxotungstates (HPOTs) and isopolyoxotungstates (IPOTs). Hitherto, an increasing number of HPOTs has been documented due to template-induced heteroatom effects, which can be rapidly obtained in high yields through a simple one-step acidification process[14]. Researches on IPOTs with the general expression [WxOy]n-are less than the plentiful HPOTs, which are potentially limited by the absence of inner heteroatoms in the IPOTs that contribute to the stabilization of structures. Even so, some examples to date, have been reported on IPOTs. The excellent examples are that Cronin’s group reported IPOTs [H4W22O74]12-and [H10W34O116]18-by adjusting pH in 2008[15]. Otherwise, rare-earth-containing IPOTs (RECIPOTs) have attracted more attention than ever before due to their potential applications in optics, catalysis, electricity and magnetism[16-17]. However, only a few examples of RECIPOTs have been addressed such as [Ce(H2O)(DMF)6(W10O32)][18], [H6Ce2(H2O)Cl(W5O18)3]7-[19], [RE4(WO4)(H2O)16{W7O22(O2)2}4]14-(RE = La3+, Pr3+)[20], [Ce2(H2O)6W22O72(OH)4]10-[21], [RE2(H2O)10W22O71(OH)2]8-(RE = La3+, Ce3+, Tb3+, Dy3+, Ho3+, Er3+, Tm3+, Yb3+, Lu3+, Y3+)[22]as well as [RE2(H2O)10W28O93(OH)2]14-(RE = Sm3+, Eu3+)[23]. Obviously, most of those previously reported RECIPOTs principally are purely inorganic whereas related reports on organic-inorganic RECIPOTs remain underdeveloped. Furthermore, the introduction of organic ligands lies in the fact that they not only can serve as structure-directing agents, but act as bridging units to connect RE and tungsten atoms to construct novel structural topologies. As a proof, in 2014, our group isolated two classes of oxalate-bridging tetra-RE-containing lacunary Lindqvist IPOTs {[RE2(C2O4) (H2O)4(OH)W4O16]2}10-and {[RE(C2O4)W5O18]4}20-(RE = Eu3+, Ho3+, Er3+, Tb3+)[24]. According to previous reports, there are few examples of using rigid organic ligands to obtain organic-inorganic hybrid RECIPOTs.

1 Experimental
1.1 Physical measurements
The IR spectrum was made from a solid sample palletized with KBr on a Bruker VERTEX 70 IR spectrometer in the range of 4 000-400 cm-1. The thermal stability was measured by thermogravimetric analysis using a Mettler Toledo TGA/SDTA 851einstrument under N2atmosphere with 10 ℃· min-1as heating rate. X-ray diffraction intensity data of1were collected on a Bruker D8 Venture Photon II detector at 150 K with Mo Kαradiation (λ= 0.710 73 nm).
1.2 Synthesis of 1
Na2WO4·2H2O (1.000 g, 3.032 mmol), K8Na2[A-α-GeW9O34]·25H2O (2.000 g, 0.680 mmol), NH2(CH3)2·HCl (1.750 g, 38.043 mmol) and HPCA (0.200 g, 1.612 mmol) were dissolved in 25.0 mL of water. The pH of the solution was adjusted to 3.20 by using 6 mol·L-1HCl solution. Then, Ce(NO3)3·6H2O (0.350 g, 0.806 mmol) was added. The final pH was adjusted to 5.00 by using 4 mol·L-1NaOH solution. The synthetic solution was stirred for 15 min, heated under 90 ℃ water bath for two hours and filtered. The filtrate was slowly evaporated at room temperature. After about thirty days, yellow block crystals of1were obtained. Yield: 0.777 g (29.2 % based on Ce(NO3)3·6H2O). Although K8Na2[A-α-GeW9O34]·25H2O was used as a staring material in the synthetic process, unexpectedly, it wasn’t observed in the structure of1. Some contrast experiments have been performed, when K8Na2[A-α-GeW9O34]·25H2O was removed from the reaction system,1can’t be afforded, which suggests that K8Na2[A-α-GeW9O34]·25H2O plays a synergistic effect in the formation of1. The specific reaction mechanism is not well understood. In the following time, we will continue to explore it.
2 Results and discussion
The crystal data of1are listed in Table 1.

Table 1 Crystal data and structural refinements of 1
2.1 Crystal structure
1crystallizes in the triclinic space groupP-1 and its molecular structural unit is a centrosymmetric dimeric polyoxoanion [Ce2(H2O)6W22O74(HPCA)2]10-(1a, Fig.1a).1a can be deemed as the fusion of two monovacant [W11O38]10-units and two supporting [Ce(H2O)3(HPCA)]3+fragments (Fig.1b). Interestingly, two [W11O38]10-units are connected in the corner-sharing mode by two W-O-W bonds, giving rise to the rarely seen docosatungstate anion [W22O74]16-. At the same time, the docosatungstate anion [W22O74]16-is supported by two metal-organic [Ce(H2O)3(HPCA)]3+fragments (Fig.1c). It should be pointed out that two Ce3+cations are located at the vacant site of the [W11O38]10-subunit. In the [Ce(H2O)3(HPCA)]3+fragment, O and N atoms on HPCA ligand simultaneously coordinate with the Ce13+cation to form one stable five-membered ring, which can enhance of structural stability of1a. The crystallographically independent Ce13+cation in1a exhibits the distorted tri-capped square antiprism geometry. To be specific, the Ce13+cation is surrounded by two oxygen atoms (O7, O12) from [W11O38]10-fragment [Ce1-O7: 0.262 1(3) nm, Ce1-O12: 0.249 4(2) nm], one oxygen atom (O26) and nitrogen atom (N1) from HPCA ligand [Ce1-O26: 0.242 9(3) nm and Ce1-N1: 0.272 6(2) nm], two oxygen atom (O2, O39) from another [W11O38]10-fragment [Ce1-O2: 0.247 5(2) nm and Ce1-O39: 0.258 8(2) nm] and three oxygen atoms (O1W, O2W, O3W) from aqua ligands [Ce1-O1W: 0.252 2 (3) nm, Ce1-O2W: 0.248 1(2) nm and Ce1-O3W: 0.251 0(3) nm ] (Fig.1d). Notably, adjacent structural units are connected each other by Ce3+cations, generating the 1D chain (Fig.1e).

Fig.1 (a-b) The [Ce2(H2O)6W22O74(HPCA)2]10- polyoxoanion in 1; (c) The docosatungstate anion [W22O74]16- supported by two metal-organic [Ce(H2O)3(HPCA)]3+ fragments; (d) The mono-capped square antiprism geometry of the Ce13+ cation; (e) The 1D chain-like structure of 1 constructed from [Ce2(H2O)6W22O74(HPCA)2]10- anions through Ce3+ linkers; Color codes: [W11O38]10- octahedra: light orange, Ce atom: purple, O atom: red, W atom: turquoise, C atom: black, N atom: blue
Further, the 3D packing arrangements of1a polyoxoanions along thea,bandcaxis are quite fascinating, which are obtained by omitting [H2N(CH3)2]+, K+, Na+and lattice water molecules. Viewing along thea,bandcaxis, the1a polyoxoanions are arranged in the -ABAB- pattern (Fig.2a-c). Each1a polyoxoanion is simplified as a parallelogram, in which W8, W10, W8A and W10A atoms are located on four vertexes of the parallelogram. As shown in Fig.2d-2f,1a polyoxoanions are arranged in the -ABAB- pattern. Further, neighbor two layers are arranged in the staggered mode so as to reduce steric hindrance.
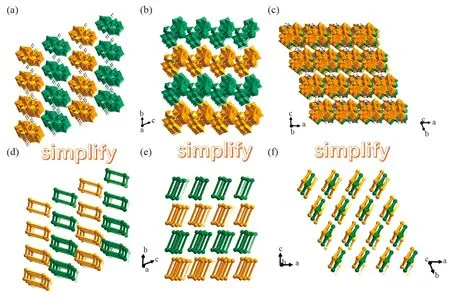
Fig.2 (a-c) Views of 3D packing arrangements of [Ce2(H2O)6W22O74(HPca)2]10- polyoxoanions along the a, b and c axes; (d-f) Simplified view of 3D packing arrangements of [Ce2(H2O)6W22O74(HPca)2]10- polyoxoanions along the a, b and c axes
2.2 IR spectrum
The structure of1was further corroborated by IR spectrum (Fig.3). It is obviously seen that the characteristic absorption peaks in the low wavenumber range (ν< 1 000 cm-1) appear at 948, 883, 812 and 708 cm-1correspond to terminal ν(W-Ot), corner-sharingν(W-Ob) and edge-sharing ν(W-Oc) vibration bands of the polyoxotugnstate anion[25-26]. In the high wavenumber region (ν > 1 000 cm-1), an intense and broad absorption band at ca. 3 435 cm-1as well as a strong absorption band at 1 620 cm-1is ascribed to stretching and bending vibration modes of water molecules[27], whereas the two weak bands appearing at 3 152 and 2 786 cm-1are assigned to theν(N-H) andν(C-H) stretching vibrations, respectively[28]. It should be noted that there is some overlap between the carboxylic absorption band of HPCA ligands and the intense bending vibration band of water molecules. The appearance of the asymmetric and symmetric vibration absorption bands of the carboxylic group at 1 582 cm-1and 1 466 cm-1is indicative of the presence of HPCA ligands[29].

Fig.3 IR spectrum of 1
2.3 Thermogravimetric analysis
Fig.4 demonstrates the thermogravimetric analysis curve for1in N2in the temperature range of 25-800 ℃, which displays that1undergoes a two-step weight. The first weight loss of 7.41% (calcd. 7.46%) occurs between 25 and 250 ℃, which can be attributed to the loss of 28 lattice water molecules. Upon continuous heating to 500 ℃, the second weight loss of 9.15% (calcd. 9.26%) happens, which can be assigned to the loss of 6 coordinated water molecules, 6 dimethylamine ligands and 2 pyrazine carboxylic acid.

Fig.4 Thermogravimetric analysis curve of 1
3 Conclusion
In summary, an organic-inorganic hybrid Ce3+-incorporated dimeric IPOT1was successfully synthesized in acidic aqueous solution. It should be noted that the dimeric [Ce2(H2O)6W22O74(HPCA)2]10-polyoxoanion is constructed from two monovacant [W11O38]10-units bridged through two [Ce(H2O)3(HPCA)]3+fragments, which is rather rare in RECIPOTs. The successful preparation of the dimeric [Ce2(H2O)6W22O74(HPCA)2]10-polyoxoanion not only enriches the structural variety of RECIPOTs, but also provides a feasible and convenient synthetic method for designing and assembling more novel organic-inorganic hybrid RECIPOTs. The further research will be made to exploit and discover some innovative IPOT fragments, which are used as the building blocks to combine rare-earth centers and functional multicarboxylic ligands, giving birth to novel organic-inorganic hybrid RECIPOTs with the aim of finding some potential optical and magnetic applications.

